Back to Journals » Patient Preference and Adherence » Volume 14
Real-World Adherence and Discontinuation of Glucagon-Like Peptide-1 Receptor Agonists Therapy in Type 2 Diabetes Mellitus Patients in the United States
Authors Weiss T , Carr RD, Pal S , Yang L, Sawhney B , Boggs R , Rajpathak S, Iglay K
Received 19 August 2020
Accepted for publication 23 October 2020
Published 27 November 2020 Volume 2020:14 Pages 2337—2345
DOI https://doi.org/10.2147/PPA.S277676
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Tracey Weiss,1 Richard D Carr,2,3 Sampriti Pal,4 Lingfeng Yang,1 Baanie Sawhney,4 Robert Boggs,1 Swapnil Rajpathak,1 Kristy Iglay1
1Center for Observational and Real-World Evidence, Merck & Co., Inc, Kenilworth, NJ, 07033, USA; 2Global Medical Affairs, Merck Sharp & Dohme Limited (MSD), Hoddesdon, EN11 9BU, UK; 3Hatter Cardiovascular Institute, University College London, London, WC1E 6HX, UK; 4Real-World Evidence, Complete HEOR Solutions (CHEORS), Pennsylvania, PA, 19454, USA
Correspondence: Tracey Weiss Tel +1 (908) 873-9697
Email [email protected]
Aim: To assess adherence and discontinuation of injectable glucagon-like peptide-1 receptor agonists (GLP-1 RA) at 12 and 24 months among adult type 2 diabetes mellitus (T2DM) patients in the United States initiating GLP-1 RA using the administrative claims-based database, Optum Clinformatics® Data Mart 7.1.
Methods: A retrospective study was conducted from 01/2009 to 12/2017. Patients were required to be continuously enrolled for 12 months prior to their first GLP-1 RA prescription. Proportion of days covered (PDC) from prescription claims ≥ 0.80 defined adherence. Discontinuation was defined as a ≥ 90-day gap from the last date of GLP-1 RA supply to the first date of subsequent prescription claim.
Results: A total of 4791 T2DM patients had ≥ 1 and 3907 had ≥ 2 GLP-1 RA prescription claims. 50.9% and 47.4% of patients were adherent at 12 and 24 months, respectively. Adherence was significantly higher among patients on weekly vs daily doses (p< 0.001). Median time to discontinuation was 13 months. The discontinuation rate was 47.7% and 70.1% at 12 and 24 months, respectively, with differences at 24 months for age and dosing frequency (p< 0.001 for both).
Conclusion: Over half of T2DM patients initiating GLP-1 RA were non-adherent and the majority (70.1%) discontinued therapy by 24 months. Reasons for non-adherence and discontinuation merit further research.
Keywords: GLP-1 RA, adherence, discontinuation, United States
Introduction
Estimates of the prevalence of type 2 diabetes mellitus (T2DM) in the United States (US) range between 9.4%-14%.1–4 Although approximately 33% of people who have diabetes are not diagnosed,1 over 23 million people in the US receive medical treatment for glycemic control.5 Cardiovascular diseases (CVD) are highly prevalent among T2DM patients. Reports of CVD vary widely across T2DM study participants and patient populations such as 21.5% of patients in a commercial database,6 51% among older US Veterans,7 and an estimated 44% based on a meta-analysis of seven studies from the US.8 Current guidelines from the American Diabetes Association (ADA) recommend a Glucagon-like Peptide-1 receptor agonist (GLP-1 RA) or Sodium-Glucose Cotransporter-2 inhibitor (SGLT-2i) with demonstrated CVD benefit as second-line therapy following metformin for T2DM patients with clinical cardiovascular disease due to their demonstrated cardiovascular benefit in clinical trials.9 Notably, the LEADER clinical trial found that when followed for a median of 3.8 years, T2DM patients on liraglutide – a GLP-1 RA – were less likely (Hazard Ratio=0.87, 95% CI (0.78, 0.97)) to experience cardiovascular events (13%) than patients receiving a placebo and standard of care (14.9%).10
However, it is well known that efficacy of medications observed in clinical trials often exceeds their real-world effectiveness due in part to patients’ non-adherence and discontinuation of therapy.11 Patients who meet the strict eligibility criteria in clinical trials are not typically representative of actual patient populations.12–14 Clinical trial participants are more likely to be healthier with fewer comorbidities, more motivated, and have resources and support to comply with treatment regimens under study.12–14 Sub-optimal adherence to diabetes medications increases medical care costs, hospitalizations,15 emergency room visits, comorbidities, and mortality.16 The primary adverse