Back to Journals » Journal of Asthma and Allergy » Volume 12
Real-world characteristics and disease burden of patients with asthma prior to treatment initiation with mepolizumab or omalizumab: a retrospective cohort database study
Authors Llanos JP , Bell CF, Packnett E, Thiel E, Irwin DE, Hahn B , Ortega H
Received 4 October 2018
Accepted for publication 27 December 2018
Published 25 January 2019 Volume 2019:12 Pages 43—58
DOI https://doi.org/10.2147/JAA.S189676
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Jean-Pierre Llanos,1 Christopher F Bell,1 Elizabeth Packnett,2 Ellen Thiel,2 Debra E Irwin,2 Beth Hahn,1 Hector Ortega3
1Respiratory, US Medical Affairs, GSK, Research Triangle Park, NC, USA; 2Truven Health Analytics, An IBM Watson Health Company, Ann Arbor, MI, USA; 3Respiratory, US Medical Affairs, GSK, La Jolla, CA, USA
Purpose: Patients with severe asthma are eligible for asthma-specific biologics as add-on therapies, such as mepolizumab and omalizumab, when optimized controller therapies are unable to control their symptoms. However, few real-world data are available to describe the characteristics and associated economic burden of patients considered to be candidates for mepolizumab or omalizumab therapy.
Methods: This retrospective cohort study investigated patients with asthma (≥12 years of age) identified at the time of first mepolizumab or omalizumab administration (index date) in the MarketScan™ Commercial Database. Data were collected during the 12-month period before the index date (baseline period) for two mutually exclusive patient groups (patients prescribed mepolizumab and omalizumab, respectively). Baseline demographics, history of exacerbations, healthcare resource utilization (HCRU), and medical costs were investigated.
Results: In total, 413 and 1,834 patients who had been prescribed mepolizumab or omalizumab, respectively, were identified. During the baseline period, patients prescribed mepolizumab experienced more exacerbations (81.4% vs 57.5%, P<0.001), had higher asthma-related HCRU for outpatient services (all P<0.01), and had higher total asthma-related healthcare costs (US$11,000 vs US$7,400, P<0.001) compared with patients prescribed omalizumab. Allergic rhinitis, atopic dermatitis, and chronic idiopathic urticaria were more common among patients prescribed omalizumab vs mepolizumab. In contrast, sinusitis, nasal polyps, and comorbid COPD were more common among patients prescribed mepolizumab vs omalizumab. Prescriptions of fixed-dose inhaled corticosteroids (ICSs) with long-acting β2-agonists (LABAs) and ICS/LABA/long-acting muscarinic antagonist triple therapy during the baseline period were higher among patients prescribed mepolizumab vs omalizumab (80.4% vs 56.8% and 27.1% vs 14.4%, respectively, both P<0.001).
Conclusion: In the 12 months prior to initiation of asthma-specific biologics, patients prescribed mepolizumab had a different prevalence of certain comorbidities, higher disease burden, higher HCRU, and higher healthcare costs compared with patients prescribed omalizumab.
Keywords: asthma, mepolizumab, biologic, healthcare resource utilization, healthcare costs
Plain language summary
Why was the study done? When patients with severe asthma are unable to control their symptoms with optimized controller therapies, such as inhaled corticosteroids (ICSs), they are eligible for additional treatment with asthma-specific biologic therapies, such as mepolizumab or omalizumab. However, few data are available to describe the disease symptoms and associated healthcare costs of patients eligible for mepolizumab or omalizumab before they start either therapy.
What did the researchers do and find? This study used data from the US MarketScan™ Commercial Database to investigate patients with asthma who were prescribed mepolizumab (Nucala®; GlaxoSmithKline LLC, The Navy Yard, PA, USA) or omalizumab (Xolair®; Novartis Pharmaceuticals, East Hanover, NJ, USA; Genentech Inc, South San Francisco, CA, USA) as an add-on therapy. Patients’ baseline characteristics, frequency of asthma attacks, healthcare utilization, and medical costs were analyzed for the 12-month period before they started treatment with mepolizumab or omalizumab.
