Back to Journals » International Journal of General Medicine » Volume 13
Real-World Analysis of Potential Pharmacokinetic and Pharmacodynamic Drug Interactions with Apixaban in Patients with Non-Valvular Atrial Fibrillation
Authors Badreldin HA , Alghamdi J , Alshaya O, Alshehri A, Alreshoud L, Altoukhi R, Vasudevan S , Ismail WW , Mohamed MSA
Received 3 May 2020
Accepted for publication 1 July 2020
Published 22 July 2020 Volume 2020:13 Pages 419—427
DOI https://doi.org/10.2147/IJGM.S260813
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Hisham A Badreldin,1 Jahad Alghamdi,2 Omar Alshaya,1 Abdulmajeed Alshehri,1 Lamya Alreshoud,1 Renad Altoukhi,1 Senthilvel Vasudevan,1 Wesam W Ismail,1,3 Mohamed Salih Aziz Mohamed4
1Department of Pharmacy Practice, College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, King Abdullah International Medical Research Center, Ministry of National Guard Health Affairs, Riyadh, Saudi Arabia; 2The Saudi Biobank, King Abdullah International Medical Research Center, King Saud Bin Abdulaziz University for Health Sciences, Ministry of National Guard Health Affairs, Riyadh, Saudi Arabia; 3Department of Pharmacy Practice and Science, College of Pharmacy, The University of Iowa, Iowa City, IA, United States; 4Adult Cardiology Department, College of Medicine, King Saud Bin Abdulaziz University for Health Sciences, King Abdullah International Medical Research Center, Ministry of National Guard Health Affairs, Riyadh, Saudi Arabia
Correspondence: Hisham A Badreldin
King Saud bin Abdulaziz University for Health Sciences, PO Box 3660, Riyadh 11481, Saudi Arabia
Tel +966 11 4299999 ext. 95103
Fax +966 11 4299999 Ext 95058
Email [email protected]
Purpose: We conducted this study to assess the real-world prevalence, nature, predictors, and clinical necessity of apixaban pharmacokinetic (PK) and pharmacodynamic (PD) drug interactions in patients with non-valvular atrial fibrillation (NVAF) at a tertiary medical institution in Saudi Arabia.
Patients and Methods: An observational retrospective cohort analysis was conducted in adult patients diagnosed with NVAF receiving apixaban for stroke prevention from the period of June 2015 to May 2019.
Results: Of the 1271 patients included in the analysis, 611 (48.1%) patients had potential PD– or PK–drug interactions with apixaban. Of those, 490 (38.6%) patients had potential PD drug–drug interactions (DDIs) and 121 (9.5%) patients had potential PK-DDIs. PD-DDIs with apixaban were mainly with antiplatelet therapy followed by non-steroidal anti-inflammatory drugs and antidepressants. PK-DDIs with apixaban were mainly with combined P-gp/CYP3A4 inhibitors or inducers. History of minor bleeding was positively correlated with PD-DDIs with apixaban, ß coefficient = 0.455 (OR 1.58; 95% CI 1.01– 2.45); p< 0.05. History of acute coronary syndrome was positively correlated with PD-DDIs with apixaban, ß coefficient = 0.515 (OR 1.60; 95% CI 1.36– 1.99); p< 0.05. History of heart failure was positively correlated with PK-DDIs with apixaban, ß coefficient = 0.459 (OR 1.58; 95% CI 1.07– 2.35); p< 0.05. Almost 15% of the included patients had no clinical indication to receive the potential interacting drug with apixaban and about 20% of them were assuming an inappropriate apixaban dose according to the product package insert.
Conclusion: Pharmacodynamics and pharmacokinetics interactions are common in more than half of the patients with NVAF receiving apixaban for stroke prevention in this real-world analysis. Some of these interacting medications are not indicated. Drug–drug interactions should always be considered and monitored with apixaban with a regular assessment of the need for any interacting medication.