effects reported from GLP-1 RA trials and observational studies are gastrointestinal disturbances (nausea, vomiting, and diarrhea).17,18 Although some of these effects are reported to subside after initial use, several studies have attributed their occurrence to lower persistence of therapy.17–19 Further, data on adherence and discontinuation of GLP-1 RAs in the real world are limited. Studies that have previously examined adherence and discontinuation to GLP-1 RAs have primarily focused on within class comparative analyses of GLP-1 RAs, non-US populations, or analyses with limited timeframes of 6 to 12 months.13 Clinical benefits of these medications have been reported following longer-term use; for example, the cardiovascular benefit of liraglutide was observed following a median exposure time of 3.8 years.10 Therefore, assessing adherence and persistence of these agents in a US T2DM population over a longer period of time is a critical component in understanding their clinical effectiveness in the real world.
To better understand real-world utilization of injectable GLP-1 RAs, this study assessed usage patterns among T2DM patients newly prescribed GLP-1 RAs over 24 months of follow-up. The analysis estimated adherence to and discontinuation of GLP-1 RAs and differences in the distributions for gender, age, and dosing frequency.
Methods
Study Design
A retrospective study was conducted among adult T2DM patients using data from the Clinformatics® Data Mart 7.1 (Optum) database.20 This administrative claims-based database includes over 65 million US patients enrolled in a single, large, closed health plan. Institutional Review Board approval was not required since this is a claims-level study using anonymized data. No primary data was collected; only a secondary data analysis was conducted. The datasets analyzed during the current study are not publicly available because they were accessed under a standard license agreement with Optum. The study period spans from January 1, 2009 until December 31, 2017. The index date was defined as the first claim for a prescription for GLP-1 RA monotherapy or dual therapy with metformin following an observed 12-month baseline period. Although an oral GLP-1 RA – oral semaglutide – was recently approved, only injectable GLP-1 RA therapies were available during the study period. Figure 1 depicts an overview of the study design.
Patient Selection
Patients were included in the study if they met the following inclusion criteria: i) prescription claim for injectable GLP-1 RA between period Jan 01, 2010 to Dec 31, 2016 ii) ≥18 years of age at index date iii) diagnosed with T2DM (based on ICD-9 codes 250.x0 or 250.x2 and ICD-10 code E11) in the 12 months prior to the index date (ie, baseline period) iv) continuous enrollment in the health care plan for the 12 months prior to and after the index date v) did not have prescription for any AHAs other than metformin within the 90 days prior and 30 days following the index date vi) was not diagnosed with other forms of diabetes including type 1 diabetes (ICD-9250.x1 or 250.x3 or ICD-10 E10), gestational diabetes (ICD-9648.8, ICD-10 O24) or other secondary diabetes illness (ICD-9249.x, ICD-10 E08 or E13) vii) was not pregnant in the 12 months prior to index date.
Adherence
Adherence was measured only among patients who had two or more GLP-1 RA prescription claims. To assess non-adherence, the study used the proportion of days covered (PDC) formula.21 The PDC was defined as the number of days covered by a GLP-1 RA prescription divided by the number of days during the measurement period. The measurement period was defined as the time from the index date until the end of 12 months or 24 months, discontinuation of GLP-1 RA medication, or exit from database, whichever came first. Patients were classified as adherent if PDC was ≥0.80 at these time points.
Discontinuation
Discontinuation of therapy was defined as having at least a 90-day gap between the last date of a supply of a GLP-1 RA and the first date of subsequent prescription claims (if any). Switching drug brands of GLP-1 RA did not constitute discontinuation unless >90 days elapsed between prescription claims. Patients who exited the database prior to the end of 24 months were censored and did not count as a GLP-1 RA discontinuation unless >90 days elapsed between prescription claims. A sensitivity analysis defining discontinuation as a treatment gap >120 days was also conducted.
