Back to Journals » Journal of Asthma and Allergy » Volume 15
Real World Biologic Use and Switch Patterns in Severe Asthma: Data from the International Severe Asthma Registry and the US CHRONICLE Study
Authors Menzies-Gow AN, McBrien C, Unni B, Porsbjerg CM, Al-Ahmad M , Ambrose CS , Dahl Assing K, von Bülow A, Busby J, Cosio BG , FitzGerald JM, Garcia Gil E, Hansen S, Heaney LG, Hew M, Jackson DJ, Kallieri M, Loukides S , Lugogo NL, Papaioannou AI , Larenas-Linnemann D , Moore WC , Perez-de-Llano LA , Rasmussen LM, Schmid JM, Siddiqui S, Alacqua M, Tran TN, Suppli Ulrik C , Upham JW , Wang E, Bulathsinhala L, Carter VA, Chaudhry I, Eleangovan N, Murray RB , Price CA, Price DB
Received 9 July 2021
Accepted for publication 23 December 2021
Published 13 January 2022 Volume 2022:15 Pages 63—78
DOI https://doi.org/10.2147/JAA.S328653
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Andrew N Menzies-Gow,1 Claire McBrien,2 Bindhu Unni,3 Celeste M Porsbjerg,4 Mona Al-Ahmad,5 Christopher S Ambrose,6 Karin Dahl Assing,7 Anna von Bülow,4 John Busby,8 Borja G Cosio,9 J Mark FitzGerald,10 Esther Garcia Gil,11 Susanne Hansen,12 Liam G aHeaney,8 Mark Hew,13,14 David J Jackson,15,16 Maria Kallieri,17 Stelios Loukides,17 Njira L Lugogo,18 Andriana I Papaioannou,17 Désirée Larenas-Linnemann,19 Wendy C Moore,20 Luis A Perez-de-Llano,21 Linda M Rasmussen,22 Johannes M Schmid,23 Salman Siddiqui,24 Marianna Alacqua,25 Trung N Tran,6 Charlotte Suppli Ulrik,26 John W Upham,27 Eileen Wang,28,29 Lakmini Bulathsinhala,3,30 Victoria A Carter,3,30 Isha Chaudhry,3,30 Neva Eleangovan,3,30 Ruth B Murray,3,30 Chris A Price,3,30 David B Price3,30,31
1UK Severe Asthma Network and National Registry, Royal Brompton & Harefield Hospitals, London, UK; 2Kingston Hospital, London, UK; 3Observational and Pragmatic Research Institute, Singapore, Singapore; 4Respiratory Research Unit, Bispebjerg University Hospital, Copenhagen, Denmark; 5Al-Rashed Allergy Center, Ministry of Health, Microbiology Department, Faculty of Medicine, Kuwait University, Kuwait, Kuwait; 6AstraZeneca, Gaithersburg, MD, USA; 7Department of Respiratory Medicine, Aalborg University Hospital, Aalborg, Denmark; 8UK Severe Asthma Network and National Registry, Queen’s University Belfast, Belfast, Northern Ireland; 9Son Espases University Hospital-IdISBa-Ciberes, Mallorca, Spain; 10The Centre for Lung Health, Vancouver Coastal Health Research Institute, UBC, Vancouver, Canada; 11AstraZeneca, Barcelona, Spain; 12Center for Clinical Research and Prevention, Bispebjerg and Frederiksberg Hospital, Copenhagen, Denmark; 13Allergy, Asthma & Clinical Immunology Service, Alfred Health, Melbourne, Australia; 14Public Health and Preventive Medicine, Monash University, Melbourne, Australia; 15UK Severe Asthma Network andNational Registry, Guy’s and St Thomas’ NHS Trust, London, UK; 16School of Immunology & Microbial Sciences, King’s College London, London, UK; 17 2nd Respiratory Medicine Department, National and Kapodistrian University of Athens Medical School, Attikon University Hospital, Athens, Greece; 18Department of Medicine, Division of Pulmonary and Critical Care Medicine, University of Michigan, Ann Arbor, MI, USA; 19Directora Centro de Excelencia en Asma y Alergia, Hospital Médica Sur, Ciudad de México, Mexico; 20Pulmonary, Critical Care, Allergy, and Immunologic Medicine, Wake Forest School of Medicine, Winston-Salem, NC, USA; 21Department of Respiratory Medicine, Hospital Universitario Lucus Augusti, Lugo, Spain; 22Allergy Clinic, Department of Dermato-Allergology, Gentofte Hospital, Copenhagen, Denmark; 23University Hospital of Aarhus, Aarhus, Denmark; 24University of Leicester, Department of Respiratory Sciences & NIHR Leicester Biomedical Research Centre (Respiratory Theme), Leicester, UK; 25AstraZeneca, Cambridge, UK; 26Department of Respiratory Medicine, Copenhagen University Hospital-Hvidovre, Hvidovre, Denmark; 27Diamantina Institute & PA-Southside Clinical Unit, The University of Queensland, Brisbane, Australia; 28Division of Allergy & Clinical Immunology, Department of Medicine, National Jewish Health, Denver, CO, USA; 29Division of Allergy & Clinical Immunology, Department of Internal Medicine, University of Colorado School of Medicine, Aurora, CO, USA; 30Optimum Patient Care, Cambridge, UK; 31Centre of Academic Primary Care, Division of Applied Health Sciences, University of Aberdeen, Aberdeen, UK
Correspondence: David B Price
Observational and Pragmatic Research Institute, 22 Sin Ming Lane, #06 Midview City, Singapore, 573969 Tel +65 3105 1489
Email [email protected]
Introduction: International registries provide opportunities to describe use of biologics for treating severe asthma in current clinical practice. Our aims were to describe real-life global patterns of biologic use (continuation, switches, and discontinuations) for severe asthma, elucidate reasons underlying these patterns, and examine associated patient-level factors.