Keywords: apixaban, drug interaction, pharmacokinetic, pharmacodynamic, atrial fibrillation
Introduction
Globally, the most commonly encountered arrhythmia in clinical settings is atrial fibrillation (AF). It is associated with higher risks of mortality, morbidity, and healthcare resources utilization.1 Stroke is one of the major complications of AF. Currently, there are many therapeutic options for stroke prevention in patients with AF.1 Direct oral anticoagulants (DOACs) are novel anticoagulants that are now recommended as first-line agents for stroke prevention in patients with non-valvular atrial fibrillation (NVAF) according to the 2019 American College of Cardiology guidelines for the management of atrial fibrillation.2 DOACs are presented as they have a lower risk of drug–drug interactions (DDI) and food–drug interactions (FDI) in comparison to vitamin-K antagonists (VKAs). VKAs, like warfarin, have many significant DDIs that might potentially limit their use, and mandate an alternative anticoagulant therapy.3 Though DOACs have clinically fewer DDIs, one should not neglect them as some of these interactions require dosing adjustments or an alternative anticoagulation strategy to avoid the risk of bleeding or thrombosis.3 The two main types of drug interactions that DOACs are subjected to are pharmacokinetic DDI (PK-DDI) and pharmacodynamic DDI (PD-DDI). PK-DDI is when another medication alters the concentration of the objected drug. Therefore, factors such as bioavailability, clearance, or distribution might be affected. Examples of such interactions with DOACs include drugs that are affected by P-glycoprotein (P-gp) and cytochrome P450-3A (CYP3A).4,5 Previous evidence showed that administering dabigatran with P-gp inhibitors resulted in higher dabigatran plasma concentrations and more bleeding episodes.6
On the other hand, PD-DDI affects the pharmacological effect of the offended DOAC by either agonizing or antagonizing its effect. Though the list of drugs that interact with DOACs is extensive, the clinical significance of said interactions is not well documented.4,5 For example, the subgroup analysis of the concomitant PD-DDI between antiplatelet therapy (aspirin and/or clopidogrel) and dabigatran in the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial was shown to increase the risk of major bleeding without any additional benefit.7 A post hoc analysis of the ARISTOTLE trial with apixaban found that patients who were using five or more concomitant drugs had higher rates of bleeding complications (1.91, 2.46, and 3.88 per 100 patient-years, respectively), thromboembolisms (1.29, 1.48, and 1.57 per 100 patient-years, for 0–5, 6–8, and ≥9 drugs, respectively), and a higher mortality rates (P<0.001).8 In comparison to the other DOACs, apixaban and rivaroxaban are substrates of both the CYP 3A4/5 hepatic isoenzyme system and P-gp efflux transporter system. This may subject individuals who are receiving these agents to a number of drug interactions.9 Since many randomized controlled trials and real-world studies assessed the safety and efficacy of DOACs but not the pattern of DDIs with apixaban, this study aimed to describe the prevalence, nature, and predictors of DDIs with apixaban, both PK-DDIs and PD-DDIs at a large, tertiary, academic medical institution.
Materials and Methods
Study Design and Study Population
This was a single-center, retrospective, observational cohort study which included all patients following up at King Abdulaziz Medical City (KAMC), Riyadh, Saudi Arabia and were diagnosed with confirmed NVAF and prescribed apixaban for stroke prevention from June 2015 to May 2019. KAMC has a bed capacity of more than 1500 beds. The Institutional Review Board at King Abdullah International Medical Research Center granted ethical approval to conduct this study (IRBC/1279/18). To identify the patients, we used the electronic hospital record system, which utilizes a medication-specific code. Each drug in the institution’s formulary is equipped with a designated medication code to aid in the identification of patients for research purposes as well as other purposes. Using the medication-coding system, we conducted a chart review and collected the following patient’s information: baseline demographics, apixaban prescribed dosage, comorbid conditions, concomitant interacting medications, documented bleeding history, and the CHA2DS2-VASc score. We categorized bleeding events according to the bleeding definition provided by the International Society for Thrombosis and Hemostasis (ISTH).10 The list of interacting medications with apixaban was collected from the United States Food and Drug Administration (US-FDA) and the European Medicines Agency (EMA) package inserts. Any patients who were on apixaban for indications other than NVAF or patients with missing information were excluded. Since apixaban is the only DOAC available at KAMC, all other DOACs were excluded from the analysis. According to the product package insert, the recommended dose of apixaban is 5 mg to be taken orally twice daily. It is recommended to reduce the dose to 2.5 mg to be taken orally twice daily in patients with at least two of the following criteria: age greater than or equal to 80 years, body weight less than or equal to 60 kg, or serum creatinine greater than or equal to 1.5 mg/dL. Moreover, it is recommended to reduce the dose by 50% when apixaban 5 mg twice daily is co-administered with drugs that are combined P-gp and strong cytochrome P450 3A4 (CYP3A4) inhibitors. In patients already taking 2.5 mg twice daily, it is recommended to avoid the co-administration of apixaban with combined P-gp and strong CYP3A4 inhibitors according to the US-FDA package insert.