Patients prescribed mepolizumab experienced more asthma attacks, had higher asthma-related healthcare utilization for outpatient services, and higher total asthma-related healthcare costs compared with patients prescribed omalizumab. Allergic rhinitis (allergies/hay fever) was more commonly seen in patients prescribed omalizumab compared with mepolizumab, while administration of medications such as fixed-dose ICSs and long-acting β2-agonists was higher in the patients prescribed mepolizumab vs omalizumab.
What do these results mean? Although these findings are reflective of the US Food and Drug Administration approved indications of these therapies, they also suggest that physicians may preferentially prescribe mepolizumab over omalizumab for more severe and difficult-to-treat asthma cases rather than across the full spectrum of eligible patients who could benefit from mepolizumab therapy.
Introduction
Poor asthma control leads to heightened medical expenses, due to increased asthma-related emergency room (ER) visits and inpatient admissions, and lost productivity.1,2 In 2014, the Healthcare Cost and Utilization Project estimated that 1.9 million ER visits and >300,000 hospitalizations in the US were related to asthma.3
Asthma severity is determined by the level of treatment needed to control symptoms and exacerbations in both the Global Initiative for Asthma (GINA) and European Respiratory Society and American Thoracic Society guidelines.4,5 Moderate asthma is defined as being well controlled with a low-dose ICS combined with a long-acting β2-agonist (LABA),4 whereas severe asthma is defined as requiring treatment with high-dose ICS plus a second controller and/or systemic corticosteroids.5
Asthma is a heterogeneous condition with multiple phenotypes, including eosinophilic asthma and allergic asthma.4,5 Guidelines recognize that asthma phenotypes may guide the use of add-on therapies when symptoms persist despite optimized treatment with controller medications.4,5 However, a notable minority of patients meet criteria for more than one asthma phenotype,6 and presently there are no guidelines on treatment strategies for these patients.4
Mepolizumab and omalizumab are two add-on therapies used to treat severe asthma. Mepolizumab is an IL-5 inhibitor indicated for the add-on maintenance treatment of severe asthma with an eosinophilic phenotype (patients ≥12 years) and for the treatment of eosinophilic granulomatosis with polyangiitis (patients ≥18 years).7 Omalizumab is an IgE inhibitor indicated for the treatment of moderate-to-severe asthma inadequately controlled with ICS with signs of allergy (patients ≥6 years), and for H1 antihistamine-refractory chronic idiopathic urticaria (CIU; patients ≥12 years).8 Both have well-established efficacy in asthma4,9–14 and have been shown to reduce asthma exacerbations, ER visits and hospitalizations, and improve health-related quality of life (HRQoL) compared with current standard of care.9–16 However, the asthma populations included in clinical trials with mepolizumab and omalizumab differed. Patients in the mepolizumab trials had asthma with a history of ≥2 exacerbations in the previous year despite regular use of high-dose ICS plus additional controller(s) with or without oral corticosteroids (OCSs), and a blood eosinophil count of either ≥150 cells/µL at screening or ≥300 cells/µL within 12 months of enrollment. In the omalizumab trials, patients had moderate to severe persistent asthma for ≥12 months and a positive skin test reaction to a perennial aeroallergen. As such, the comparative efficacy of mepolizumab and omalizumab is unclear, and no head-to-head comparisons have been conducted to date. Indirect comparisons using network meta-analyses have indicated broadly similar efficacy and safety profiles in patients with severe asthma, although population differences and high rates of outcome heterogeneity precluded any definitive conclusions.17,18 More recently, a single-arm study showed that patients not optimally controlled with omalizumab who were switched directly to mepolizumab and followed for 32 weeks achieved clinically meaningful improvements from baseline in exacerbation rates, asthma control, HRQoL, and lung function, with no new safety issues identified.19,20
Currently, there are a lack of definitive guidelines for selecting between mepolizumab and omalizumab in patients eligible for both treatments. As such, real-world data on patients’ characteristics, burden of disease, and healthcare resource utilization (HCRU) prior to being prescribed mepolizumab or omalizumab would be valuable for improving understanding of current treatment patterns and the clinical events driving prescription of these therapies. Using real-world data derived from a large, geographically diverse employer medical claims database, this study aimed to describe the baseline demographic and clinical characteristics, as well as HCRU and costs, in patients with asthma during a 12-month period prior to being prescribed mepolizumab or omalizumab.