Methods: This was a historical cohort study including adults with severe asthma enrolled into the International Severe Asthma Registry (ISAR; http://isaregistries.org, 2015– 2020) or the CHRONICLE Study (2018– 2020) and treated with a biologic. Eleven countries were included (Bulgaria, Canada, Denmark, Greece, Italy, Japan, Kuwait, South Korea, Spain, UK, and USA). Biologic utilization patterns were defined: 1) continuing initial biologic; 2) stopping biologic treatment; or 3) switching to another biologic. Reasons for discontinuation/switching were recorded and comparisons drawn between groups.
Results: A total of 3531 patients were included. Omalizumab was the most common initial biologic in 2015 (88.2%) and benralizumab in 2019 (29.6%). Most patients (79%; 2791/3531) continued their first biologic; 10.2% (356/3531) stopped; 10.8% (384/3531) switched. The most frequent first switch was from omalizumab to an anti–IL-5/5R (49.6%; 187/377). The most common subsequent switch was from one anti–IL-5/5R to another (44.4%; 20/45). Insufficient efficacy and/or adverse effects were the most frequent reasons for stopping/switching. Patients who stopped/switched were more likely to have a higher baseline blood eosinophil count and exacerbation rate, lower lung function, and greater health care resource utilization.
Conclusion: The description of real-life patterns of continuing, stopping, or switching biologics enhances our understanding of global biologic use. Prospective studies involving structured switching criteria could ascertain optimal strategies to identify patients who may benefit from switching.
Keywords: severe asthma, biologics, prescribing, cohort study, management, international
Introduction
With the advent of personalized medicine, biologic therapy is becoming more widely used for a number of diseases, including severe asthma.1 However, there is a paucity of literature on both the frequency and patterns of biologic use in severe asthma, as well as the characterization of pre-biologic patient factors associated with stopping or switching versus continuation of the initial biologic.
Omalizumab was the first available biologic therapy for severe asthma, targeting immunoglobulin E (IgE) and therefore the allergic asthma phenotype. In recent years, four more monoclonal antibodies have been added to the biologic repertoire. For the eosinophilic phenotype, there are three available biologic agents.Mepolizumab and reslizumab both target interleukin-5 (IL-5), whereas benralizumab binds to the alpha subunit of the IL-5 receptor.2 The most recently-approved biologic, dupilumab, inhibits both IL-4 and IL-13 pathways by binding to the alpha subunit of the IL-4 receptor.3 Due to the different mechanisms of action, selection of the optimal initial biologic for each individual depends on accurate phenotyping using clinical characteristics (age of onset, allergy-related symptoms) and appropriate biomarkers such as blood eosinophil count (BEC) and Fractional exhaled Nitric Oxide (FeNO).4–7 Other considerations include: 1) route of delivery (reslizumab is intravenous whereas all the other biologics are administered subcutaneously); 2) frequency of administration (which, during the maintenance phase, varies between every two weeks and every eight weeks); 3) adverse effect profile; 4) patient preferences; and 5) comorbidities, such as atopic dermatitis and nasal polyposis. Prescribers are also constrained by licensing and availability of biologics and influenced by prescribing and reimbursement guidelines issued at international, national and/ or local levels. In general, biologics are indicated for individuals with severe asthma, who experience uncontrolled disease despite optimized standard medical care and for whom other factors that could contribute to the lack of asthma control (including comorbidities, incorrect inhalation technique and non-adherence) have been ruled out or appropriately optimized. In appropriately selected patients, biologics can result in a significant reduction in annualized exacerbation frequency.8 Additional benefits can include reduced exposure to systemic corticosteroids and improvements in lung function and quality of life/symptom control.9 Reduced exposure to systemic corticosteroids is particularly desirable due to their numerous short- and long-term adverse effects, including increased risk of diabetes mellitus, infections and osteoporosis.10
Despite a growing body of evidence with regards to the efficacy of biologics in asthma management, detailed knowledge of how biologics are actually used in real life is lacking. In particular, it is important to understand what proportion of patients continue, stop and switch from their initial biologic and the patient factors associated with biologic stopping or switching versus continuation.
Until recently we were unable to answer these questions. Small national and regional severe asthma registries did exist, but they were discrete and did not share information between them. The situation has now changed with the availability of data from two large databases, the International Severe Asthma Registry (ISAR; http://isaregistries.org/) and the CHRONICLE study (USA). ISAR, the largest adult severe asthma registry in the world, retrospectively and prospectively collects patient level, standardized variables from 29 countries around the world;11–14 at the time of writing it included >11,500 patients and covers diverse health care systems. It, therefore, has sufficient power to investigate, quantify, and describe biologic patterns of use, facilitating the generalizability of findings to the wider severe asthma population. Patients entered into the ISAR database represent, in the UK: a convenience sample from the UK Severe Asthma Registry, and in all other countries: all patients in severe asthma centres. The CHRONICLE study (ClinicalTrials.gov: NCT03373045) is a real-world, prospective, non-interventional cohort study of US specialist-treated patients with severe asthma that is aligned with ISAR and collects primary health care provider-reported and patient-reported data on patient characteristics, comorbidities, laboratory and imaging assessments, medical treatments, health care utilization (HCRU), and health outcomes from a large, geographically diverse cohort of US patients and providers.15,16 Per protocol, sites approach all eligible patients for enrollment. Although ISAR has a US site, there is no expected overlap between ISAR and CHRONICLE as the ISAR site is not included in CHRONICLE.
This study sought to describe biologic treatment frequency, patterns of use and reasons for biologic treatment discontinuation and switching in a real-life severe asthma cohort. Patient demographic and clinical characteristics (prior to initial biologic prescription) were also captured according to patterns of biologic use to identify individual patient-level factors associated with biologic stopping or switching versus continuation.
Materials and Methods
Study Design and Data Source
This was a historical cohort study using data from patients enrolled into ISAR (2015–2020) or CHRONICLE (2018–2020). Eleven countries had at least two biologics available (Bulgaria, Canada, Denmark, Greece, Italy, Japan, Kuwait, South Korea, Spain, UK, and the USA). Longitudinal de-identified patient data relating to these countries were extracted from CHRONICLE (USA) and ISAR Italy in February 2020, from ISAR UK in December 2019; and from the other ISAR countries included in the analysis in September 2019.