Endpoints
This study’s major endpoints were to investigate the prevalence and nature of patients who received a PK or PD interacting medication with apixaban at discharge. Also, we assessed patient-specific factors that could contribute to these interactions by comparing those who received PD- or PK-interacting medication to those who did not receive any interacting medication. Moreover, we assessed the clinical indication of these interacting medications. The clinical indication was defined as the presence of documented index event that necessitates the administration of these interacting medications. Finally, in patients who received a PK or PD interacting medication with apixaban, we assessed the inappropriateness of the apixaban administered dose according to the US-FDA product package insert.
Statistical Analysis
Continuous data were presented as means and standard deviation, or medians and interquartile range where appropriate. Categorical data were presented as frequency and percentages. Prevalence of patients who received a PK or PD interacting medication with apixaban was calculated by dividing the number of patients who received a PK or PD interacting medication with apixaban by the total number of patients with NVAF receiving apixaban for stroke prevention over the study period. To investigate the patient-specific factors that could contribute to these interactions, we have used two types of analysis, bivariate and multivariate analyses. The bivariate analysis was conducted to find the association between categorical variables by using the Chi-Square test and to compare the mean difference between continuous variables between groups by using independent samples t-test. Significant variables obtained by the bivariate analyses were taken and included in the final multivariate logistic regression analysis to find the potential predicting factors for PK and PD drug interactions among patients receiving apixaban. The backward elimination method was used to find the potential influencing factors associated with PK and PD drug interactions among patients receiving apixaban. The goodness of fit was assessed, and variables were reviewed. Odds ratios (ORs) and their 95% confidence intervals (CIs) were reported. Statistical significance was fixed at p < 0.05. Apixaban dose inappropriateness was calculated by dividing the number of patients who received a PK or PD interacting medication with inappropriately dosed apixaban according to the US-FDA package insert by the total number of patients with NVAF who received a PK or PD interacting medication with apixaban. The collected data were compiled using Microsoft Excel 2010 (Office 365, Microsoft Ltd., USA) and analyzed by using Statistical Package for Social Sciences 20.0 version (SPSS Inc. Chicago, USA).
Results
Patients Baseline Demographics
We were able to identify 1932 patients who received apixaban during the study period. We excluded 577 patients as they were receiving apixaban for an indication other than NVAF. Also, we excluded 84 patients due to missing information. A total of 1271 patients were included in the final analysis. Of the included patients, 61.4% were males with a mean age of 66.8 years. The mean body mass index was 26.3 kg/m2, and the mean calculated CHA2DS2-VASc score was 3.3. Almost 90% of the included patients were receiving a dose of 5 mg of apixaban. Other pertinent baseline demographics are shown in (Table 1).
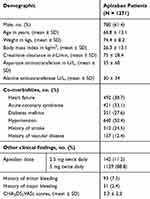 |
Table 1 Patients Demographics |
Potential Pharmacokinetic and Pharmacodynamics Drug Interactions with Apixaban
Overall, 611 (48.1%) of the included patients had potential PD- or PK-DDIs with apixaban in which, 490 (38.6%) patients had potential PD-DDIs, 121 (9.5%) patients had potential PK-DDIs with apixaban, and 87 (6.1%) patients had potential PD-DDIs and PK-DDIs with apixaban. Of the 490 patients who had potential PD-DDIs with apixaban, 122 (25%) patients were receiving non-steroidal anti-inflammatory drugs, 101 (20.6%) were receiving either selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI), 126 (25.7%) patients were receiving aspirin, 32 (6.5%) patients were receiving clopidogrel, 109 (22.2%) were receiving clopidogrel and aspirin. Of the 490 patients who had potential PD-DDIs with apixaban, 260 (53%) had a history of acute coronary syndrome, 179 (36.5%) had a history of stroke and 77 (15.7%) had a history of depression. Figure 1 shows a comprehensive list of PD-DDIs with apixaban.