Patient Characteristics
In addition to age and gender, data on relevant comorbidities, laboratory results and GLP-1 RA prescribed dosing frequencies (weekly, daily) were extracted. Comorbidities were identified from diagnostic codes, procedure codes, and other disease-specific algorithms. Comorbidities include microvascular and macrovascular complications, kidney, liver, and pancreatic diseases, hyperlipidemia, and others listed in Table 1. The Optum database did not have sufficient information to accurately estimate the prevalence of obesity; diagnostic codes were sparse in claims data. For a subset of patient records, clinical laboratory test results were available; the most recent test results preceding the index date were extracted and reported.
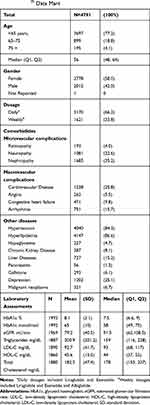 |
Table 1 Baseline Characteristics Among Patients with Type 2 Diabetes Initiating GLP-1 Receptor Agonist, in the Optum Clinformatics® Data Mart |
Statistical Analysis
Frequency distributions were generated for categorical data and means, standard deviations, 95% confidence intervals, medians, and quartiles were generated for continuous variables.
Cross-tabulations were generated for adherence and discontinuation by age, gender, and prescribed dosing frequency (weekly vs daily). Survival analysis was used to produce a Kaplan–Meier curve of time until discontinuation over 24 months of follow-up. Chi-Square tests were used to detect differences in adherence and discontinuation between genders, age and dosing frequency (weekly or daily).
Results
A total of 4791 T2DM patients met all inclusion criteria and initiated GLP-1 RA treatment during the study period. Among these patients, 3709 (81.5%) had at least two prescription claims to assess adherence. The majority of patients were <65 years of age (77.2%) and the median age was 56 (Table 1). More than half of the cohort was female (58%). Two thirds (66%) of patients received prescriptions for daily-dose GLP-1 RAs.
The prevalence of several comorbidities in this sample were disproportionately higher than the general population, but consistent with other patient populations with diabetes from real-world data6,15 and survey studies.22 Nephropathy (35.2%) was the most frequent microvascular complication, followed by neuropathy (22.6%) and retinopathy (4.0%). Of the macrovascular complications, the prevalence of cardiovascular disease (25.8%) was highest and 15.7% of patients had arrhythmia. Most patients had hypertension (84.3%) or hyperlipidemia (86.6%). The proportion of patients with diagnostic codes for liver diseases (15.2%) was higher than chronic kidney disease (8.1%). Diagnosis codes for depression were present in 25.1% of patient records.
As listed in Table 1, laboratory blood test results were reported for a subset of patients. The mean and median values for hemoglobin A1c were 8.1% and 7.5%, respectively. The median (159 mg/dL, IQR [116, 228]) and mean (200 mg/dL, standard deviation [201.2]) triglyceride levels were above normal. HDL-C was borderline low (mean=45.6 mg/dL standard deviation [13.0]; median = 44mg/dL, IQR [37,52]). The mean LDL-C of 92.7 mg/dL (SD=41.7, median=93 mg/dL, IQR [68,117]), was within guidelines for healthy adults, but markedly above current recommendations for patients at high risk of cardiovascular events (<70 mg/dL).23
Adherence
For patients who had two or more GLP-1 RA prescription claims, the mean PDC was 74.7% and 71.8% at 12 and 24 months, respectively. One year following initiation of GLP-1 RA therapy, the prevalence of adherence (PDC ≥80%) was 50.9%. This decreased slightly to 47.4% cumulatively over the two-year study period (Figure 2). Adherence varied by gender, age, and prescribed dosing frequency, and generally worsened over time. At 12 months, significantly fewer females (48.4%) than males (54.2%) (p<0.0001) were adherent and after 24 months, adherence prevalence decreased to 44.6% among females and 51.1% among males (p<0.001). Significantly fewer patients younger than age 65 (49.7%) were classified as adherent at 12 months compared to patients 65–74 years of age (54.7%) or at least 75 years of age (56.4%) (p=0.019). Cumulative adherence prevalence at 24 months decreased to 45.8% among patients <65 years; 52.2% among patients ages 65–74 years (p<0.01); it remained at 56.4% for patients ≥75 years (p=0.01).