Patients
Patients were required to be aged ≥18 years old at the date of biologic initiation, have severe asthma (ie receiving treatment at Global Initiative for Asthma [GINA] 2018 Step 5 or with uncontrolled asthma at GINA Step 4)17 and treated with a biologic (ie omalizumab, mepolizumab, reslizumab, benralizumab, or dupilumab). Patients were required to have a minimum of six months of follow-up after biologic initiation.
The majority of countries included had at least two biologics available by 2018. With regard to patterns of biologic use after initiation, information regarding subjects who stopped or switched in 2018 or later was assessed. All subjects included in these analyses were treated in countries that, in 2018, had access to at least two biologics, therefore each of the possible patterns of biologic use (continuation, stopping, or switching) was feasible.
Outcomes (Definition of Biologic Continuation/Switching/Stopping)
Information on biologic use (availability, frequency, and duration of biologic use as well as reasons for switching/stopping) were collected. The percentages of patients who continued, stopped, or switched their first biologic were calculated overall and by country. “Continued biologic” was defined as the ongoing use of one biologic for at least 6 months at the time of data extraction without data indicating switching to another biologic. “Switched biologic” indicated those who received >1 biologic during follow-up; there was no restriction on how much time elapsed between the different biologics. “Stopped biologic” referred to individuals who received one biologic, stopped and did not commence another biologic during their follow-up period. Patients with ongoing biologic use of <6 months at the time of data extraction were excluded, as they would not meet the criteria for Continued, Switched, or Stopped.
Demographic and Clinical Characteristics Pre-First Biologic Treatment
A full description of pre-biologic demographic and clinical variables collected is provided in Table E1 including details on asthma exacerbations, hospitalizations and emergency visits, presence of comorbidities, test results (eg IgE, BEC, and FeNO), and treatment regimen (eg long-term oral corticosteroid (OCS) use and other add-on therapies to inhaled corticosteroid (ICS)/long-acting β2-agonist (LABA)).
Analyses
Patterns of Biologic Use
The time to cessation of first biologic after initiation (for those that stopped or switched in 2018 or later) was assessed. Patterns of biologic use were established for the total population and by country. Switch patterns (aggregated by biologic class) were analysed for all switched patients, including those who switched once and more than once. Additionally, first switch patterns were analyzed by age, long-term OCS use, age of asthma onset and presence of nasal polyps, factors which were identified as likely to affect outcomes. Demographic and clinical features stratified by patterns of biologic use were explored. Patients who switched or stopped were compared to patients who continued using univariate chi-square tests. Data from the closest preceding visit of the initial biologic start date were used. For emergency visits, hospitalization, BEC, and other asthma therapy, data within the 12 months preceding first biologic initiation were analyzed to align data collection methods from both ISAR and CHRONICLE.
Sensitivity Analyses
Analyses were repeated for three subpopulations within the larger cohort: 1) Prospective population (all countries, n=2656) assessed to ensure only patients with active data collection were used (to minimize information bias); 2) US only (n=2127 – predominantly prospective) as these patients made up a large proportion of the dataset (60%), and the US had the largest deviation from other countries included in the study; 3) Non-US (n=1404, prospective).
Results
Patients
A total of 3531 patients with severe asthma were included, 2295 from ISAR and 1236 from CHRONICLE (Figure 1). The three sub-populations each contained >1000 patients: 1) Prospective population (n=2656) (Figure E1); 2) US populations (n=2127); 3) Non-US (n=1404) (Figure E2).
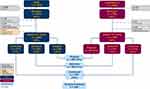 |
Figure 1 Subject disposition and pattern of biologic use of patients enrolled into ISAR or CHRONICLE. Abbreviations: ISAR, International Severe Asthma Registry; Bx, biologic. |
Biologic Availability and Overall Utilization
Biologic availability increased over time from 2015 to 2019 (Figure E3). All countries had ≥2 biologics available as of May 2019 (Table E2; Figure E3). Omalizumab and mepolizumab were available in all 11 countries. Benralizumab, reslizumab and dupilumab were available in 82% (9/11), 64% (7/11), and 55% (6/11) of countries, respectively. Most countries (8/11) recommended assessment of biologic efficacy at 4–12 months after patient initiation.
Omalizumab was used by 96.4% (n=756/784) of patients in 2015; its proportional use steadily declining to 45.5% (n=1510/3319) of users by 2019 (Figure E4). During the same time frame the proportion of patients prescribed mepolizumab increased from 3.6% (n=28/784) to 35.4% (n=1174/3319), whilst use of benralizumab increased from 0.9% (n=22/2266) of patients in 2017 to 12.8% (n=423/3319) in 2019. Reslizumab use stayed <3% throughout 2017–2019, whereas dupilumab was prescribed to 354/3272 patients (10.8%) in 2018, increasing to 425/3319 patients (12.8%) in 2019 (Figure E4).
First Biologic
Of the 229 patients who newly started a biologic in 2015, the majority commenced omalizumab (88.2%; n=202/229). However, as the number of available biologics increased, so too did the proportion of patients starting on an anti–IL-5/5R; by 2019, most patients started with an anti–IL-5/5R (56.9%; n=202/356) (Figure 2). By 2019, the highest proportion of patients (29.6%; n=105/356) started with benralizumab, followed by mepolizumab (24.5%; n=87/356) and dupilumab (23.1%; n=82/356). Meanwhile, the proportion of patients starting on omalizumab declined to 20% (71/356). A similar pattern of first biologic prescription was noted for the prospective and non-US populations (ie decreasing first prescription of omalizumab and increasing prescription of anti–IL-5/5R over time), but in the case of the non-US population, a much higher proportion of patients started with anti–IL-5/5R (83.6%; n=61/73) by 2019 as dupilumab was not yet available for many of these countries (ie UK, Spain, Bulgaria, South Korea, and Greece) (Figure E5).