 |
Figure 1 Comprehensive list of PD-DDIs with apixaban (N = 490). |
Of the 121 patients who had potential PK-DDIs with apixaban, 106 (87.6%) patients were receiving agents that might increase blood levels of apixaban, and 15 (12.4%) patients were receiving other agents that might reduce blood levels of apixaban. For the 106 (87.6%) patients who were receiving agents that might increase blood levels of apixaban, 103 (97.2%) patients were receiving either a combined P-gp inhibitor and moderate CYP3A4 inhibitor agent or strong CYP3A4 inhibitor agent alone, and 3 (2.8%) patients were receiving a combined P-gp inhibitor and strong CYP3A4 inhibitor agent. For the 15 (12.4%) patients who were receiving other agents that reduce blood levels of apixaban, 4 (26.7%) patients were receiving a combined P-gp inducer and strong CYP3A4 inducer agent, 11 (73.3%) patients were receiving a strong CYP3A4 inducer agent. Figure 2 shows a comprehensive list of PD-DDIs with apixaban.
 |
Figure 2 Comprehensive list of PK-DDIs with apixaban (N = 121). |
Predictors of Pharmacodynamics Drug Interactions with Apixaban
In the univariate analysis of categorical variables, several patient’s characteristics showed statistical significance, including heart failure, hypertension, acute coronary syndrome, history of minor bleeding event, and history of major bleeding event. Other categorical variables did not show any statistical significance. In the univariate analysis of continuous variables, several patient’s characteristics showed statistical significance, including CHA2DS2-VASc score and aspartate aminotransferase. Other continuous variables did not show any statistical significance. Statistically significant categorical and continuous variables were incorporated in the multivariate logistic regression analysis (Table 2). Results from that analysis showed a statistically significant positive correlation between pharmacodynamic drug interactions with apixaban and patients with a history of minor bleeding, ß = 0.455 (OR 1.58; 95% CI 1.01–2.45); p < 0.05. We also found a statistically significant positive correlation between pharmacodynamic drug interactions with apixaban and patients with acute coronary syndrome, ß = 0.515 (OR 1.60; 95% CI 1.36–1.99); p < 0.05. The other variables, like heart failure, hypertension, history of major bleeding, aspartate aminotransferase, and CHA2DS2VASc score, did not show any statistical correlation. In this binary logistic regression analysis, under the Hosmer and Lemeshow test, the Chi-Square value was 4.219 with the p-value was 0.837, which was not statistically significant with p-value >0.05.
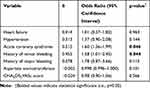 |
Table 2 Multivariate Logistic Regression Analysis of Predictors Associated with Pharmacodynamic Drug Interactions with Apixaban |
Predictors of Pharmacokinetic Drug Interactions with Apixaban
In the univariate analysis of categorical variables, several patient’s characteristics showed statistical significance, including heart failure, diabetes mellitus, history of stroke, and history of minor bleeding event. Other categorical variables did not show any statistical significance. In the univariate analysis of continuous variables, several patient’s characteristics showed statistical significance, including CHA2DS2VASc score and aspartate aminotransferase. Other continuous variables did not show any statistical significance. Statistically significant categorical and continuous variables were incorporated in the multivariate logistic regression analysis (Table 3). Results from that analysis showed a statistically significant positive correlation between pharmacokinetic drug interactions with apixaban and patients with heart failure, ß = 0.459 (OR 1.58; 95% CI 1.07–2.35); p < 0.05. The other variables, including diabetes mellitus, history of stroke, and history of minor bleeding event, aspartate aminotransferase, and CHA2DS2VASc score, did not show any statistical significant correlation. In this binary logistic regression analysis, under the Hosmer and Lemeshow test, the Chi-Square value was 8.256, with the p-value was 0.409, which was not statistically significant with p-value >0.05.
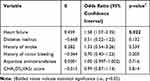 |
Table 3 Multivariate Logistic Regression Analysis of Predictors Associated with Pharmacokinetic Drug Interactions with Apixaban |
Clinical Indication
When we investigated the documented index events that necessitate the administration of a potential interacting medication, the clinical indication to administer a potential PD- or PK-DDIs with apixaban was present in 518 (84.8%) of the 611 patients who had a potential PD- or PK-DDIs with apixaban. Of the 490 patients who had potential PD-DDIs with apixaban, 407 (83.1%) patients had a clinical indication to administer the potential PD-DDIs with apixaban. Of the remaining 83 (16.9%) patients, 72 (86.7%) patients were receiving an NSAID, and 11 (13.3%) patients were receiving an SSRI regularly without a documented clinical indication. Of the 121 patients who had potential PK-DDIs with apixaban, 111 (91.7%) patients had a clinical indication to administer the potential PK-DDIs with apixaban.
