Back to Journals » Clinical Ophthalmology » Volume 9
Real-world assessment of intravitreal dexamethasone implant (0.7 mg) in patients with macular edema: the CHROME study
Authors Lam W, Albiani D, Yoganathan P, Chen JC, Kherani A, Maberley D, Oliver A, Rabinovitch T, Sheidow T, Tourville E, Wittenberg L, Sigouin C, Baptiste D
Received 8 January 2015
Accepted for publication 26 March 2015
Published 10 July 2015 Volume 2015:9 Pages 1255—1268
DOI https://doi.org/10.2147/OPTH.S80500
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Wai-Ching Lam,1 David A Albiani,2 Pradeepa Yoganathan,3 John Chanchiang Chen,4 Amin Kherani,5 David AL Maberley,6 Alejandro Oliver,7 Theodore Rabinovitch,3 Thomas G Sheidow,8 Eric Tourville,9 Leah A Wittenberg,10 Chris Sigouin,11 Darryl C Baptiste12
1Department of Ophthalmology and Vision Sciences, University of Toronto, Toronto, ON, 2West Coast Retinal Consultants, Vancouver, BC, 3North Toronto Eye Care, North York, ON, 4Department of Ophthalmology, McGill University, Montreal, QC, 5Southern Alberta Eye Center, Calgary, AB, 6Department of Ophthalmology and Visual Sciences, University of British Columbia, Vancouver, BC, 7Timmins and District Hospital, Timmins, ON, 8Ivey Eye Institute, London, ON, 9Center Oculaire de Quebec, Quebec City, QC, 10Retina Surgical Associates, New Westminster, BC, 11Clinwest Research Inc, Burlington, ON, 12Allergan Inc., Markham, ON, Canada
Background: The purpose of this study was to evaluate the real-world use, efficacy, and safety of one or more dexamethasone intravitreal implant(s) 0.7 mg (DEX implant) in patients with macular edema (ME).
Methods: This was a retrospective cohort study of patients with ME secondary to retinal disease treated at ten Canadian retina practices, including one uveitis center. Best-corrected visual acuity (BCVA), central retinal thickness (CRT), intraocular pressure (IOP), glaucoma and cataract surgery, and safety data were collected from the medical charts of patients with ≥3 months of follow-up after the initial DEX implant.
Results: One hundred and one patient charts yielded data on 120 study eyes, including diagnoses of diabetic ME (DME) (n=34), retinal vein occlusion (RVO, n=30; branch in 19 and central in 11), and uveitis (n=23). Patients had a mean age of 60.9 years, and 73.3% of the study eyes had ME for a duration of ≥12 months prior to DEX implant injection(s). Baseline mean (± standard error) BCVA was 0.63±0.03 logMAR (20/86 Snellen equivalents) and mean CRT was 474.4±18.2 µm. The mean number of DEX implant injections was 1.7±0.1 in all study eyes; 44.2% of eyes had repeat DEX implant injections (reinjection interval 2.3–4.9 months). The greatest mean peak changes in BCVA lines of vision occurred in study eyes with uveitis (3.3±0.6, P<0.0001), followed by RVO (1.3±0.5, P<0.01) and DME (0.7±0.5, P>0.05). Significant decreases in CRT were observed: -255.6±43.6 µm for uveitis, -190.9±23.5 µm for DME, and -160.7±39.6 µm for RVO (P<0.0001 for all cohorts). IOP increases of ≥10 mmHg occurred in 20.6%, 24.1%, and 22.7% of DME, RVO, and uveitis study eyes, respectively. IOP-lowering medication was initiated in 29.4%, 16.7%, and 8.7% of DME, RVO, and uveitis study eyes, respectively. Glaucoma surgery was performed in 1.7% of all study eyes and cataract surgery in 29.8% of all phakic study eyes receiving DEX implant(s).
Conclusion: DEX implant(s) alone or combined with other treatments and/or procedures resulted in functional and anatomic improvements in long-standing ME associated with retinal disease.
Keywords: diabetic macular edema, posterior segment inflammatory disease, retinal vein occlusion, registry, sustained-release dexamethasone implant, Ozurdex®
Introduction
Macular edema (ME) most often results from retinal diseases such as diabetic retinopathy, retinal vein occlusion (RVO), exudative age-related macular degeneration, and uveitis, and commonly presents with symptoms of blurred or reduced central vision. Research into the pathophysiology of ME has led to a greater understanding of the underlying mechanisms and the role of inflammatory mediators that facilitate cellular damage, leading to accumulation of fluid within the retina.1,2 The anti-inflammatory, antiangiogenic, and antipermeability effects of corticosteroids counteract three key pathologic processes involved in the development of ME.2,3 However, the efficacy of corticosteroids is greatly affected by the route of administration. Direct intravitreal delivery of corticosteroids, which bypasses the blood–retinal barrier, results in a high local drug concentration and improved systemic safety.3 The ability to safely deliver therapeutic drug levels to the posterior segment of the eye without the need for frequent redosing remains a challenge with the currently available treatment options for ME.