Methods
Study design
This was a retrospective cohort study of patients with asthma with commercial insurance coverage identified from a US administrative insurance database (MarketScan™ Commercial Claims and Encounters Database; Truven Health Analytics, Ann Arbor, MI, USA). The database contains the healthcare experience of privately insured individuals covered under a variety of fee-for-service, fully capitated, and partially capitated health plans. Data are contributed by employers and health plan organizations. Further information on this database can be found in the supplementary materials.
Patients were identified between November 1, 2015 and March 31, 2017 (patient identification period) at the time of first mepolizumab or omalizumab administration (index date). Data were examined for each patient during the 12 months prior to, and including, the index date (baseline period); exacerbation rates were also examined for the 3-month period immediately prior to index. Patient assignment to treatment groups is described in the supplementary materials.
All database records were fully de-identified and fully compliant with US patient confidentiality requirements, including the Health Insurance Portability and Accountability Act of 1996. As patients could not be identified, no patient consent was required.
Patients
Eligible patients were ≥12 years of age with a medical or pharmacy claim with a Healthcare Common Procedure Coding System (HCPCS) or National Drug Code (NDC) indicating first administration of mepolizumab (HCPCS: C9473, J2182; NDC: 00173-0881-01) or omalizumab (HCPCS: J2357; NDC: 50242-0040-62, 50242-0040-01) during the patient identification period. HCPCS is a standardized coding system used to describe specific healthcare procedures or services provided; NDCs are universal product identifiers for human drugs in the US. Eligible patients also had 12 months of continuous enrollment with medical and pharmacy benefits prior to and including the date of the first administration of mepolizumab or omalizumab (index date). Details on the exclusions of patients are shown in the supplementary materials.
End points and assessments
Demographics were reported at the index date. End points were assessed for two different periods: the baseline period (12 months prior to and including the index date) and the 3-month period immediately prior to the index date. End points in the baseline period included: comorbid conditions, treatment history, any asthma exacerbation and asthma exacerbation requiring hospitalization, all-cause and asthma-related HCRU, and all-cause and asthma-related costs. In addition, exacerbation rates were assessed for the 3-month period immediately prior to the index date.
Comorbid conditions were identified using diagnosis codes present on medical claims during the baseline period; patients were required to have at least one nondiagnostic medical claim with the diagnosis (ICD–9/ICD-10 codes) during the baseline period.
Exacerbations were defined as requiring 1) an outpatient or ER visit with asthma diagnosis recorded in any position on the claim and at least one dispensing of systemic corticosteroids (oral, intravenous, or intramuscular) within 7 days before or after the encounter or 2) hospitalization with asthma as a primary diagnosis. Exacerbations requiring hospitalization were defined as those requiring hospitalization with asthma as a primary diagnosis. Exacerbations within 14 days of each other were considered a single exacerbation.
HCRU included inpatient services, outpatient services, and pharmacy services. Inpatient services were based on the presence of insurance claims occurring in an inpatient setting during a hospitalization. All services administered during the hospital stay are included. Outpatient services included encounters and claims for services rendered in a doctor’s office, hospital outpatient facility, ER, or other outpatient facility (eg, radiology services, laboratory tests, outpatient infusion). Outpatient pharmacy services were identified by prescription claims.
All-cause and asthma-related costs were based on medical costs associated with inpatient services (including claims associated with the admission such as hospital claims, physician claims, surgeon claims, and claims from independent laboratories), outpatient services, and outpatient pharmacy services. All costs were adjusted for inflation using the Consumer Price Index and standardized to 2016 US dollars. Further details of asthma-related and all-cause costs can be found in the supplementary materials.
Statistical analysis
All study variables were analyzed descriptively, stratified by treatment group and presented as percentages for categorial variables and mean (SD) or median for continuous variables. Statistical comparisons of mepolizumab vs omalizumab were provided using appropriate tests based on the distribution of the measure (Student’s t-test, Mann–Whitney U test, or chi-squared test), with statistical significance evaluated at the α=0.05 level. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Ethics approval and informed consent
This study utilized de-identified retrospective claims data, and as such, this study did not require institutional review board review and approval or informed consent.