Apixaban Dose Inappropriateness
Of the 611 (63.3%) included patients who had potential PD- or PK-DDIs with apixaban, 121 (19.8%) patients were receiving an inappropriate apixaban dose according to the US-FDA package insert. Of the 121 patients, 108 (89.3%) patients were receiving 5 mg twice daily, and they were supposed to be switched to 2.5 mg twice daily. The remaining 13 (10.7%) patients were receiving 2.5 mg twice daily, and alternative anticoagulant therapy should have been considered instead of apixaban.
Discussion
In this study, we evaluated the real-world prevalence, nature, predictors, and clinical indication of PD- or PK-DDIs with apixaban in patients with NVAF at a large, tertiary, academic medical center. It is noteworthy to mention that our study is one of the few studies that evaluated interactions with apixaban specifically, whereas other studies included DOACs as a class, dabigatran or rivaroxaban alone.
Our findings showed that 611 (48.1%) of our patients were receiving at least one interacting medication with apixaban, which is consistent with previous studies that showed that the prevalence of drug interactions with DOACs ranges between (37–78%).11–15 It is important to highlight that the prevalence of drug interactions with apixaban remains high and is consistent with other studies. One explanation of this high percentage could be due to polypharmacy in patients with atrial fibrillation. Evidence has shown that patients with atrial fibrillation are more likely to be receiving five or more medications.16 Moreover, it has been reported that the prevalence of polypharmacy in atrial fibrillation patients ranges between (40–76%), and as the number of medications increases, mortality, stroke, and major bleeding were more frequent.8,16
PD-DDIs were more frequent in our patients compared to PK-DDIs (38.6% versus 9.5%, respectively), which is similar to other studies that reported the same finding.12,17 One study reported that (29%) of patients have at least one PD-DDIs. However, the percentage (38.6%) was slightly higher in our study, which could be attributed to the increased use of antiplatelet therapy in our population (21%) compared to the other study (3.3%).12 This could be explained by the fact that atherosclerotic cardiovascular diseases were more prevalent in our population, which may necessitate the use of an antiplatelet for primary or secondary prevention purposes. When it comes to other PD-DDI, SSRI/SNRI interactions were also not uncommon. This could be explained by the lack of clinical evidence that correlates SSRI/SNRI with bleeding when used concomitantly with apixaban with the exception of the package insert that includes a caution of potential interaction. However, an increased risk of bleeding was reported due to SSRI/SNRI interactions with other anticoagulants but not apixaban in AF patients.18 In a study that evaluated rivaroxaban interactions, NSAIDs were the most frequent interacting class of medications, and it significantly increased the risk of bleeding in AF patients.17 Our results were comparable; however, NSAIDs were the second most frequent PD-DDIs after antiplatelet therapy. The drug–drug interaction due to NSAIDs use could be minimized by using the lowest effective dose of the safest NSAIDs (eg, naproxen) for the shortest period of time.19 Although a 50% increase in apixaban AUC and a 60% increase in Cmax have been reported with the use of naproxen (a P-gp inhibitor), this was not associated with clinically relevant prolongation of bleeding time. Patients with ACS were more likely to be on a pharmacodynamic interacting medication, ß coefficient = 0.515 (OR 1.60; 95% CI 1.36–1.99); p<0.05 which could be due to that the majority of these patients require dual or triple antithrombotic therapy according to their index event and the risk of bleeding.20 Furthermore, minor bleeding was a significant predictor of PD-DDIs with apixaban, ß coefficient = 0.455 (OR 1.58; 95% CI 1.01–2.45); p<0.05. This finding could be due to a lack of long-term consequences of minor bleeding and underestimating such interactions.
In terms of PK-DDIs with apixaban, 121 patients had at least one interacting medications with apixaban. The majority of them (87.6%) were using a combined P-gp/CYP3A4 inhibitor agent, which increases the blood level of apixaban. Previous study reported that 59.8% of patients were on at least one combined P-gp/CYP3A4 inhibitor agent.20 So, drugs inhibiting or inducing P-gp/CYP3A4 remains the main mechanism of PK-DDIs. Patients with heart failure were more likely to have pharmacokinetic drug interactions with apixaban, ß coefficient = 0.459 (OR 1.58; 95% CI 1.07–2.35); p<0.05. This is an important finding because patients with heart failure are known to have altered pharmacokinetics of many medications.21 This is due to the reduction in both the volume of distribution and the perfusion to the various sites of drug clearance, such as the liver and the kidneys.21 Moreover, patients with heart failure tend to have several comorbidities that require additional drug therapy treatment, thus producing a heavy pill burden and might increase the risk of PK-DDIs.22 That being said, possible potentiation or reduction in apixaban effects could occur due to altered pharmacokinetics.