Dexamethasone (DEX) is a corticosteroid with anti- inflammatory activity up to six fold greater than prednisolone or triamcinolone, 25-fold greater than hydrocortisone, and similar to fluocinolone acetonide.4 Injection of DEX into the vitreous humor has been shown to produce high drug concentrations with low toxicity; however, the half-life of DEX following intravitreal injection is short (approximately 3 hours), limiting its usefulness.5–7 The 0.7 mg intravitreal DEX implant (Ozurdex®, Allergan, Inc., Irvine, CA, USA) is a biodegradable solid polymer drug delivery system.8 A single-use applicator with a 22-gauge needle that leaves a sutureless self-sealing wound is used to place the DEX implant into the vitreous cavity.9 The sustained-release formulation was designed to release DEX from the implant for up to 6 months,10 as the poly(D,L-lactide-co-glycolide) polymer matrix (Novadur®, Allergan, Inc.) degrades into lactic acid and glycolic acid, and then metabolizes to water and carbon dioxide. Therefore, sequential DEX implants can be safely administered as an office-based procedure without the need for surgical removal.
In an earlier study, DEX implant treatment of eyes with persistent ME secondary to various retinal diseases produced significant improvements in best-corrected visual acuity (BCVA), macular thickness, and fluorescein leakage when compared with no treatment.11 The efficacy of the DEX implant for ME following branch or central RVO (BRVO, CRVO) was established in two identical, prospective, multicenter, randomized, sham-controlled, 6-month clinical trials, in which treatment with a single DEX 0.7 mg implant injection significantly improved BCVA and anatomic outcomes compared with sham treatment.12,13 In another prospective, multicenter, randomized, sham-controlled, 6-month clinical trial, treatment with a single DEX implant for noninfectious intermediate or posterior uveitis resulted in a significant proportion of study eyes demonstrating complete resolution of vitreous haze and a significant percentage achieving three or more lines of vision gain throughout the entire study period.14 The DEX implant is administered with the intention to treat ME associated with uveitis and does not control the underlying disease. The sustained release of DEX into the vitreous space provided by the DEX implant has also been shown to control ME over several months in vitrectomized eyes.15,16
Elevations in intraocular pressure (IOP) and cataract formation are common concerns with intravitreal corticosteroid injections. In the RVO and uveitis DEX implant phase III trials, a transient increase in IOP was observed (typically peaking 60 days after DEX implant injection), and no more than 24% of eyes required treatment with topical IOP-lowering medication. Six months after a single DEX implant was injected, no significant increases in cataract adverse events were observed.12,14 However, a significant increase in cataract (7.3%–29.8%) was seen following retreatment with the DEX implant.13 Reported adverse event rates for cataract and cataract surgery following an average of five DEX implants over a 3-year period for the treatment of diabetic ME (DME) were 67.9% and 59.2%, respectively.17
Several retrospective case series conducted in the USA and Europe have been published evaluating the use of DEX implants for the treatment of ME, particularly in patients with RVO18–20 but also in DME21,22 and uveitis.23 In Canada, at the time the present study was conducted, the DEX implant was approved only for treatment of ME following CRVO, and for treatment of noninfectious uveitis affecting the posterior segment of the eye,24 and there was a lack of observational studies assessing the efficacy and safety of DEX implants in the clinical setting. We conducted a retrospective study to explore the use of DEX implant by Canadian retina and uveitis specialists in a real-world clinical practice setting. The objectives of this study were to describe the demographics and ophthalmic medical history of patients with ME treated with the DEX implant, to assess treatment patterns for use of the DEX implant, and to evaluate the functional, anatomic, and safety outcomes after one or more DEX implant injections in patients with ME.
Materials and methods
Study design
This was a multicenter, retrospective, open-label, exploratory chart review of data collected from patients with ME treated with one or more DEX implants (0.7 mg) at ten Canadian retina practices, including one uveitis center (ClinicalTrials.gov identifier NCT01805323). The study was conducted according to the International Conference on Harmonisation Good Clinical Practice guidelines and all applicable regulatory authority requirements and national laws. The protocol was reviewed and approved by the institutional review board or independent ethics committee of each site prior to commencing the study. Patients enrolled in the study were assigned an identification number (consisting of a site and a patient number), and no patient-identifiable information was collected.
Eligibility criteria and data collection
Medical records were included in the study if the patient met all the following criteria: had a diagnosis of retinal disease involving ME in the study eye(s); received at least one DEX implant and had follow-up data for a minimum duration of 3 months (12±2 weeks) after the first injection; had data collected from December 1, 2010 through December 1, 2012 inclusive; and had signed an informed consent form prior to first collection of study data. Patient charts were excluded if “no” was the answer to any of the four inclusion criteria.
Data were collected from the patient charts for three types of visits (Table S1): visit 1 – medical history visit prior to DEX implant injection (screening visit); visit 2 – baseline first injection visit or subsequent DEX implant injection visits (day 1); visit 3 – post-injection follow-up visits from 2 to up to 26 weeks after each DEX implant injection or until the next DEX implant injection. No data were collected beyond 26 weeks (6 months) for patients who were still within the study interval but were not scheduled to receive additional DEX implants. Any ocular procedures performed following DEX implant injection (eg, laser photocoagulation, cataract surgery) were captured from records of post-injection follow-up visits.