 |
Figure 2 Proportion of patients with severe asthma enrolled into ISAR or CHRONICLE (n= 3531) on each biologic (first use) by year. Abbreviation: ISAR, International Severe Asthma Registry. |
Patterns of Biologic Use
Most patients included in the overall analysis continued their first biologic for at least 6 months (79%; n=2791/3531), whereas a small proportion stopped (10.2%; n=356/3531) or switched (10.8%; n=384/3531) during the course of follow up (Figure 1; Table 1). A numerically higher proportion of patients in the US cohort proceeded to stop biologic treatment than patients from the other cohorts (% who stopped - prospective: 5.3% [n=139/2656]; US: 13.1% [n=279/2127]; non-US: 5.5% [n=77/1404]) (Table 1).
 |
Table 1 Pattern of Biologic Use Overall and by Country for Patients with Severe Asthma Enrolled into ISAR and CHRONICLE |
Duration of Biologic Use in Stoppers and Switchers
Most patients who initiated biologic therapy in 2018 and subsequently stopped or switched (n=117), stopped their first biologic within 12 months (Figure 3). This was true for 85% of omalizumab (n=29/34) and mepolizumab (n= 34/40) recipients and 91% (n=39/43) of benralizumab recipients. Among those who switched, the time for which patients received their initial biologic varied numerically between drugs, with median durations of 6.8 months for omalizumab, 4.3 months for mepolizumab, and 6.0 months for benralizumab.
Japan and the US had numerically the highest proportions of users in whom biologic treatment was stopped (17.7% and 13.1% respectively) (Table 1). The US, UK, and Spain had higher proportions of patients stopping biologics versus switching; Kuwait, Bulgaria, Canada, Denmark, South Korea, Italy, and Greece had higher proportions switching biologic versus stopping; Japan had an equal proportion of patients stopping and switching.
Patterns of Switching
First Switch
Of those patients who stopped or switched their first biologic, the most common first switch was from omalizumab to (or, rarely, combined with) an anti–IL-5/5R (49.6%; n=187/377); (Figure 4). The second most common switch was within class, adding or switching from one anti–IL-5/5R to another (30.8%; n=116/377). A similar first switch pattern was noted for the prospective, US only and non-US cohorts (Figure E6).
Figure E7 describes the variability in switch patterns between the different biologic classes. For example, the cohort of subjects who switched from anti–IL-5-IL5/5R to anti–IL-4-IL4 contained a numerically higher proportion of patients taking long-term OCS, compared to other switch patterns.
Subsequent Switch
For the 45 patients who switched more than once, the most common subsequent biologic switch was from one anti–IL-5/5R to another (44.4%; n=20/45); (Figure E8). The next most common second switch was from an anti–IL-5/5R to (or combined with) an anti–IL-4 (22.4%; n=11/45). 17.5% of these patients (n=8/45) switched more than twice.
Description of Those Who Switched or Stopped Their First Prescribed Biologic
Comparing the pre-biologic characteristics of individuals who continued their first prescribed biologic to those who switched (Table 2), switchers had a numerically higher BEC (both on and off long-term OCS). Switchers also had a higher FeNO than continuers (p=0.007) and were more likely to be using theophylline as add-on therapy (3.8% vs 1.3%, p=0.003). In patients not on long-term OCS, switchers had more exacerbations than continuers (p=0.004). Switchers were numerically more likely than continuers to have used more healthcare resources: more invasive ventilation episodes (p=0.079), emergency visits (p=0.010), and hospitalizations (p<0.001) Switchers also showed a higher proportion of patients with eosinophilic chronic rhinosinusitis (19.8%) than continuers (11.0%) (p=0.020).
 |  |  |
Table 2 Demographic and Clinical Characteristics of Patients with Severe Asthma Enrolled into ISAR or CHRONICLE, Prior to First Biologic, According to Pattern of First Biologic Use |
Comparing the pre-biologic characteristics of individuals who continued their first prescribed biologic to those who stopped biologic therapy (Table 2), stoppers were numerically less likely to be on long-term OCS (p=0.007) and to be using LTRA as add-on therapy (p=0.005). In patients not on long-term OCS, switchers had more exacerbations than continuers (p=0.002). Similar to switchers, stoppers tended to have used more healthcare resources than continuers: more invasive ventilation episodes (p=0.002), emergency visits (p=0.142), and hospitalizations (p=0.004). Although the proportion of patients with chronic rhinosinusitis was higher in stoppers (93.2%) than in continuers (75.0%), the proportion of patients who had chronic rhinosinusitis with nasal polyps was higher in continuers (60.1%) than in stoppers (38.4%) (p<0.001).
Reasons for Stop or Switch
The most commonly cited reason for stopping or switching a biologic was due to insufficient clinical efficacy (72 of 113 stopped patients [63.7%]%) and (158 of 183 switched patients [86.3%], %) respectively. The presence of adverse outcomes potentially caused by biologic was also cited by approximately 15.9% (n=18) and 7.7% (n=14) of all stopped and switched individuals, respectively (Table 3).
 |
Table 3 Reasons Why eCRF Patients with Severe Asthma Enrolled into ISAR or CHRONICLE Stopped or Switched Their First Prescribed Biologic |
Discussion
The severe asthma biologic landscape is becoming increasingly complex due to the array of monoclonal antibodies available to prescribers and the lack of head-to-head studies to inform clinical decision-making when an individual patient qualifies for more than one biologic option. To our knowledge, this is the largest study to investigate biologic treatment patterns for severe asthma in real-life, spanning 11 countries and 3 continents. Within this cohort, we have assessed both initial biologic treatment choice and subsequent prescribing patterns. When analyzed globally and at the country level, a low rate of biologic switching is a consistent finding, albeit with some inter-country variability. The data set provided sufficient depth of information to investigate first and subsequent switch patterns at the biologic class level, to describe reasons for this behavior and to assess the spread of baseline clinical factors (eg presence of nasal polyps, age of asthma onset, and long-term OCS use) across subjects undergoing different biologic utilization patterns.