Dosing frequency exhibited the largest difference in adherence between groups (Figure 2). After 12 months of follow-up, 43.8% of patients prescribed daily dosing were adherent in contrast to 64.2% of patients prescribed weekly injections (p<0.0001). The cumulative prevalence of adherence at 24 months decreased to 40.8% for daily doses and to 59.8% for weekly doses (p<0.01).
Discontinuation
Figure 3 displays the Kaplan-Meier plot of days until GLP-1 RA therapy discontinuation. Patients discontinued at median of 406 days (95% CI 386–424) after initiating therapy.
Overall, 47.7% of the cohort discontinued GLP-1 RA therapy by 12 months of follow-up (Figure 4). The proportions of patients were comparable with respect to gender (~47%, p=0.48) and prescribed dosing frequency (~48%, p=0.73). A higher proportion of patients ≥75 years (57.4%), however, discontinued therapy at 12 months than patients ages 65–74 years (49.1%) and younger than 65 years (46.8%) (p=0.01).
After 24 months of follow-up, 70.1% of patients overall discontinued therapy (Figure 4). Most patients younger than 65 years of age (68.2%) discontinued GLP-1 RA therapy at 24 months, while 75.3% and 82.6% of those 65–74 years and ≥75 years of age, respectively, discontinued therapy (p=0.01). Approximately 70% of both males and females discontinued GLP-1 RAs at two years. Significantly more patients on weekly dosing discontinued (73.2%) therapy than daily dosing (68.5%) (p<0.01). In sensitivity analysis, (>120-day gap between prescription claims), the overall proportion of patients who discontinued therapy was 44.6% at 12 months and 67.4% at 24 months.
Discussion
This study investigated GLP-1 RA therapy adherence and discontinuation among patients with T2DM using real-world data from a large administrative claims database of prescription claims in a single closed health insurance plan. Approximately half of patients overall were non-adherent. Adherence patterns differed significantly with respect to gender and age, but the difference in adherence by dosing frequency (daily vs weekly) was the most pronounced; adherence was lower among with patients prescribed daily doses. In contrast, discontinuation by dosing frequency was comparable for weekly and daily doses at 12 months and more frequent among weekly users at 24 months. The majority (70.1%) of patients discontinued GLP-1 RA therapy within 24 months of initiating therapy.
These findings for adherence are consistent with previous reports regarding antihyperglycemic agents (AHAs). A meta-analysis of 13 studies covering all classes of oral AHAs estimated the pooled mean medication possession ratio (MPR) to be 75.3% (95% CI [68.8–81.7%]) over 6–24 months of follow-up.24 This suggests that non-adherence is not unique to GLP-1 RAs. For GLP-1 RAs specifically, it has been noted that adherence varies significantly across studies.13 That said, several studies using administrative and electronic medical record datasets from a number of European countries and the US have observed a similar trend to this study with increased GLP-1 RA dosing frequency increasing non-adherence.13,25–28
However, the findings for discontinuation diverged substantially from past studies. As noted previously, past real-world studies have generally characterized persistence over a shorter time frame (6 or 12 months) or in specific GLP-1 RA agents. Studies that assessed discontinuation rates among specific agents are challenging to compare to this study as this study did not consider switching to another agent within the GLP-1 RA class to be discontinuation. However, a similar study that examined GLP-1 RAs as an overall class (not by specific agent) reported a 12-month GLP-1 RA discontinuation rate of 29.5% and 36.4% in the UK and Germany, respectively.28 Comparatively, the current study found that 47.7% of patients discontinued GLP-1 RAs within 12 months.