Efficacy and safety assessments
Efficacy was measured by calculating the peak mean change in BCVA and central retinal thickness (CRT) using optical coherence tomography (OCT) from baseline to 2–26 weeks following the last DEX implant injection. OCT instruments used at the ten study sites included Cirrus HD-OCT (n=5; Carl Zeiss Meditec Inc, Dublin, CA, USA); Spectralis (n=3; Heidelberg Engineering Inc, Heidelberg, Germany); RTVue (n=1; Optovue, Fremont, CA, USA); and 3D OCT-2000 (n=1; Topcon Medical Systems Inc, Oakland, NJ, USA). Peak BCVA line change from baseline was calculated by converting logMAR values to number of lines as described by Holladay et al.25 A clinically significant decrease in BCVA (defined as a reduction of ≥10 letters compared with baseline), a clinically significant increase in CRT (defined as an increase of ≥50 μm compared with baseline), or a failure of the DEX implant to produce the expected/intended effect leading to an adverse outcome for the patient in the opinion of the investigator were to be reported as an adverse event.
Safety was assessed by monitoring changes in IOP, use of IOP-lowering medications, incidence of glaucoma and cataract surgery, and investigator-reported adverse events such as injection-related events. IOP elevations of ≥5 mmHg from baseline were considered to be corticosteroid-related responses and reported as adverse events. All adverse event terms recorded in patient medical charts were mapped to Preferred Terms and System Organ Classes using the Medical Dictionary for Regulatory Activities.
Data analysis and statistical methods
Data were retrospectively collected from patients’ medical charts. Given the exploratory nature of the study, most analyses were descriptive for treatment patterns and safety outcomes with the DEX implant and concurrent therapies. Efficacy outcomes after DEX implant injection are presented by retinal disease subgroups in cohorts that had sufficient data for analysis; subgroup analyses of phakic and pseudophakic and non-vitrectomized and vitrectomized eyes were also performed.
Continuous variables were summarized using descriptive statistics, including sample size, mean, standard error (SE), median, minimum, and maximum. Categorical variables were summarized by frequency and percentage tables. Peak mean change in BCVA and CRT analyses only included patients with observations available for the last DEX implant injection from 2 to 26 weeks with a minimum follow-up of 12±2 weeks; the 95% confidence interval and statistical significance were analyzed using a generalized estimating equations model with a correlation structure. The nature and frequency of adverse events were tabulated throughout the study and summarized using descriptive statistics. Use of and access to data were supervised by a scientific advisory committee and Allergan Inc.
Results
Baseline demographics
In all, 101 patient charts were eligible for inclusion in the study with a total of 120 study eyes for analysis (Table 1). No medical charts from patients who met the eligibility criteria were excluded from the analysis. Retinal disease subgroups with a sufficient number of study eyes for meaningful analysis of functional and anatomic outcomes (>20) included DME, RVO (BRVO and CRVO), and uveitis. Cohorts with other ocular diagnoses were not large enough for separate analysis (Table 2), but were combined in the all eyes study group. The overall patient age (mean ± standard deviation) was 60.9±14.8 years. Males comprised two-thirds of the study population (65.3%), and the majority of patients were Caucasian (77.2%). Systemic hypertension was the most common comorbid condition in the study population (55.4%).
  | Table 2 Ocular diagnoses other than diabetic macular edema, retinal vein occlusion, and uveitis in study eyes included in the all eyes cohort |
DEX implants were most frequently used to treat study eyes with an indication of DME (28.3%), followed by RVO (25.0%) and uveitis (19.2%). Prior to the first DEX implant injection, 73.3% of all eyes had persistent ME for a period of 12 months or more (Table 3). Mean (± SE) BCVA, CRT, and IOP were 0.63±0.03 logMAR (20/86 Snellen equivalents), 474.4±18.2 μm, and 14.4±0.4 mmHg, respectively, at baseline. Overall, 25.0% of the study eyes had a history of steroid response, defined as an IOP elevation of ≥5 mmHg following prior topical or intravitreal steroid exposure. The uveitis cohort had the greatest proportion (34.8%) of study eyes known to have a history of steroid response.
Prior treatments and ocular procedures
The most commonly reported previous treatments in study eyes with DME and RVO were intravitreal bevacizumab (47.1% and 36.7%, respectively) and intravitreal triamcinolone acetonide (IVTA, 38.2% and 30.0%, respectively, Table 3). Study eyes with uveitis were most commonly treated with IVTA (65.2%) and sub-Tenon’s triamcinolone acetonide (43.5%); systemic prednisone (43.5%), mycophenolate mofetil (26.1%), methotrexate (30.4%), cyclosporine (13.0%), and methylprednisolone (8.7%) had also been prescribed prior to DEX implant.
Prior cataract surgery was particularly prevalent in eyes with a diagnosis of DME (67.6%). Prior glaucoma surgery was most frequently reported in the uveitis cohort (17.4%). Before administration of the first DEX implant injection, laser surgery (focal/grid or panretinal photocoagulation) and vitrectomy had been performed frequently in eyes with DME (55.9% for each), and vitrectomy was also commonly reported in eyes with RVO (40.0%).