Results
Patient population and demographics
A total of 413 and 1,834 patients were included in the patients prescribed mepolizumab and omalizumab, respectively (Figure 1). Patient demographics at the index date are shown in Table 1. Compared with patients prescribed omalizumab, patients prescribed mepolizumab were slightly older, with a greater proportion of patients ≥55 years, and more likely to have an index year that was later in the patient identification period (both P<0.001). No significant differences were found between patients prescribed mepolizumab or omalizumab for type of commercial insurance (P=0.174).
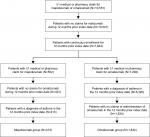  | Figure 1 Patient flow diagram. |
Comorbidities and asthma treatment during the 12-month baseline period
The most commonly observed comorbidities were allergic rhinitis, respiratory infections, sinusitis, and COPD for both treatment cohorts (Table 2). The occurrence of respiratory infections was similar in both groups (P=0.784). Compared with patients prescribed omalizumab, patients prescribed mepolizumab had significantly lower prevalence of allergic rhinitis, atopic dermatitis, and CIU (all P≤0.002). However, a significantly higher proportion of patients prescribed mepolizumab vs omalizumab had sinusitis, COPD, nasal polyps, and hypereosinophilic syndrome (all P≤0.001).
The most common asthma treatments (used by >50% of patients) in both cohorts were OCS for acute and chronic use, short-acting β2-agonists (SABA), fixed-dose combinations of ICS with a LABA, and leukotriene receptor antagonists (Table 2). The number of patients prescribed immunosuppressive therapies in the 12-month baseline period was ~1% among patients prescribed mepolizumab and omalizumab (supplementary materials).
Asthma exacerbations during the baseline periods
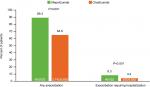
During the 3-month and 12-month baseline periods, significantly more patients that were prescribed mepolizumab had any exacerbation compared with those prescribed omalizumab (both P<0.001) (Figure 2A, B). The proportion of patients who experienced exacerbations requiring hospitalization was numerically higher in the mepolizumab group vs the patients prescribed omalizumab at 3 months and 12 months, but the differences were not statistically significant (P=0.505 and P=0.121, respectively).
  | Figure 2 Proportion of patients with exacerbations during the (A) 3-month and (B) 12-month baseline period. |
HCRU and expenditure during the 12-month baseline period
A high proportion of patients in both groups (>86%) had an asthma-related outpatient office visit or pharmacy prescription during the 12-month baseline period; however, these services were accessed by a significantly higher proportion of patients receiving mepolizumab compared with omalizumab (Figure 3). A comparable proportion of patients in both groups experienced an asthma-related inpatient admission or an asthma-related ER visit (Figure 3). Similar patterns were observed regarding the mean number of services accessed (Table 3).
A similar proportion of patients in both groups (>98%) had an all-cause outpatient visit or pharmacy prescription during the 12-month baseline period (Figure S1). However, the mean number of all-cause outpatient office visits and pharmacy prescriptions was significantly higher in patients who were subsequently prescribed mepolizumab (Table S1). All-cause inpatient admissions were seen in a significantly higher proportion of patients prescribed mepolizumab, and these patients also had a significantly longer inpatient stay vs patients subsequently prescribed omalizumab (Figure S1 and Table S1). The proportion of patients with ≥1 all-cause ER visit was significantly higher in the patients prescribed omalizumab (43.1% vs 37.0%; P=0.025); however, the mean number of ER visits per patient was significantly higher in the patients prescribed mepolizumab (Figure S1 and Table S1).
The mean total asthma-related healthcare costs incurred during the baseline period were significantly higher among patients prescribed mepolizumab (US$11,000) compared with patients prescribed omalizumab (US$7,400, P<0.001) (Figure 4A). The cost categories that were significantly higher for patients prescribed mepolizumab vs omalizumab included outpatient costs (US$6,295 vs US$4,114, P=0.002) and outpatient pharmacy costs (US$3,044 vs US$1,909, P<0.001). Outpatient costs for office visits and other outpatient services were both significantly higher in the patients prescribed mepolizumab vs omalizumab (both P≤0.005). ER visit costs were comparable between the two groups.
  | Figure 4 Asthma-related (A) and total (B) healthcare expenditure during the 12-month baseline period. |
Similar to the asthma-related healthcare costs, all-cause healthcare costs during the 12-month baseline period were significantly higher for patients prescribed mepolizumab compared with the omalizumab, including total healthcare costs (US$34,825 vs US$25,559, P<0.001), outpatient costs (US$18,690 vs US$14,660, P=0.020), and outpatient pharmacy costs (US$8,719 vs US$5,754, P<0.001) (Figure 4B).