For both the PD- and PK-DDIs predictors, the variables selected for the logistic regression models were very good, and goodness fit was fulfilled. It should be highlighted that this study has been conducted at a tertiary hospital, and the prevalence of DDIs with apixaban might be higher in a real-world setting of patients discharged from minor hospitals. Moreover, apixaban has been used only as a paradigmatic treatment since drug interactions are common to other anticoagulants and, in general, to all drugs.
Our study has several strengths. First, we assessed the clinical indication of administering a potential interacting medication with apixaban. The clinical indication was justified in the majority of the patients who had a potential PD- or PK-DDIs. Interestingly, 15.2% (93/611) of patients who had a potential PD- or PK-DDIs had no clinical indication for the interacting medication. Thus, regular monitoring and assessment of the need for medications being co-administered with apixaban should be encouraged, notably after we identified that more than 60% of patients using apixaban are receiving at least one interacting medication. Also, it is crucial to frequently assess the risk versus the benefit of switching to another anticoagulant such as warfarin, especially if the interacting drug with apixaban is essential and has no alternative. Second, our study is one of the few studies that evaluated the prevalence and predictors of apixaban interactions from both pharmacokinetics and pharmacodynamics perspectives. Our results emphasize the importance of considering these interacting medications and their possible impacts on clinical outcomes, which could help to create strategies in apixaban dosing tailored for patient-specific needs. Third, it is good to highlight that the sample size in our study may be considered relatively high compared to other observational studies that evaluated DOACs interactions in AF patients.
There are several limitations to our study, including retrospective study design, single-center, and the possibility of documentation bias. Also, we only assessed the potential DDIs with apixaban, and the results of this study should not be extrapolated to the other DOACs. Moreover, this study was designed to identify the prevalence and predictors of interactions not to report the clinical effects of such interactions, such as bleeding or thrombotic events. We plan to reassess our cohort for the clinical effects and outcomes of PK- and PD-DDIs with apixaban in a future study. This could provide further guidance for clinicians whenever they prescribe this agent. Besides, polypharmacy was not addressed in our study, which has been reported to be an essential predictor of pharmacodynamics and pharmacokinetic interactions.23 It could also lead to multi-mechanism DDIs, which have been reported to result in more serious adverse clinical outcomes compared to single mechanism DDIs.24 That being said, we believe that this study represents one of the largest real-world evidence investigating the prevalence of potential DDIs with apixaban. The results of this study will be utilized to implement several institutional strategies and interventions in an effort to assist the prescribing clinicians in overcoming this perplexity in the future.
Conclusion
The prevalence of pharmacodynamics and pharmacokinetics drug interactions with apixaban is common in almost half of the patients with atrial fibrillation receiving apixaban for stroke prevention. Moreover, some of these interacting medications could have no clinical indication to be administered. Drug–drug interactions should always be carefully considered and monitored with apixaban with a regular assessment of the need for any interacting medication.
Compliance with Ethical Standards
Approval was obtained by King Abdullah International Medical Research Center Institutional Review Board Committee : (IRBC reference: 1279/18). Giving the retrospective nature of this study, informed consent was not required.
Data Sharing Statement
The authors declare that they had full access to all of the data in this study, and the authors take complete responsibility for the integrity of the data and the accuracy of the data analysis. Data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
All authors expressed sincere gratitude to the Data Management Department at King Abdullah International Medical Research Center’s for their cooperation in obtaining and extracting patient’s data.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Morillo CA, Banerjee A, Perel P, Wood D, Jouven X. Atrial fibrillation: the current epidemic. J Geriatr Cardiol. 2017;14(3):195–203. doi:10.11909/j.issn.1671-5411.2017.03.011
2. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2019;74(1):104–132. doi:10.1016/j.jacc.2019.01.011
3. Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55–61. doi:10.15420/aer.2017.50.1
4. Roberts AG, Gibbs ME. Mechanisms and the clinical relevance of complex drug-drug interactions. Clin Pharmacol. 2018;10:123–134. doi:10.2147/CPAA.S146115
5. Nutescu EA, Burnett A, Fanikos J, Spinler S, Wittkowsky A. Pharmacology of anticoagulants used in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41(1):15–31. doi:10.1007/s11239-015-1314-3
6. Bernier M, Lancrerot SL, Rocher F, et al. Major bleeding events in octagenarians associated with drug interactions between dabigatran and P-gp inhibitors. J Geriatr Cardiol. 2019;16(11):806–811.