DEX implant treatment patterns
The most frequently reported rationales for choosing DEX implant therapy by the Canadian physicians participating in this study were to improve visual acuity, to decrease ME, and to rescue eyes that had proved to be nonresponsive to other available therapies. The mean (± SE) number of injections for study eyes with DME, RVO, and uveitis was 1.6±0.1, 1.7±0.1, and 1.7±0.2, respectively. The mean (± SE) time to the first and second DEX implant reinjection intervals (ie, second and third DEX implants) was longest in DME (5.8±0.5 months and 5.6±1.0 months, respectively) and RVO (4.9±0.3 months and 7.2±2.3 months, respectively) study eyes. The uveitis cohort had the shortest first and second DEX implant reinjection intervals (4.7±0.3 months and 3.4±0.4 months, respectively).
Concomitant treatments and procedures
In the DME, RVO, and uveitis cohorts, repeat DEX implant injections were administered to 44.2%, 50.0%, and 43.5% of study eyes, respectively, and adjunctive treatments and/or procedures were administered to 41.2%, 26.7%, and 78.3% of study eyes, respectively (Table 4). The most common adjunctive intravitreal treatments administered with DEX implants among DME, RVO, and uveitis study eyes were bevacizumab (8.8%, 10.0%, and 13.0%, respectively) and IVTA (20.6%, 13.3%, and 4.3%, respectively). Systemic treatments were primarily administered to patients with a diagnosis of uveitis, with the most common systemic therapy in this group being mycophenolate mofetil (47.8%).
Few surgical procedures were performed during the study period. Vitrectomy was the most commonly reported procedure in study eyes with DME (11.8%) and vitrectomy with membrane peeling was most prevalent in study eyes with DME (8.8%). Focal/grid laser was most prevalent in study eyes with RVO (10.0%) and DME (5.9%). One eye with uveitis was switched to a fluocinolone acetonide implant (Retisert®, Bausch & Lomb Inc, Rochester, NY, USA) by a clinician who believed a longer-acting intraocular steroid was needed. This same eye underwent prophylactic tube-shunt glaucoma surgery at conclusion of surgical placement of the fluocinolone acetonide implant.
Efficacy of DEX injections in DME, RVO, and uveitis cohorts
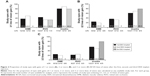
The greatest peak mean line gains from baseline after DEX implant injection were observed in study eyes with uveitis (P<0.0001), followed by RVO (P<0.01). Significant peak mean line gains were also observed in subgroups of non-vitrectomized and vitrectomized eyes with uveitis and non-vitrectomized eyes with RVO (Figure 1). For study eyes with DME, average peak gains in lines of vision were not statistically significant compared with baseline for all eyes or for non-vitrectomized and vitrectomized eyes (Figure 1). When analyzed by lens status at baseline, in the DME cohort, a peak mean loss of 0.6±0.6 lines in phakic eyes and a statistically significant peak mean gain of 1.4±0.5 lines (P<0.05) in pseudophakic eyes was reported.
Study eyes with a diagnosis of uveitis had the highest proportion of eyes gaining one or more lines of vision (81.0%, mean baseline vision, 0.76±0.08 logMAR; 20/115 Snellen equivalents), followed by RVO (44.4%, mean baseline vision, 0.69±0.08 logMAR; 20/97 Snellen equivalents) and DME (37.5%, mean baseline vision, 0.80±0.09 logMAR; 20/125 Snellen equivalents, Figure 2A). A similar pattern for two or more and three or more lines of vision gains was observed following one to three DEX implant injections, with the highest proportions achieved by uveitis study eyes (Figure 2B and C). The proportions of DME, RVO, and uveitis study eyes that had ME for a duration of ≥12 months and had 0 or fewer lines of vision change from baseline were 46.7% (mean baseline vision, 0.58±0.08 logMAR; 20/76 Snellen equivalents), 42.1% (mean baseline vision, 0.57±0.12 logMAR; 20/74 Snellen equivalents); and 9.1% (mean baseline vision, 0.54 logMAR; 20/70 Snellen equivalents), respectively.
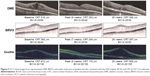
Study eyes with uveitis demonstrated the greatest decrease in CRT, followed by study eyes with DME and RVO (P<0.0001 for each group compared with baseline, Figure 3). In the DME, RVO, and uveitis cohorts, significant decreases in CRT were observed in both non-vitrectomized and vitrectomized study eyes (Figure 3). The percentage of uveitis eyes with ME (ie, CRT >300 μm) at baseline that showed both a reduction in CRT and improved vision (ie, >0 lines gained) following treatment with DEX implant was 66.7% (14/21). The baseline mean (± SE) CRT and vision for these eyes were 546.3±38.8 μm and 0.8±0.1 logMAR (20/128 Snellen equivalents), respectively. Following treatment with the DEX implant, the mean (± SE) peak improvement in CRT was −274.3±42.3 μm and the corresponding vision improved +4.0 lines for a final mean (± SE) vision of 0.4±0.1 logMAR (20/51 Snellen equivalents). Figure 4 shows OCT images from representative DME, BRVO, and uveitis cases following treatment with the DEX implant.