Other studies have attempted to elucidate real-world biologic prescribing patterns. For example, a survey performed among allergists using a semi-structured 10-item self-administered web-based questionnaire, published in 2020, found that omalizumab was the most commonly-prescribed biologic for asthma (98%), and that “uncontrolled asthma despite adherence to controller medication” was the most common indication.18 The selection bias in this study, favouring omalizumab over alternative biologic agents, was considerable, given that the questionnaire was directed toward allergists. A cross-sectional observational study including six countries analysed a cohort of 670 patients with severe asthma, recruited between December 2014 and May 2015. Biologic eligibility was 31–41% for omalizumab, 20% for mepolizumab, and 5% for reslizumab. Substantial overlap was noted between groups; for example, out of 101 patients who were eligible for mepolizumab (and not currently receiving omalizumab), 27–37% were also eligible for omalizumab.19 A separate retrospective cohort study investigated the characteristics of 1834 individuals who commenced treatment with either omalizumab or mepolizumab between November 2015 and March 2017. Compared to patients prescribed omalizumab, those prescribed mepolizumab were more likely to have experienced an exacerbation in the 12 months leading up to biologic initiation (81.4% vs 57.5%) and were more likely to have conditions such as sinusitis (35.4% vs 26.3%) and nasal polyps (19.1% vs 6.9%) but less likely to have allergic rhinitis (69.7% vs 77%), atopic dermatitis (2.2% vs 8.1%), or chronic idiopathic urticaria (0.5% vs 21.2%).20
Despite a rapid increase in the availability of biologic therapies for asthma with different mechanisms of action, 79% of subjects in this study remained on the first biologic that they were prescribed (biologic 1). Overall, the percentage of patients who switched to another biologic was low. These findings naturally trigger the question: “Is the first biologic prescribed to a patient usually the best one for that individual, or are we under-switching?” Although in many cases, continuation of the first biologic may reflect appropriate biomarker-guided biologic selection and good clinical efficacy of the initial biologic, a substantial proportion of patients qualify for one or more alternative biologic agents. The response to the un-tried agent(s) is unknown and perhaps a subset would be better served by switching biologics.
It may be that the careful selection of an appropriate first biologic based on clinical characteristics and biomarkers resulted in adequate clinical efficacy in the majority of patients. It is, however, possible to speculate on other reasons for the evident low rate of switching once a patient is established on the initial agent, with possibilities including: 1) a high initial logistical hurdle to biologic prescription that may lead individuals to persist with the first biologic, rather than invest further time and effort in pursuing an alternative agent; 2) conservative thresholds for response, eg, a 50% reduction in exacerbation rate and /or maintenance oral corticosteroid dose in the UK for anti–IL-5/5R: although an individual’s response to their initial biologic may not be transformative, if it is better than their previous treatment they may be unwilling to change treatment, due to underestimation of the effect which may be achieved with a different biologic and fear of losing the marginal improvement seen with the first choice of biologic and; 3) there is currently limited evidence with regards to the potential benefits of switching biologic. All of these reasons underscore the need for further research into which patients respond best to the available biologics.
It is important to note that within this study most switches occurred within 12 months of initial biologic prescription. This is likely related to GINA and national payer recommendations to review biologic response within 4–12 months after initiation. In contrast, the UK’s National Institute for Health and Care Excellence (NICE) recommendation to review at 12 months is often misinterpreted as “must treat for 12 months”, a phenomenon that may in some instances provide another explanation for the low rates of switching. International variation in practice was observed. We saw more switchers in South Korea (although based on low numbers), Japan, and Denmark, and a low prevalence of switchers in Spain and Bulgaria. We also saw the highest proportion of stoppers in Japan and the US, perhaps due to higher patient co-pay practices in both healthcare systems. Regional or national prescribing restrictions and local policies with regard to biologic initiation and switching are other important factors. Full exploration of these factors are beyond the scope of this study.
Some switch patterns are associated with different comorbidities. For example, individuals with chronic rhinosinusitis with nasal polyposis (CRSwNP) had the highest likelihood of switching to an anti–IL-4R agent, possibly due to early evidence that anti–IL-4R may treat CRSwNP.21 Additionally, switchers were more likely to have more severe disease (shown by more exacerbations and greater healthcare resource utilization [HCRU] pre-biologic) and evidence of eosinophilic disease (ie high BEC and FeNO). These individuals were likely switching from an anti-IgE to an anti–IL-5/5R or anti–IL-4R in an effort to personalize their medicine and specifically target the underlying drivers of their severe asthma.
Clinicians should be alert to factors present at baseline that are predictive of a subsequent decision to switch biologics, such as a high BEC, high exacerbation rate and high HCRU. These factors may be associated with biologic switching simply because such patients are likely to qualify for more than one drug, eg subjects commencing omalizumab who are known to have a high BEC will have the option to switch to anti–IL-5/5R therapy, and within the eosinophilic phenotype, there is scope to switch between different anti–IL-5/5R agents. Patients with one or more of these characteristics may benefit from tailored pre-biologic counselling regarding the decision to stop or switch drug, a decision which is often due to perceived insufficient clinical efficacy. Conversely, patients who were found in this study to be less likely to stop or switch biologic may benefit from increased scrutiny with regards to the clinical efficacy of their initial biologic. For example, patients are more likely to continue on their initial biologic if they have a low baseline exacerbation rate. Such individuals may not be flagged as experiencing insufficient clinical efficacy due to an ongoing low exacerbation rate following initiation of biologic therapy. This, however, does not guarantee that their initial biologic choice is the best available option for them; there is a risk of accepting mediocre improvement when a better outcome is possible. A “treat-to-target” approach may be beneficial. In such a treatment paradigm, patients who do not reach a predefined threshold of ‘success’ (prospectively agreed between the clinician and the patient) after an appropriate trial period and who qualify for one or more alternative biologic agents, should be offered the opportunity to switch biologic. As treatment for severe asthma continues to improve, clinical remission on treatment is an aggressive treatment target that may help guide clinical decision-making.22
The data collected in this study carry some inherent limitations. The clinical profile of ISAR patients in the intervening time between switches cannot be ascertained as patients are followed up annually, and most switches occur during the course of the first year. As such, data on clinical outcomes is limited. Further real-world studies examining clinical outcomes (eg, exacerbation rates) before and after switching from one biologic to another could potentially provide valuable information regarding any potential benefits that switching biologics may yield for individuals. The criteria used to define stopping and switching were somewhat arbitrary but we do not expect this to negatively influence the results. Future studies using time-to-event analysis might help further refine the patterns that we observed in this descriptive study.