7. Dans AL, Connolly SJ, Wallentin L, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Circulation. 2013;127(5):634–640. doi:10.1161/CIRCULATIONAHA.112.115386
8. Jaspers Focks J, Brouwer MA, Wojdyla DM, et al. Polypharmacy and effects of apixaban versus warfarin in patients with atrial fibrillation: post hoc analysis of the ARISTOTLE trial. BMJ. 2016;353:i2868. doi:10.1136/bmj.i2868
9. Rose DK, Bar B. Direct oral anticoagulant agents: pharmacologic profile, indications, coagulation monitoring, and reversal agents. J Stroke Cerebrovasc Dis. 2018;27(8):2049–2058. doi:10.1016/j.jstrokecerebrovasdis.2018.04.004
10. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119–2126. doi:10.1111/jth.13140
11. Hirsh Raccah B, Rottenstreich A, Zacks N, et al. Drug interaction as a predictor of direct oral anticoagulant drug levels in atrial fibrillation patients. J Thromb Thrombolysis. 2018;46(4):521–527. doi:10.1007/s11239-018-1738-7
12. Forbes HL, Polasek TM. Potential drug-drug interactions with direct oral anticoagulants in elderly hospitalized patients. Ther Adv Drug Saf. 2017;8(10):319–328. doi:10.1177/2042098617719815
13. Chin PK, Vella-Brincat JW, Walker SL, Barclay ML, Begg EJ. Dosing of dabigatran etexilate in relation to renal function and drug interactions at a tertiary hospital. Intern Med J. 2013;43(7):778–783. doi:10.1111/imj.12170
14. Armbruster AL, Buehler KS, Min SH, Riley M, Daly MW. Evaluation of dabigatran for appropriateness of use and bleeding events in a community hospital setting. Am Health Drug Benefits. 2014;7(7):376–384.
15. Sidman E, Probst LA, Darko W, Miller CD. Evaluation of dabigatran utilization and risk among hospitalized patients. Ann Pharmacother. 2014;48(3):349–353. doi:10.1177/1060028013513722
16. Proietti M, Raparelli V, Olshansky B, Lip GYH. Polypharmacy and major adverse events in atrial fibrillation: observations from the AFFIRM trial. Clin Res Cardiol. 2016;105(5):412–420. doi:10.1007/s00392-015-0936-y
17. Kreutz R, Haas S, Holberg G, et al. Rivaroxaban compared with standard thromboprophylaxis after major orthopaedic surgery: co-medication interactions. Br J Clin Pharmacol. 2016;81(4):724–734. doi:10.1111/bcp.12836
18. Quinn GR, Singer DE, Chang Y, et al. Effect of selective serotonin reuptake inhibitors on bleeding risk in patients with atrial fibrillation taking warfarin. Am J Cardiol. 2014;114(4):583–586. doi:10.1016/j.amjcard.2014.05.037
19. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9(1):143–150. doi:10.14336/AD.2017.0306
20. Gargiulo G, Goette A, Tijssen J, et al. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J. 2019;40(46):3757–3767. doi:10.1093/eurheartj/ehz732
21. Shammas FV, Dickstein K. Clinical pharmacokinetics in heart failure. An updated review. Clin Pharmacokinet. 1988;15(2):94–113. doi:10.2165/00003088-198815020-00002
22. Mastromarino V, Casenghi M, Testa M, et al. Polypharmacy in heart failure patients. Curr Heart Fail Rep. 2014;11(2):212–219. doi:10.1007/s11897-014-0186-8
23. Admassie E, Melese T, Mequanent W, Hailu W, Srikanth BA. Extent of poly-pharmacy, occurrence and associated factors of drug-drug interaction and potential adverse drug reactions in Gondar Teaching Referral Hospital, North West Ethiopia. J Adv Pharm Technol Res. 2013;4(4):183–189. doi:10.4103/2231-4040.121412
24. Palleria C, Di Paolo A, Giofre C, et al. Pharmacokinetic drug-drug interaction and their implication in clinical management. J Res Med Sci. 2013;18(7):601–610.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