Safety of intravitreal DEX implant injections
A total of 35 treatment-related adverse events were reported following the first DEX implant in patients with a diagnosis of DME (33.3%; 8/24), RVO (37.9%; 11/29), and uveitis (25.0%; 5/20). The most commonly reported adverse event was increased IOP, with a total of 24 events for DME (25.0%; 6/24), RVO (27.6%; 8/29), and uveitis (10.0%; 2/20) patients (Table 5). Other treatment-related adverse events occurring in >1% of patients were subcapsular cataracts and retinal detachments (5.0%; 1/20 each in uveitis patients) and reduced visual acuity (6.9%; 2/29 in RVO patients). The eye that developed a retinal detachment underwent surgical repair and treatment with the DEX implant was continued. Endophthalmitis was reported as a serious adverse event related to DEX implant treatment in a patient with BRVO, and a uveitis flare was reported as a serious adverse event unrelated to DEX implant treatment in a patient with uveitis. The lone case of endophthalmitis resolved following treatment with intravitreal injection of vancomycin, and treatment with the DEX implant was discontinued. No serious adverse events were reported among patients diagnosed with DME.
  | Table 5 Treatment-related adverse events occurring in >1% of all patients |
The proportion of eyes in the DME, RVO, and uveitis subgroups with IOP increases of at least 10 mmHg ranged from 20.6% to 24.1% (Figure 5). Those with an absolute IOP reading of ≥25 mmHg or ≥35 mmHg recorded at any follow-up visit after DEX implant(s) ranged from 17.4% to 26.7% and from 2.9% to 6.7%, respectively (Figure 5). The proportions of DME, RVO, and uveitis study eyes requiring topical IOP-lowering therapy at any point following treatment with the DEX implant in the study were 29.4%, 16.7%, and 8.7%, respectively (Figure 6). Cases of new onset elevated IOP were reported in 70.0% of DME, 80.0% of RVO, and 50.0% of uveitis study eyes. In this set of eyes, only one DME eye with a history of steroid response had new onset elevated IOP.
The proportions of DME, RVO, and uveitis eyes that had a history of steroid response and also experienced an IOP increase of at least 10 mmHg following DEX implant treatment(s) were 40.0%, 20.0%, and 37.5%, respectively. The proportions of DME, RVO, and uveitis eyes that had a history of steroid response and also presented at follow-up visits with absolute IOP readings ≥25 mmHg were 20.0%, 20.0%, and 37.5%, respectively. Only study eyes with uveitis (12.5%) and other ocular diagnoses (28.6%; not including those with DME or RVO) had both a history of steroid response and an absolute IOP reading ≥35 mmHg recorded at any follow-up visit after treatment with the DEX implant(s). The proportions of DME, RVO, and uveitis eyes with a history of steroid response requiring topical IOP-lowering medications following treatment with the DEX implant in the study were 30.0%, 40.0%, and 62.5%, respectively.
In the uveitis study eye cohort, four eyes had glaucoma surgery prior to treatment with the DEX implant. The adverse event report revealed that one eye had an IOP increase related to DEX implant injection that resolved following treatment with topical IOP-lowering medication. Another eye had ongoing mild pain deemed by the investigator to be unrelated to the DEX implant injection and did not require any treatment. The other two uveitis eyes that had undergone prior glaucoma surgery did not have any new safety concerns following treatment with DEX implant(s) over the duration of the study.
In total, 1.7% (2/120) of study eyes receiving the DEX implant underwent glaucoma surgery during the study. One eye with CRVO and no history of prior steroid response underwent glaucoma surgery at 2.3 months because the IOP had increased from 24 mmHg at baseline to 33 mmHg before the surgery, despite ongoing treatment with three IOP-lowering medications. One other study eye (not in the DME, RVO, or uveitis cohorts) with a history of steroid response had glaucoma surgery at 3.4 months because IOP had increased by greater than 10 mmHg following DEX implant injection (from 11 mmHg at baseline to 23 mmHg), despite treatment with a fixed combination IOP-lowering medication.
Cataract surgery was performed in 29.8% (14/47) of all phakic study eyes receiving at least one DEX implant injection over a maximum follow-up period of 19.3 months; 27.3% (3/11) of DME eyes (two with prior vitrectomy), 7.7% (1/13) of RVO eyes, and 45.5% (5/11) of uveitis eyes had cataract surgery. Almost half of these surgeries (42.9%) were performed 5–12 months after starting treatment with the DEX implant.
Discussion
The Chart Review of Ozurdex® in Macular Edema (CHROME) study reports experience with the DEX implant by retina and uveitis specialists in the Canadian clinical setting. This study evaluated use of the DEX implant in patients with persistent ME secondary to a wide variety of conditions. The results of similar studies focusing on specific patient populations in other countries have recently been published. A large retrospective study was conducted in patients with BRVO-related or CRVO-related ME (n=289) in the USA who received two or more DEX implant injections. The patients received a mean of 3.2 (range 2–9) DEX implant injections, alone or combined with other therapies, and experienced improvements in CRT and visual acuity with each subsequent injection.19 In a retrospective study conducted in Germany of RVO patients (n=102) receiving a single DEX implant injection, significant improvements in BCVA and reductions in CRT were observed.18 In other ocular indications, a small retrospective study concerning noninfectious uveitis (27 patients, 38 study eyes) found that repeat DEX implant injections improved retinal thickness and resolved inflammation, which resulted in improved ocular function.23 Functional and anatomic improvements after DEX implant treatment have also been reported in several case series evaluating patients with persistent DME.21,22,26
In Canada, the DEX implant was approved for treatment of ME secondary to CRVO in 2011 and for treatment of noninfectious uveitis affecting the posterior segment of the eye in 2012.24 The recent approval for the treatment of adult pseudophakic DME granted by Health Canada, came in 2015, well after the conclusion of the CHROME study. The data presented herein covers a time frame when the Canadian experience with the DEX implant was very limited. Herein, the most common use of the DEX implant was off-label treatment for DME, followed by treatment of study eyes with RVO and uveitis, to improve visual acuity and to resolve ME, and as rescue therapy for eyes unresponsive to other therapies. The fact that almost three- quarters of patients had persistent ME for a period of at least 12 months prior to treatment with the DEX implant suggests DEX implant injections were being used primarily as salvage therapy. At the time the study was conducted, with the exception of the province of Quebec, the DEX implant was not included in government-funded drug formularies in Canada, which greatly limited access to the drug and was likely an important factor that restricted its earlier use or choice as first-line therapy for ME in these patients.