By combining data from two sizeable registries, the large sample size and broad international representation within this study is a significant strength. Some differences between subpopulations were noted, in particular between US and non-US populations, which were likely due to differences in biologic availability and healthcare structure. Additionally, only countries with at least two biologics available as of 2018 were included in this study, to reduce the magnitude of confounding from limited biologic access. To describe the duration of use of a biologic, only biologic initiations from 2018 and after were included as all 11 countries had at least two biologics available for the treatment of severe asthma. Further studies will be needed to assess the independent impact of country of residence, healthcare structure, and biologic accessibility on varying biologic use patterns.
Conclusions
This study illustrates that the majority of patients with severe asthma continue treatment with their initial biologic. In the era of precision medicine, it is important that we direct our most targeted therapies (ie, biologics) to the right patients, thereby ensuring the greatest possible benefits with respect to clinical and healthcare economic outcomes. Further research is needed to investigate in more detail the reasons for biologic stopping and switching and to more accurately predict those most likely to stop or switch and conversely those most likely to benefit from continuing each biologic. Additionally, real-world longitudinal evidence of outcomes following various switch patterns would help to inform appropriate clinical decisions regarding if and when to switch biologics, with the ultimate aim of ensuring that every individual receives the best available treatment at the earliest opportunity.
Abbreviations
BEC, blood eosinophil count; CRSwNP, chronic rhinosinusitis with nasal polyposis; FeNO, Fractional exhaled Nitric Oxide; GINA, Global Initiative for Asthma; HCRU, health care utilization; ICS, inhaled corticosteroid; IgE, immunoglobulin E; IL-4, interleukin-4; IL4R, interleukin 4 receptor; IL-5, interleukin-5; IL5/5R, interleukin 5/5 receptor; IL-13, interleukin-13; ISAR, International Severe Asthma Registry; LABA, long-acting β2-agonist; NICE, National Institute for Health and Care Excellence; OCS, oral corticosteroid.
Data Sharing Statement
In line with ISAR governance restrictions, sharing individual deidentified participant data is subject to the consent of the ISAR steering committee (ISC) members in accordance with patient consent, patient confidentiality and ethical considerations. The study documents (protocol, statistical analysis plan, clinical study report) will be made available in accordance with the criteria of the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance ([EUPAS32724]). Proposals should be directed to [email protected]; to gain access, if approved by the regulatory boards data requestors will need to sign a data access agreement. Specific approval for this research study was granted by the Anonymised Data Ethics Protocols and Transparency committee (ADEPT approval reference: ADEPT1119).
Ethics Approval and Informed Consent
This study was designed, implemented, and reported in compliance with the European Network Centres for Pharmacoepidemiology and Pharmacovigilance Code of Conduct (EMA 2014; EUPAS32724) and with all applicable local and international laws and regulation. Registration of the ISAR database with the European Union Electronic Register of Post-Authorization studies was also undertaken (ENCEPP/DSPP/23720). ISAR has ethical approval from the Anonymised Data Ethics Protocols and Transparency (ADEPT) committee (ADEPT0218). Governance was provided by The Anonymous Data Ethics Protocols and Transparency (ADEPT) committee (registration number: ADEPT1119). All data collection sites in the International Severe Asthma Registry (ISAR) have obtained regulatory agreement in compliance with specific data transfer laws, country-specific legislation, and relevant ethical boards and organizations.
Acknowledgments
We would like to acknowledge Dr. Ghislaine Scelo (PhD) and Dr. Nasloon Ali (PhD) of the Observational and Pragmatic Research Institute (OPRI), Singapore, for their assistance in statistical data analysis, and Ms. Audrey Ang (BSc, Hons) of the Observational and Pragmatic Research Institute (OPRI), Singapore, for editorial and formatting assistance that supported the development of this publication.
Funding
This study was conducted by the Observational and Pragmatic Research Institute (OPRI) Pte Ltd and was partially funded by Optimum Patient Care Global and AstraZeneca Ltd. No funding was received by the Observational & Pragmatic Research Institute Pte Ltd (OPRI) for its contribution.