We also conducted a sensitivity analysis in which patients who had received omalizumab or mepolizumab during the baseline period were included. Patient demographics, clinical characteristics, asthma exacerbations, and HCRU were generally consistent with the main analysis (Figures S2 and S3 and Tables S2–S4). Although total asthma-related healthcare costs were significantly higher among patients prescribed omalizumab compared with patients prescribed mepolizumab (Figure S4), this likely reflects the fact that there was a greater proportion of patients with prior omalizumab use in the omalizumab group (who would have corresponding pharmacy costs for biologic therapy) than the mepolizumab group.
Discussion
This study described the baseline characteristics, disease burden, and HCRU of patients with asthma in the 12 months prior to initiation of mepolizumab or omalizumab in a real-world setting. Overall, disease burden and HCRU in the prior year were higher for patients who were subsequently prescribed mepolizumab compared with those subsequently prescribed omalizumab. These patterns were also seen in healthcare expenditures; patients subsequently prescribed mepolizumab had both greater asthma-related and all-cause healthcare expenditures than those subsequently prescribed omalizumab.
The differences observed between patients prescribed mepolizumab or omalizumab reflect the US Food and Drug Administration approved indications of the two asthma-specific biologics.4,5,7,8 This difference was also reflected in the extent of asthma medications prescribed in the 12 months prior to mepolizumab or omalizumab prescription. For example, a higher proportion of patients subsequently prescribed mepolizumab vs omalizumab were prescribed ICS-based therapy in the prior year, although it should be noted that no information on relative dose levels was collected. Additionally, HCRU and costs in the previous year were much higher for patients prescribed mepolizumab than omalizumab. This is likely due to higher numbers of patients with uncontrolled severe asthma who experienced exacerbations in the mepolizumab vs omalizumab cohort (81.4% vs 57.5%, respectively). The relationship between healthcare costs, exacerbations, and asthma control was previously described in a UK and US database study, which reported that the mean asthma-related healthcare cost per exacerbation in patients with severe uncontrolled asthma was almost double that found in patients without severe uncontrolled asthma (US$911 vs US$486).21 Another explanation for the lower burden of disease among patients subsequently prescribed omalizumab could be that ~20% of these patients had comorbid CIU. As such, these patients may have had milder asthma, requiring lower levels of ICS maintenance therapy, and lower HCRU and costs.
Drivers for prescription of mepolizumab or omalizumab to patients with severe asthma include exacerbation risk reduction and OCS dose sparing. Randomized controlled trials have demonstrated reductions in exacerbation rates with mepolizumab9,10 or omalizumab,12–14 and a reduction in maintenance OCS dose with mepolizumab, in patients with asthma.10 An observational study and a pooled analysis of two randomized controlled trials also suggested an OCS dose-sparing effect with omalizumab.22,23 In the current study, a notable proportion of patients prescribed mepolizumab or omalizumab had not experienced an exacerbation in the 12 months (19% and 46% of patients, respectively) or 3 months (49% and 65% of patients, respectively) prior to prescription. Add-on therapy use may be influenced by factors other than exacerbation risk, such as OCS sparing effect or, for patients prescribed omalizumab, CIU diagnosis. Moreover, treatment decisions may have been influenced by additional clinical information that may not be reflected in the claims database. While OCS treatment can be useful when patients fail to respond to controller medications,4,5 there is the potential for debilitating side effects with chronic use.4 A previous real-world study of asthma-specific biologic therapy eligibility in patients with severe asthma showed that mepolizumab- and omalizumab-eligible populations have broadly similar demographics but did report more frequent maintenance OCS use in mepolizumab-eligible patients.24 However, the relative proportion of patients receiving maintenance vs short-term OCS in this study were not recorded.