The majority of study eyes had persistent ME despite prior treatment and procedures. Subsequent treatment with the DEX implant improved visual acuity and ME. In the DME subgroup, more than 90% of study eyes had had ME for at least 12 months before entering the study. Compared with baseline, decreases in CRT were statistically significant (P<0.0001). However, this anatomic improvement is not reflected in a statistically significant improvement in visual acuity for the DME cohort. A closer look at our data revealed that the improvement in visual acuity was more pronounced in pseudophakic eyes than in phakic eyes. These findings are in line with results from clinical trials of DEX implant injections for the treatment of patients with DME.17 We suspect that phakic eyes with DME did not show significant visual acuity improvement following treatment with the DEX implant because of development of cataracts. Cataract is known to be more prevalent among elderly diabetic patients.27 Moreover, in this study, more diabetic patients with ME underwent vitrectomy prior to treatment with the DEX implant than patients with other pathologies, further exacerbating the risk of cataract development.28
Results from two large, randomized, sham-controlled Phase III trials (together comprising the Macular Edema Assessment of implantable Dexamethasone in diabetes or the MEAD study) evaluating the efficacy and safety of the DEX implant in patients with DME have recently been reported.17 Similar to the CHROME study, patients received DEX implant reinjections approximately every 6 months; however, in the MEAD study, patients were administered up to seven DEX implants over a period of 3 years and no concomitant treatments were permitted. In both studies, rates of glaucoma surgery were low (0% in CHROME study DME eyes; 1.4% in MEAD study eyes), and the proportion of study eyes with increased IOP (IOP change ≥10 mmHg, absolute IOPs ≥25 mmHg or ≥35 mmHg) was similar. As in the current study, treatment with the DEX implant resulted in significant improvement in BCVA and CRT compared with sham treatment in the MEAD study. However, vision gains following DEX implant reinjections were likely confounded by cataract progression in phakic eyes, given that subgroup analysis of pseudophakic DME eyes demonstrated consistent significant improvements in BCVA relative to sham-treated eyes over the 3-year study period.17
Study eyes with uveitis demonstrated the largest gain in BCVA (P<0.0001) and the highest proportion of eyes gaining ≥1, ≥2, and ≥3 lines of vision after treatment with the DEX implant in our study. This group of study eyes also demonstrated the greatest decreases in CRT (P<0.0001) following DEX implant injections. These improvements occurred often in patients with ME resistant to traditional therapy of IVTA and periocular steroid injections.29 The uveitis cohort received treatment with the DEX implant earlier than other cohorts (56.6% of uveitis study eyes had had ME for at least 12 months prior to the DEX implant compared with 73.3% of all study eyes). This finding suggests the uveitis eyes in the present study had comparatively less irreversible damage to retinal structures than the other more refractory retinal disease cohorts, which potentially explains the improved functional changes. Greater improvements in the uveitis subgroup could also be due to the lower BCVA (mean logMAR 0.71±0.07; 20/102 Snellen equivalents) and greater CRT (517.2±40.3 μm) of these patients at baseline. In the Phase III DEX implant RVO study, sham-treated control patients who completed the double-masked 180-day phase and were eligible (BCVA <84 letters or CRT >250 μm) for a delayed first treatment with DEX implant (BCVA) demonstrated less improvement in BCVA than that seen in patients who had their first DEX implant treatment 6 months earlier.13 These results suggest that delaying treatment may decrease the ability of patients with ME to benefit from treatment with the DEX implant.
In the present study, eyes with RVO had significant improvements in visual acuity and anatomic changes following DEX implant injections. RVO patients were most likely to have repeat DEX implant injections (50%, 15/30 eyes) and to be treated with no concomitant medication or procedure other than the DEX implant (73.3%, 22/30 eyes). Our results are similar to those of a US-based retrospective study, which evaluated the efficacy and safety of two or more DEX implant injections for treatment of ME in RVO patients, and reported improvements in visual acuity and CRT with each subsequent DEX implant injection (administered alone or combined with other therapies).19 Sharareh et al recently published a small retrospective chart review involving 18 patients with RVO who received at least two intravitreal bevacizumab injections before treatment with the DEX implant.20 The study identified two groups of bevacizumab-resistant patients, non-responders and partial responders, who had recurrent ME despite continued treatment. Both groups responded to subsequent DEX implant therapy, with reductions in central foveal and cube average thickness and improvements in visual acuity. Taken together, these findings suggest that, in clinical practice, patients with ME secondary to RVO may benefit from treatment with DEX implants, including patients with recalcitrant ME after anti-vascular endothelial growth factor (VEGF) therapy.