Disclosure
Andrew N. Menzies-Gow has attended advisory boards for AstraZeneca, GlaxoSmithKline, Novartis, Roche, Sanofi and Teva, and has received speaker fees from AstraZeneca, Novartis, Teva, and Sanofi. He has participated in research with AstraZeneca for which his institution has been remunerated and has attended international conferences with Teva. He has had consultancy agreements with AstraZeneca and Sanofi. Claire McBrien has attended an international conference with Boehringer Ingelheim and a national meeting with TEVA. Celeste M. Porsbjerg has attended advisory boards for AstraZeneca, Novartis, TEVA, and Sanofi-Genzyme; has given lectures at meetings supported by AstraZeneca, Novartis, TEVA, Sanofi-Genzyme, and GlaxoSmithKline; has taken part in clinical trials sponsored by AstraZeneca, Novartis, MSD, Sanofi-Genzyme, GlaxoSmithKline, and Novartis; and has received educational and research grants from Astra Zeneca, Novartis, TEVA, GlaxoSmithKline, ALK, and Sanofi-Genzyme. Mona Al-Ahmad has received advisory board and speaker fees from AstraZeneca, Sanofi, Novartis, and GlaxoSmithKline. Anna von Bülow reports speakers fees and consultancy fees from AstraZeneca and Novartis, outside the submitted work. She has also attended advisory board for Novartis. Borja G. Cosio declares grants from Chiesi and GSK; personal fees for advisory board activities from Chiesi, GSK, Novartis, Sanofi, Teva, and AstraZeneca; and payment for lectures/speaking engagements from Chiesi, Novartis, GSK, Menarini, and AstraZeneca, outside the submitted work. Liam G. Heaney declares he has received grant funding, participated in advisory boards and given lectures at meetings supported by Amgen, AstraZeneca, Boehringer Ingelheim, Circassia, Hoffmann la Roche, GlaxoSmithKline, Novartis, Theravance, Evelo Biosciences, Sanofi, and Teva; he has received grants from MedImmune, Novartis UK, Roche/Genentech Inc, and Glaxo Smith Kline, Amgen, Genentech/Hoffman la Roche, Astra Zeneca, MedImmune, Glaxo Smith Kline, Aerocrine and Vitalograph; he has received sponsorship for attending international scientific meetings from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK and Napp Pharmaceuticals; he has also taken part in asthma clinical trials sponsored by Boehringer Ingelheim, Hoffmann la Roche, and GlaxoSmithKline for which his institution received remuneration; he is the Academic Lead for the Medical Research Council Stratified Medicine UK Consortium in Severe Asthma which involves industrial partnerships with a number of pharmaceutical companies including Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Hoffmann la Roche, and Janssen. Mark Hew declares grants and other advisory board fees (made to his institutional employer) from AstraZeneca, GlaxoSmithKline, Novartis, Sanofi, Teva, and Seqirus, for unrelated projects. David J Jackson has received advisory board and speaker fees from AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Teva, Napp, Chiesi, Novartis, Sanofi and research grant funding from AstraZeneca. Stelios Loukides received honorarium form Novartis, Astra, GSK; received grants from GSK. Njira Lugogo received consulting fees for advisory board participation from Amgen, AstraZeneca, Genentech, GSK, Novartis, Regeneron, Sanofi, and Teva; honoraria for non-speakers bureau presentations from GSK and Astra Zeneca; and travel support from Astra Zeneca and GSK; her institution received research support from Amgen, AstraZeneca, Avillion, Evidera, Gossamer Bio, Genentech, GSK, Janssen, Regeneron, Sanofi, Novartis and Teva. She is an honorary faculty member of Observational and Pragmatic Research Institute (OPRI) but does not receive compensation for this role. Andriana I Papaioannou has received fees and honoraria from Menarini, GSK, Novartis, Elpen, Boehringer Ingelheim, AstraZeneca, and Chiesi. Désirée Larenas Linnemann reports personal fees from Allakos, Amstrong, Astrazeneca, Chiesi, DBV Technologies, Grunenthal, GSK, Mylan/Viatris, Menarini, MSD, Novartis, Pfizer, Sanofi, Siegfried, UCB, Gossamer, Carnot and grants from Sanofi, Astrazeneca, Novartis, Circassia, GSK, Purina institute, Abvvie, Lilly, Pfizer, outside the submitted work. Wendy C. Moore is on advisory boards for and reports grant and personal fees from AstraZeneca, Sanofi Regeneron, Genentech, Gossamer Bio Inc, Cumberland NHLBI/NHLBI, and GlaxoSmithKline. She is also a member of the writing and steering committee for the CHRONICLE study as well as a PI of the clinical trial at Wake Forest.). Luis Perez-de-Llano declares non-financial support, personal fees, and grants from Teva; non-financial support and personal fees from Boehringer Ingelheim, Esteve, FAES, GlaxoSmithKline, Mundipharma, and Novartis; personal fees and grants from AstraZeneca and Chiesi; personal fees from Sanofi, MSD, Techdow Pharma Leo-Pharma, GEBRO, and GILEAD; and non-financial support from Menarini outside the submitted work. Linda M. Rasmussen declares speakers fees from AstraZeneca, Boehringer Ingelheim, TEVA, ALK, GlaxoSmithKline, and Mundipharma, outside the submitted work and attended advisory board for AstraZeneca, Sanofi and Teva. Salman Siddiqui declares personal fees from AstraZeneca, GlaxoSmithKline, Novartis, Chiesi, Knopp Biosciences, CSL Behring, Mundipharma, ERT Medical, Owlstone Medical, Boehringer Ingelheim, outside the submitted work. Christopher S. Ambrose and Trung N. Tran are employees of AstraZeneca, sponsor and funder of the CHRONICLE study and a co-funder of the International Severe Asthma Registry. Esther Garcia Gil and Marianna Alacqua were employees of AstraZeneca at the time this study was completed. Charlotte Suppli Ulrik has attended advisory boards for AstraZeneca, ALK-Abello, GSK, Boehringer-Ingelheim, Novartis, Chiesi, TEVA, and Sanofi-Genzyme; has given lectures at meetings supported by AstraZeneca, Sandoz, Mundipharma, Chiesi, Boehringer-Ingelheim, Orion Pharma, Novartis, TEVA, Sanofi-Genzyme, and GlaxoSmithKline; has taken part in clinical trials sponsored by AstraZeneca, Novartis, Merck, InsMed, ALK-Abello, Sanofi-Genzyme, GlaxoSmithKline, Boehringer-Ingelheim, Regeneron, Chiesi and Novartis; and has received educational and research grants from AstraZeneca, MundiPharma, Boehringer-Ingelheim, Novartis, TEVA, GlaxoSmithKline and Sanofi-Genzyme. John W. Upham has received speaker fees and consulting fees from Novartis, AstraZeneca, GSK, Sanofi, and Boehringer Ingelheim. Eileen Wang has received honoraria from AstraZeneca, GlaxoSmithKline, Clinical Care Options, and Wefight. She has been an investigator on clinical trials sponsored by AstraZeneca, Optimum Patient Care, Sanofi, Sema4, GlaxoSmithKline, Genentech, Novartis, Teva, and National Institute of Allergy and Infectious Diseases (NIAID) for which her institution has received funding. Karin Dahl Assing, Susanne Hansen, Maria Kallieri, Johannes Schmid, John Busby, J. Mark FitzGerald, and Ruth B. Murray declare no relevant conflicts of interest concerning this paper. Victoria A. Carter and Chris A. Price are employees of Optimum Patient Care Global, a co-funder of the International Severe Asthma Registry. Neva Eleangovan is an employee of the Observational and Pragmatic Research Institute (OPRI), which conducted this study in collaboration with Optimum Patient Care and AstraZeneca. OPRI has also conducted paid research in respiratory disease on behalf of the following other organisations in the past 3 years: Aerocrine, AKL Research and Development Ltd, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mapi Group, Meda, Mylan, Mundipharma, Napp, Novartis, Orion, Regeneron, Roche, Takeda, Teva, and Zentiva (a Sanofi company). Lakmini Bulathsinhala, Isha Chaudhry, and Bindhu Unni were employees of the Observational and Pragmatic Research Institute, which conducted this study in collaboration with Optimum Patient Care and AstraZeneca. David Price has advisory board membership with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals, Thermofisher; consultancy agreements with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Novartis, Pfizer, Teva Pharmaceuticals, Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Teva Pharmaceuticals, Theravance, UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals; payment for the development of educational materials from Mundipharma, Novartis; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartis, Thermofisher; funding for patient enrolment or completion of research from Novartis; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the Efficacy and Mechanism Evaluation programme, and Health Technology Assessment; and was an expert witness for GlaxoSmithKline. The authors report no other conflicts of interest in this work.