GINA guidelines recommend add-on therapies for asthma that is uncontrolled on step 4 treatment, which includes moderate- to high-dose ICS/LABA maintenance treatment, medium-dose ICS/formoterol as maintenance and reliever, and high-dose ICS plus a second controller. Anti-IL-5 add-on therapies (eg, mepolizumab) are recommended for severe eosinophilic asthma that is uncontrolled despite step 4 treatment, whereas omalizumab add-on therapy is recommended for uncontrolled severe allergic asthma with elevated IgE levels.4 Based on these guidelines, physicians treating patients who remain uncontrolled on step 4 treatment may select omalizumab for patients with an allergic phenotype and mepolizumab for those with an eosinophilic phenotype. In the current study, comorbid conditions associated with upper respiratory eosinophil involvement (nasal polyps and sinusitis25,26) as well as other respiratory conditions (COPD) were more frequent in the patients prescribed mepolizumab than omalizumab. In contrast, allergic conditions (allergic rhinitis, atopic dermatitis, and CIU) were more frequent in the patients prescribed omalizumab. These data suggest that the distinct GINA guidelines for severe allergic and severe eosinophilic asthma phenotypes did influence prescription patterns of add-on therapy. However, evidence from real-world settings shows that eosinophilic and allergic asthma phenotypes are overlapping rather than discrete conditions, and that a considerable proportion of patients with severe asthma (~37%) are eligible for both mepolizumab and omalizumab.24 Similarly, a post hoc analysis of the Phase III MENSA and MUSCA studies, which assessed the effect of subcutaneous mepolizumab 100 mg plus standard of care in patients with severe eosinophilic asthma, found that 27% of 936 patients included in the analysis were also eligible for omalizumab therapy according to US prescribing criteria.27 Addition of mepolizumab was also associated with improvements vs placebo in asthma control, HRQoL and OCS use irrespective of prior omalizumab use.28 More recently, a prospective, single-arm study confirmed that patients who are suboptimally controlled on omalizumab and who switch directly to mepolizumab add-on treatment can achieve significant improvement in exacerbation rates, lung function, health status, and asthma control, with 77% of patients experiencing the minimal clinically important difference of ≥0.5-point reduction in Asthma Control Questionnaire-5 score.19,20 Together, these data suggest that mepolizumab could be a suitable option for those patients who exhibit severe uncontrolled asthma with both allergic and eosinophilic phenotype markers and are, therefore, eligible for both mepolizumab and omalizumab.
Limitations of this study include: first, data do not represent the entire US population, given that these data were obtained from patients with commercial insurance in the US and no patients insured through Medicare and Medicaid were included. Second, during the 12-month baseline period, the asthma severity classification of each patient was not defined, making it difficult to determine if eligibility requirements of each asthma-specific biologic were being correctly followed. Third, the US approval of omalizumab for severe asthma in 200329 precedes the approval of mepolizumab30 by 12 years, meaning that physicians have additional years of prescribing experience with omalizumab, which could influence prescribing habits. Fourth, some patients may not have been receiving optimized controller therapy, which may have impacted on asthma control and subsequent HCRU and costs. Alternatively, the nature of the study design and data availability may also have impacted this output. Fifth, data on asthma-related exacerbations, HCRU, and costs may have been influenced by nonasthma diagnoses also included on the claim. Finally, among patients prescribed omalizumab, 21.2% had comorbid CIU, which is also indicated for omalizumab treatment. It is possible that some of these patients had milder asthma, which would not warrant omalizumab treatment in the absence of the CIU diagnosis. This would have resulted in a lower burden of disease and thereby lower HCRU and healthcare costs in this cohort compared with the mepolizumab cohort. In contrast, 15.3% of patients prescribed mepolizumab had features of hypereosinophilic syndrome, which may have been associated with more severe disease, higher HCRU, and higher healthcare costs.
Conclusion
The results of this real-world US study show that, in the 12 months prior to prescription, patients who were subsequently prescribed mepolizumab had a greater disease burden than those subsequently prescribed omalizumab. Patients prescribed mepolizumab also had significantly greater prior asthma-related HCRU and healthcare costs compared with those prescribed omalizumab. These results suggest that physicians may currently preferentially prescribe mepolizumab in more refractory cases of severe eosinophilic asthma, rather than across the full spectrum of eligible patients who could benefit from mepolizumab therapy. In addition, perceptions about the allergic phenotype may still play a role in the decision to prescribe omalizumab. As more experience with mepolizumab is gained among treating physicians, a shift toward mepolizumab prescription across the full range of eligible patients with severe asthma might be seen.