No new safety concerns were observed compared with earlier studies,11–14,19 although results from the CHROME study are to be viewed cautiously because of their retrospective nature, thus being limited to safety information captured on patient charts included for analysis. The most common adverse event was increased IOP reported in 20.8% of patients; 22.9% of study eyes had IOP increases ≥10 mmHg from baseline and 17.5% of study eyes required use of IOP-lowering medications during the course of the study for control of new or worsening IOP following treatment with the DEX implant. These results are in line with the Phase III trials of the DEX implant where by the end of the study period, no more than 24% of RVO and 23% of uveitis study eyes required use of IOP-lowering medications following treatment with the DEX implant.12,14 Elevations in IOP after the DEX implant were managed using four or less topical IOP-lowering medications, where the majority of study eyes required the use of just one or two IOP-lowering medications. Few study eyes had glaucoma surgery post-DEX implant. Cataract surgery was performed in 29.8% of the 47 phakic eyes evaluated in the study. However, it is not possible to attribute this rate strictly to the DEX implant, given that lens opacity at baseline and during the study was not collected in the medical charts reviewed in this retrospective study, and many of the eyes in our study received prior and concurrent steroid treatments (IVTA, sub-Tenon’s triamcinolone, and systemic prednisone) in addition to the DEX implant. Prior vitrectomy was performed in almost half (47.5%, 57/120) of the study eyes which, along with repeated DEX implant treatment, may have increased cataract development. There were too few patients with phakic eyes in the DME (n=3), RVO (n=1), and uveitis (none) cohorts that had cataract surgery following treatment with the DEX implant to allow a meaningful analysis of vision-related improvements after cataract surgery compared with baseline.
Results from the CHROME study provide new data on the real-world utilization and effect of the DEX implant across retinal indications in Canada. Since the date of final study data collection, reimbursement programs have remained the same and DEX implant treatment patterns have not significantly changed in the centers involved in the present study. Anti-VEGF therapies remain the first-line option for treatment of retinal disease, but significant proportions of patients who either do not respond optimally to anti-VEGF therapy or have disease recurrence have the need for frequent anti-VEGF injections that can become a significant burden. At the time of data collection for the CHROME study, bevacizumab treatments were primarily captured; however, now we would likely see more use of ranibizumab and aflibercept prior to or administered concomitantly with DEX implants.
The major limitations of our study are its retrospective and open-label design. No standardized assessments were defined prior to treatment across the centers, assessment tools such as OCT instruments were not normalized, and adverse events were limited to those reported on the medical charts. Additionally, per patient data collected depended on the number of DEX implant injections, frequency of treatment, and duration of follow-up. Analyses were limited to include data captured in patient medical charts; consequently, some assessments such as evaluation of changes in vitreous haze could not be assessed.
Nevertheless, DEX implant(s) provided a one-line to three-line gain in visual acuity from baseline, along with significant CRT improvements across all indications evaluated in this study. No new safety concerns were observed with the DEX implant, and increases in IOP, common to corticosteroids, were manageable using topical IOP-lowering medications. Additional studies will help to further elucidate the efficacy and safety of the DEX implant in various patient populations in the clinical setting.
Acknowledgments
This study was supported by Allergan Inc., Markham, ON, Canada, and is published on behalf of the CHROME Study Group. Writing and editorial assistance was provided by Kakuri Omari, PhD, of Evidence Scientific Solutions, Philadelphia, PA, and was funded by Allergan, Inc., Irvine, CA, USA. All authors met the International Committee of Medical Journal Editors authorship criteria. Neither honoraria nor payments were made for authorship.
Disclosure
W-CL has served as an advisor, consultant, and speaker, and has received travel reimbursement from Allergan Inc. and Novartis, has served as an advisor and received travel reimbursement and institutional grant(s) from Bayer, has served as an advisor and speaker for Alcon, and has served as a speaker for Bausch & Lomb. DAA has served as a consultant to Bausch & Lomb and Bayer, has served as a speaker for Novartis, and received a grant from Allergan Inc. related to the study. PY is a consultant for Bayer and has received honoraria for speaking and developing educational material, and has served as an advisor and speaker for Alcon, Allergan Inc., Bausch & Lomb, and Novartis. JCC has served as an advisor and consultant to Bayer, and received travel reimbursement from Allergan Inc. related to this study. AK has served as an advisor to Allergan Inc. DALM has served as an advisor to Alcon, Allergan Inc., and Bayer. AO has served as a consultant and a speaker, and has received grant(s) from Novartis, and has served as a consultant to Allergan Inc. TR has served as a consultant and speaker, and received honoraria for developing educational material from Alcon, has been a speaker for Allergan Inc., and AMO, and has received honoraria for developing education material from Bausch & Lomb. TGS has served as an advisor to Alcon, Allergan Inc., Bayer, and Novartis. ET has served as an advisor and consultant to Allergan Inc. LW has served as a consultant to Allergan Inc. CS is a statistical consultant to Allergan Inc., and DCB is an employee of Allergan Inc.