Discontinuation rates observed in this study deviate even further from those reported in clinical trials. For example, the median time of exposure to liraglutide in the LEADER trial was 3.8 years.10 In the SUSTAIN6 trial, 22.6% of patients on semaglutide prematurely discontinued therapy during the 24-month trial period.29 Yet, in this real-world study, 70.1% of patients who initiated GLP-1 RA therapy discontinued therapy within 24 months. The sensitivity analysis expanding the allowable gap in days of supply between two GLP-1 RA prescription claims to 120 days before classifying a patient as discontinued negligibly altered the percent of patients considered discontinued (67.4% at 24 months). This result further supports that the majority of patients, in fact, discontinue GLP-1 RA therapy within 24 months.
In addition, this study deviates from several analyses of real-world data from Europe and the US with respect to discontinuation and dosing frequency. Previous studies have observed an increase in discontinuation for patients on more frequent dosing regimens of GLP-1 RAs. For example, a retrospective study of GLP-1 RAs from Italy compared five GLP-1 RA treatment regimens. Rates of persistence were highest for weekly dosing at six months as compared to one or two daily doses.30 Similarly, another retrospective study of a German and UK population observed that an increased dosing frequency of GLP-1 RAs was associated with greater discontinuation at 12 months.28 Further, a retrospective study in a US-based population found that patients on twice daily exenatide were more likely to discontinue therapy at 12 months than those on once-daily liraglutide.26 In contrast, the proportions of patients in the current study who discontinued at 12 months post-initiation of GLP-1 RA therapy were comparable for weekly and daily doses and, at 24 months, significantly more patients on weekly dosing compared to daily discontinued therapy. As a retrospective database study, it is difficult to ascertain why this study found that patients were more likely discontinue weekly therapy at 24 months as this seems inconsistent with previous findings. However, given no other study examined this phenomenon at 24 months, it is possible this may be reflecting longer-term results of cost, side effects, or patients/provider preferences with regards to weekly GLP-1 RA agents.
In T2DM patients at high risk for cardiovascular events in the LEADER clinical trial, those who received the GLP-1 RA liraglutide experienced fewer events in the primary composite outcome (the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) than the placebo group, with a median follow-up of 42 months.10 Our findings suggest that patients on GLP-1 RAs often discontinue therapy in the real world and this could impact the effectiveness of the treatment. Limited studies have evaluated real-world effectiveness of GLP-1 RAs to date and those that have yielded inconsistent results, possibly related to inconsistent approaches to accounting for adherence and discontinuation.12,31–35 Future studies should consider evaluating the impact of adherence and persistence on glycemic effectiveness to better understand potential gaps in translating randomized trials efficacy into real-world effectiveness.
This study has several limitations. First, the use of prescription claims data to assess adherence lacks sensitivity because it tracks prescription claims. Determining if patients actually took their medication or capturing adjustments to dosing outside of the prescription (for example, physicians gradually up-titrating patients’ doses) was not possible. Acknowledging this as a limitation of observational research, different formulae can be applied to administrative claims data as proxy measures for adherence. This study used the Proportion of Days Covered (PDC) because it is more sensitive to capturing uncovered days compared to the Medication Possession Ratio.21 Another limitation to this study is that it is descriptive and did not consider potential confounding factors as the database lacked information to fully characterize patients. Specifically, determining comorbidities was restricted to the presence of diagnostic codes and thresholds of some laboratory tests, including HbA1c, were available for only half of the cohort. Baseline obesity was not available. Future studies may wish to investigate additional factors and their impact on GLP-1 RA adherence. Finally, information that may explain the underlying reasons for these findings was unavailable. For example, it is possible that the gastro-intestinal side effects experienced when initiating therapy reported in clinical trials explains the low adherence and high discontinuation rates seen in this study, particularly in year 1.19,36 This is supported by the finding that, from the total cohort of 4709 patients in this study, 18.5% did not have a second prescription claim in their records. Generally, though, it is difficult to ascertain the reasons patients discontinue therapy in a claims database study as information on out of pocket expenses associated with these medications, side effects, or patient/provider preferences cannot be obtained. Future studies may wish to investigate the impact of additional factors, such as HbA1c lowering, weight loss, side effects, and route of administration on GLP-1 RA adherence and discontinuation. Finally, this patient population is not generalizable to other patient populations in the US because all patients were in the same closed health care plan. This study also limited the analysis to those on GLP-1 RA monotherapy and dual therapy with metformin in order to minimize the influence of multiple AHAs on adherence and discontinuation; as a result, it may not be generalizable to all patients on GLP-1 RA therapy. Despite the shortcomings of claims data, using this real-world data nevertheless provided insights about patterns of patient use and clinical practice.