Data availability
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data, and for clinical studies not listed, please submit an enquiry via the website.
Acknowledgments
We would like to thank Christina Larson-Chebili for her assistance during the data acquisition phase of the study and SAS programming. Editorial support (in the form of writing assistance, including development of the initial draft based on author direction, assembling tables and figures, collating and incorporating authors’ comments, grammatical editing, and referencing) was provided by Mary E Morgan, PhD, of Fishawack Indicia Ltd, UK, and was funded by GSK. This study was funded by GSK (study identifier: HO-16–16497).
Author contributions
All authors contributed to the conception and the design of the study. EP, ET, and DEI were involved in data acquisition; BH, CFB, J-PL, and HO analyzed and interpreted the data. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
J-PL, CFB, and BH are GSK employees and hold stock/shares. HO was an employee of GSK and holds stocks/shares in GSK during the conduct of this study and is now employed by Gossamer Bio. EP, ET, and DEI are Truven Health Analytics, An IBM Watson Health Company employees. The authors report no other conflicts of interest in this work.
References
Accordini S, Corsico AG, Braggion M, et al. The cost of persistent asthma in Europe: an international population-based study in adults. Int Arch Allergy Immunol. 2013;160(1):93–101. | ||
Global Initiative for Asthma. Online Appendix – Global Strategy for Asthma Management and Prevention; 2018. Available from: https://ginasthma.org/2017-online-appendix-global-strategy-for-asthma-management-and-prevention/. Accessed September 18, 2018. | ||
Agency for Healthcare Research and Quality. Healthcare cost and utilization project; 2017. Available from: https://hcupnet.ahrq.gov/#setup. Accessed September 18, 2018. | ||
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention; 2018. Available from: https://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/. Accessed September 18, 2018. | ||
Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. | ||
Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368(9537):804–813. | ||
GlaxoSmithKline. Nucala prescribing information; 2017. Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Nucala/pdf/NUCALA-PI-PIL.PDF. Accessed September 18, 2018. | ||
Genentech USA. Xolair prescribing information; 2018. Available from: https://www.gene.com/download/pdf/xolair_prescribing.pdf. Accessed September 18, 2018. | ||
Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. | ||
Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. | ||
Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3B trial. Lancet Respir Med. 2017;5(5):390–400. | ||
Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184–190. | ||
Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–316. | ||
Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573–582. | ||
Yancey SW, Ortega HG, Keene ON, et al. Meta-analysis of asthma-related hospitalization in mepolizumab studies of severe eosinophilic asthma. J Allergy Clin Immunol. 2017;139(4):1167–1175.e2. | ||
Corren J, Casale T, Deniz Y, Ashby M. Omalizumab, a recombinant humanized anti-IgE antibody, reduces asthma-related emergency room visits and hospitalizations in patients with allergic asthma. J Allergy Clin Immunol. 2003;111(1):87–90. | ||
Cockle SM, Stynes G, Gunsoy NB, et al. Comparative effectiveness of mepolizumab and omalizumab in severe asthma: an indirect treatment comparison. Respir Med. 2017;123:140–148. | ||
Nachef Z, Krishnan A, Mashtare T, Zhuang T, Mador MJ. Omalizumab versus mepolizumab as add-on therapy in asthma patients not well controlled on at least an inhaled corticosteroid: a network meta-analysis. J Asthma. 2018;55(1):89–100. | ||
Albers FC, Liu MC, Chipps BE, et al. Efficacy and safety of mepolizumab in uncontrolled patients with severe eosinophilic asthma following a switch from omalizumab (OSMO study): asthma control, quality of life and lung function outcomes. J Allergy Clin Immunol. 2018;141(2):AB408. | ||
Galkin D, Liu MC, Chipps BE, et al. Efficacy and safety of mepolizumab in uncontrolled patients with severe eosinophilic asthma following a switch from omalizumab (OSMO study): exacerbation and safety outcomes. J Allergy Clin Immunol. 2018;141(2):AB409. | ||