References
1. McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199:433–445. doi:10.1164/rccm.201810-1944CI
2. Hillas G, Fouka E, Papaioannou AI. Antibodies targeting the interleukin-5 signaling pathway used as add-on therapy for patients with severe eosinophilic asthma: a review of the mechanism of action, efficacy, and safety of the subcutaneously administered agents, mepolizumab and benralizumab. Expert Rev Respir Med. 2020;14(4):353–365. doi:10.1080/17476348.2020.1718495
3. Le Floc’h A, Allinne J, Nagashima K, et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy Eur J Allergy Clin Immunol. 2020;75(5):1188–1204. doi:10.1111/all.14151
4. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi:10.1056/NEJMoa1403290
5. Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, Phase 3 trials. Lancet Respir Med. 2015;3(5):355–366. doi:10.1016/S2213-2600(15)00042-9
6. FitzGerald JM, Bleecker ER, Menzies-Gow A, et al. Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir Med. 2018;6:51–64. doi:10.1016/S2213-2600(17)30344-2
7. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi:10.1056/NEJMoa1804092
8. Ramonell RP, Iftikhar IH. Effect of anti-IL5, anti-IL5R, anti-IL13 therapy on asthma exacerbations: a network meta-analysis. Lung. 2020;198(1):95–103. doi:10.1007/s00408-019-00310-8
9. Menzella F, Ruggiero P, Galeone C, et al. Significant improvement in lung function and asthma control after benralizumab treatment for severe refractory eosinophilic asthma. Pulm Pharmacol Ther. 2020;64:101966. doi:10.1016/j.pupt.2020.101966
10. Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. 2018;52(4):1800703. doi:10.1183/13993003.00703-2018
11. Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the international severe asthma registry. Chest. 2020;157(4):790–804. doi:10.1016/j.chest.2019.10.053
12. Canonica GW, Alacqua M, Altraja A, et al. International severe asthma registry: mission Statement. Chest. 2020;157(4):805–814. doi:10.1016/j.chest.2019.10.051
13. Fitzgerald JM, Tran TN, Alacqua M, et al. International severe asthma registry (ISAR): protocol for a global registry. BMC Med Res Methodol. 2020;20. doi:10.1186/s12874-020-01065-0
14. Bulathsinhala L, Eleangovan N, Heaney LG, et al. Development of the International Severe Asthma Registry (ISAR): a modified Delphi study. J Allergy Clin Immunol Pract. 2019;7:578–588.e2. doi:10.1016/j.jaip.2018.08.016
15. Ambrose C, Chipps BE, Moore WC, et al. The CHRONICLE study of US adults with subspecialist-treated severe asthma: objectives, design, and initial results. Pragmatic Obs Res. 2020;11:77–90.
16. Moore WC, Panettieri RA, Trevor J, et al. Biologic and maintenance systemic corticosteroid therapy among US subspecialist-treated patients with severe asthma. Ann Allergy Asthma Immunol. 2020;125(3):294–303.e1. doi:10.1016/j.anai.2020.04.004
17. Global Initiative for Asthma. Global statement for asthma management and prevention 2018. Available from: https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf.
18. Kuruvilla M, Ariue B, Oppenheimer JJ, Singh U, Bernstein JA. Clinical use of biologics for asthma treatment by allergy specialists: a questionnaire survey. Ann Allergy Asthma Immunol. 2020;125(4):433–439. doi:10.1016/j.anai.2020.06.041
19. Albers FC, Müllerová H, Gunsoy NB, et al. Biologic treatment eligibility for real-world patients with severe asthma: the IDEAL study. J Asthma. 2018;55(2):152–160. doi:10.1080/02770903.2017.1322611
20. Llanos JP, Bell CF, Packnett E, et al. Real-world characteristics and disease burden of patients with asthma prior to treatment initiation with mepolizumab or omalizumab: a retrospective cohort database study. J Asthma Allergy. 2019;12:43–58. doi:10.2147/JAA.S189676
21. Kim J, Naclerio R. Therapeutic potential of dupilumab in the treatment of chronic rhinosinusitis with nasal polyps: evidence to date. Ther Clin Risk Manag. 2020;16:31–37. doi:10.2147/TCRM.S210648
22. Menzies-Gow A, Bafadhel M, Busse WW, et al. An expert consensus framework for asthma remission as a treatment goal. J Allergy Clin Immunol. 2020;145(3):757–765. doi:10.1016/j.jaci.2019.12.006
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