In conclusion, this study of 4791 T2DM patients on injectable GLP-1 RAs found that approximately half of patients were non-adherent, half also discontinued therapy by the end of 12 months and the majority (70%) discontinued by the end of the 24-month follow-up period. The real-world clinical benefit of adherence to these agents merits further investigation.
Acknowledgments
This study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. The authors thank Irene Doherty, PhD, of Complete HEOR Solutions, for manuscript writing assistance. The abstract of this paper was presented at the American Diabetes Association Scientific Session meeting in 2019 as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Diabetes 2019 Jun; 68 (Supplement 1). https://doi.org/10.2337/db19-984-P.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Authors Weiss, Yang, Boggs, Rajpathak and Iglay are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Carr is an employee of MSD UK, London, UK and holds an honorary position at University College, London, UK. Authors Sawhney and Pal were external contractors whose analysis services were paid for by Merck & Co., Inc. The authors report no other conflicts of interest in this work.
References
1. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314:1021. doi:10.1001/jama.2015.10029
2. Cheng YJ, Kanaya AM, Araneta MRG, et al. Prevalence of diabetes by race and ethnicity in the United States, 2011–2016. JAMA. 2019;322:2389. doi:10.1001/jama.2019.19365.
3. Benoit SR, Hora I, Albright AL, Gregg EW. New directions in incidence and prevalence of diagnosed diabetes in the USA. BMJ Open Diabetes Res Care. 2019;7:e000657. doi:10.1136/bmjdrc-2019-000657
4. Beckles GL, Chou C-F. Disparities in the prevalence of diagnosed diabetes — United States, 1999–2002 and 2011–2014. MMWR Morb Mortal Wkly Rep. 2016;65:1265–1269. doi:10.15585/mmwr.mm6545a4
5. National diabetes statistics report | data & statistics | diabetes | CDC; 2019. Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html.
6. Iglay K, Hannachi H, Howie PJ, et al. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32:1243–1252. doi:10.1185/03007995.2016.1168291
7. Raghavan S, Vassy JL, Ho Y, et al. Diabetes mellitus–related all‐cause and cardiovascular mortality in a national cohort of adults. J Am Heart Assoc. 2019;8. doi:10.1161/JAHA.118.011295
8. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17:83. doi:10.1186/s12933-018-0728-6
9. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61:2461–2498. doi:10.1007/s00125-018-4729-5
10. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi:10.1056/NEJMoa1603827
11. Nordon C, Karcher H, Groenwold RHH, et al. The “efficacy-effectiveness gap”: historical background and current conceptualization. Value Health. 2016;19:75–81. doi:10.1016/j.jval.2015.09.2938
12. Carls GS, Tuttle E, Tan R-D, et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1 RA and DPP-4 therapies in patients with type 2 diabetes. Diabetes Care. 2017;40:1469–1478. doi:10.2337/dc16-2725
13. Guerci B, Charbonnel B, Gourdy P, et al. Efficacy and adherence of glucagon-like peptide-1 receptor agonist treatment in patients with type 2 diabetes mellitus in real-life settings. Diabetes Metab. 2019;45:528–535. doi:10.1016/j.diabet.2019.01.006
14. Pratley RE. The efficacy and effectiveness of drugs for diabetes: how do clinical trials and the real world compare? Diabetologia. 2014;57:1273–1275. doi:10.1007/s00125-014-3263-3
15. Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166:1836–1841. doi:10.1001/archinte.166.17.1836
16. Kennedy-Martin T, Boye KS, Peng X. Cost of medication adherence and persistence in type 2 diabetes mellitus: a literature review. Patient Prefer Adherence. 2017;11:1103–1117. doi:10.2147/PPA.S136639
17. Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34:S279–84. doi:10.2337/dc11-s231
18. McGovern A, Tippu Z, Hinton W, Munro N, Whyte M, de Lusignan S. Comparison of medication adherence and persistence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2018;20:1040–1043. doi:10.1111/dom.13160
19. Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context. 2015;4:1–19. doi:10.7573/dic.212283