Suruki RY, Daugherty JB, Boudiaf N, Albers FC. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017;17(1):74. | ||
Bhutani M, Yang WH, Hébert J, de Takacsy F, Stril JL. The real world effect of omalizumab add on therapy for patients with moderate to severe allergic asthma: the ASTERIX observational study. PLoS One. 2017;12(8):e0183869. | ||
Karpel J, Massanari M, Geba GP, Kianifard F, Inhaber N, Zeldin RK. Effectiveness of omalizumab in reducing corticosteroid burden in patients with moderate to severe persistent allergic asthma. Ann Allergy Asthma Immunol. 2010;105(6):465–470. | ||
Albers FC, Müllerová H, Gunsoy NB, et al. Biologic treatment eligibility for real-world patients with severe asthma: the IDEAL study. J Asthma. 2018;55(2):152–160. | ||
Shah SA, Ishinaga H, Takeuchi K. Pathogenesis of eosinophilic chronic rhinosinusitis. J Inflamm (Lond). 2016;13(1):11. | ||
Tashkin DP, Wechsler ME. Role of eosinophils in airway inflammation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2018;13:335–349. | ||
Liu M, Humbert M, Bratton D. Effect of mepolizumab (100 mg subcutaneous) on exacerbation rate in patients with severe eosinophilic asthma by omalizumab eligibility (US criteria), and immunoglobulin E and eosinophilic subgroups. Am J Respir Crit Care Med. 2018;197(A7657). | ||
Magnan A, Bourdin A, Prazma CM, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy. 2016;71(9):1335–1344. | ||
Genentech.. FDA Approves Xolair, biotechnology breakthrough for asthma; 2003. Available from: https://www.gene.com/media/press-releases/6287/2003-06-20/fda-approves-xolair-biotechnology-breakt. Accessed September 18, 2018. | ||
GlaxoSmithKline. GSK’s Nucala (mepolizumab) receives approval from US FDA; 2015. Available from: https://us.gsk.com/en-us/media/press-releases/2015/gsk-s-nucala-mepolizumab-receives-approval-from-us-fda/. Accessed September 18, 2018. |
Supplementary materials
Methods
MarketScan™ commercial claims and encounters database
This database contains information about privately insured individuals with fee-for-service, fully capitated, and partially capitated health plans. The plans include preferred provider organizations, point-of-service plans, indemnity plans, and health maintenance organizations.
Assignment of patients to treatment group
Assignment to treatment groups was hierarchical to ensure that each patient would be included in one treatment group. The patients administered mepolizumab during the patient identification period were selected first regardless of omalizumab use in that same time period; the remaining omalizumab patients, with no evidence of mepolizumab use during the patient identification period, were assigned to the omalizumab treatment group. After this initial assignment, patients prescribed mepolizumab with evidence of omalizumab use in the patient identification period were excluded.
Exclusion criteria
Patients were excluded if they did not have a diagnosis of asthma in the 12 months prior to, or on, the index date (ICD-9 493.xx or ICD-10 J45.xx), or if they used omalizumab during the 12 months prior to the index date. Patients were also excluded if they had a chronic idiopathic urticaria diagnosis in the absence of an asthma diagnosis in the 12 months prior to initiating omalizumab.
Asthma-related costs
Asthma-related costs were a subset of all-cause costs calculated from claims with an asthma diagnosis in any position on outpatient/emergency room claims and in the primary position on inpatient claims. Costs were calculated from paid amounts of adjudicated claims, including insurer and health plan payments, as well as patient cost-sharing in the form of co-payment, deductible, and co-insurance.
Results
Immunosuppressive therapy use in the 12-month baseline period
Overall, the number of patients receiving immunosuppressive therapies (tumor necrosis factor inhibitors, interleukin inhibitors, abatacept, alemtuzumab, belimumab, canakinumab/pf, glatiramer acetate, interferon-β-1a, interferon-β-1a/albumin human, interferon-β-1b, peginterferon-β-1a, rituximab, tofacitinib citrate, vedolizumab, natalizumab, and ocrelizumab) during the 12-month baseline period was similar (only 1.0%–1.2%).
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.