References
Kleinman ME, Baffi JZ, Ambati J. The multifactorial nature of retinal vascular disease. Ophthalmologica. 2010;224 Suppl 1:16–24. | ||
Wolfensberger TJ, Gregor ZJ. Macular edema – rationale for therapy. Dev Ophthalmol. 2010;47:49–58. | ||
Sarao V, Veritti D, Boscia F, Lanzetta P. Intravitreal steroids for the treatment of retinal diseases. Scientific World Journal. 2014;2014:989501. | ||
Hardman JG, Limburd LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman and Gilman’s: The Pharmacological Basis of Therapeutics. 9th ed. New York, NY, USA: McGraw-Hill; 1996. | ||
Kwak HW, D’Amico DJ. Evaluation of the retinal toxicity and pharmacokinetics of dexamethasone after intravitreal injection. Arch Ophthalmol. 1992;110:259–266. | ||
Maxwell DP Jr, Brent BD, Diamond JG, Wu L. Effect of intravitreal dexamethasone on ocular histopathology in a rabbit model of endophthalmitis. Ophthalmology. 1991;98:1370–1375. | ||
Nabih M, Peyman GA, Tawakol ME, Naguib K. Toxicity of high-dose intravitreal dexamethasone. Int Ophthalmol. 1991;15:233–235. | ||
Ozurdex (dexamethasone intravitreal implant 0.7 mg). [Package insert]. Irvine, CA, USA. Allergan, Inc.; 2014. | ||
Haller JA, Dugel P, Weinberg DV, Chou C, Whitcup SM. Evaluation of the safety and performance of an applicator for a novel intravitreal dexamethasone drug delivery system for the treatment of macular edema. Retina. 2009;29:46–51. | ||
Chang-Lin JE, Attar M, Acheampong AA, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52:80–86. | ||
Kuppermann BD, Blumenkranz MS, Haller JA, et al. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol. 2007;125:309–317. | ||
Haller JA, Bandello F, Belfort R Jr, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–1146. | ||
Haller JA, Bandello F, Belfort R Jr, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118:2453–2460. | ||
Lowder C, Belfort R Jr, Lightman S, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129:545–553. | ||
Boyer DS, Faber D, Gupta S, et al. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31:915–923. | ||
Shaikh AH, Petersen MR, Sisk RA, Foster RE, Riemann CD, Miller DM. Comparative effectiveness of the dexamethasone intravitreal implant in vitrectomized and non-vitrectomized eyes with macular edema secondary to central retinal vein occlusion. Ophthalmic Surg Lasers Imaging Retina. 2013;44:28–33. | ||
Boyer DS, Yoon YH, Belfort R Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–1914. | ||
Bezatis A, Spital G, Hohn F, et al. Functional and anatomical results after a single intravitreal Ozurdex injection in retinal vein occlusion: a 6-month follow-up – the SOLO study. Acta Ophthalmol. 2013;91: e340–e347. | ||
Capone A Jr, Singer MA, Dodwell DG, et al. Efficacy and safety of two or more dexamethasone intravitreal implant injections for treatment of macular edema related to retinal vein occlusion (SHASTA study). Retina. 2014;34:342–351. | ||
Sharareh B, Gallemore R, Taban M, Onishi S, Wallsh J. Recalcitrant macular edema after intravitreal bevacizumab is responsive to an intravitreal dexamethasone implant in retinal vein occlusion. Retina. 2013;33:1227–1231. | ||
Dutra Medeiros M, Postorino M, Navarro R, Garcia-Arumi J, Mateo C, Corcostegui B. Dexamethasone intravitreal implant for treatment of patients with persistent diabetic macular edema. Ophthalmologica. 2014;231:141–146. | ||
Zucchiatti I, Lattanzio R, Querques G, et al. Intravitreal dexamethasone implant in patients with persistent diabetic macular edema. Ophthalmologica. 2012;228:117–122. | ||
Tomkins-Netzer O, Taylor SR, Bar A, et al. Treatment with repeat dexamethasone implants results in long-term disease control in eyes with noninfectious uveitis. Ophthalmology. 2014;121:1649–1654. | ||
Ozurdex (dexamethasone intravitreal implant 0.7 mg). [Product monograph]. Markham, ON, Canada; Allergan Inc.; 2015. | ||
Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997;13:388–391. | ||
Zalewski D, Raczynska D, Raczynska K. Five-month observation of persistent diabetic macular edema after intravitreal injection of Ozurdex implant. Mediators Inflamm. 2014;2014:364143. | ||
Petrash JM. Aging and age-related diseases of the ocular lens and vitreous body. Invest Ophthalmol Vis Sci. 2013;54:ORSF54–ORSF59. | ||
Bhatnagar P, Schiff WM, Barile GR. Diabetic vitrectomy: the influence of lens status upon surgical outcomes. Curr Opin Ophthalmol. 2008; 19:243–247. | ||
Kruh J, Foster CS. Corticosteroid-sparing agents: conventional systemic immunosuppressants. Dev Ophthalmol. 2012;51:29–46. |
Supplementary material
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.