20. Clinformatics® Data Mart User Manual. 7.1. Optum; 2017.
21. Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–574. doi:10.1002/pds.1230
22. Kalyani RR, Saudek CD, Brancati FL, Selvin E. Association of diabetes, comorbidities, and A1C with functional disability in older adults: results from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Diabetes Care. 2010;33:1055–1060. doi:10.2337/dc09-1597
23. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285–350. doi:10.1016/j.jacc.2018.11.003
24. Iglay K, Cartier SE, Rosen VM, et al. Meta-analysis of studies examining medication adherence, persistence, and discontinuation of oral antihyperglycemic agents in type 2 diabetes. Curr Med Res Opin. 2015;31:1283–1296. doi:10.1185/03007995.2015.1053048
25. Qiao Q, Ouwens MJ, Grandy S, Johnsson K, Kostev K. Adherence to GLP-1 receptor agonist therapy administered by once-daily or once-weekly injection in patients with type 2 diabetes in Germany. Diabetes Metab Syndr Obes Targets Ther. 2016;9:201–205. doi:10.2147/DMSO.S99732
26. Malmenäs M, Bouchard JR, Langer J. Retrospective real-world adherence in patients with type 2 diabetes initiating once-daily liraglutide 1.8 mg or twice-daily exenatide 10 μg. Clin Ther. 2013;35:795–807. doi:10.1016/j.clinthera.2013.03.021
27. Divino V, Boye KS, Lebrec J, DeKoven M, Norrbacka K. GLP-1 RA treatment and dosing patterns among type 2 diabetes patients in six countries: a retrospective analysis of pharmacy claims data. Diabetes Ther. 2019;10:1067–1088. doi:10.1007/s13300-019-0615-5
28. Wilke T, Mueller S, Groth A, et al. Non-persistence and non-adherence of patients with type 2 diabetes mellitus in therapy with GLP-1 receptor agonists: a retrospective analysis. Diabetes Ther. 2016;7:105–124. doi:10.1007/s13300-015-0149-4
29. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi:10.1056/NEJMoa1607141
30. Federici MO, McQuillan J, Biricolti G, et al. Utilization patterns of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus in Italy: a retrospective cohort study. Diabetes Ther. 2018;9:789–801. doi:10.1007/s13300-018-0396-2
31. Evans M, McEwan P, O’Shea R, George L. A retrospective, case-note survey of type 2 diabetes patients prescribed incretin-based therapies in clinical practice. Diabetes Ther. 2013;4:27–40. doi:10.1007/s13300-012-0015-6
32. Nyeland ME, Ploug UJ, Richards A, et al. Evaluation of the effectiveness of liraglutide and sitagliptin in type 2 diabetes: a retrospective study in UK primary care. Int J Clin Pract. 2015;69:281–291. doi:10.1111/ijcp.12575
33. Dhesi B, Chauhan H, Basu A. Audit of clinical practice in the use of incretin mimetic agents for the management of patients with type 2 diabetes. Pract Diabetes. 2013;30:159–162. doi:10.1002/pdi.1767
34. Coleman CI, Pandya S, Wang L, et al. Treatment patterns, glycemic control and bodyweight with canagliflozin 300 mg versus GLP1RAs in Type II diabetes patients. J Comp Eff Res. 2019;8:889–905. doi:10.2217/cer-2019-0002
35. Thomsen RW, Baggesen LM, Søgaard M, et al. Early glycaemic control in metformin users receiving their first add-on therapy: a population-based study of 4734 people with type 2 diabetes. Diabetologia. 2015;58:2247–2253. doi:10.1007/s00125-015-3698-1
36. Iepsen EW, Lundgren J, Dirksen C, et al. Treatment with a GLP-1 receptor agonist diminishes the decrease in free plasma leptin during maintenance of weight loss. Int J Obes. 2015;39:834–841. doi:10.1038/ijo.2014.177
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.