Back to Journals » OncoTargets and Therapy » Volume 13
Reactive Oxygen Species Modulator 1 is Associated with Poor Survival in Patients with Non-Small Cell Lung Cancer After Stereotactic Fractionated Radiosurgery: A Retrospective Pilot Study
Authors Kong M , Sung JY, Lee SH
Received 5 June 2020
Accepted for publication 30 July 2020
Published 18 August 2020 Volume 2020:13 Pages 8173—8180
DOI https://doi.org/10.2147/OTT.S266344
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Moonkyoo Kong,1 Ji-Youn Sung,2 Seung Hyeun Lee3
1Division of Lung & Head and Neck Oncology, Department of Radiation Oncology, Kyung Hee University Medical Center, Kyung Hee University College of Medicine, Seoul, Republic of Korea; 2Department of Pathology, Kyung Hee University Medical Center, Kyung Hee University College of Medicine, Seoul, Republic of Korea; 3Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kyung Hee University Medical Center, Kyung Hee University College of Medicine, Seoul, Republic of Korea
Correspondence: Seung Hyeun Lee
Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kyung Hee University Medical Center, Kyung Hee University College of Medicine, 23 Kyungheedae-Gil, Dongdaemoon-Gu, Seoul 02447, Republic of Korea
Tel +82-2-958-8511
Fax +82-2-958-2848
Email [email protected]
Purpose: It has been reported that the overexpression of reactive oxygen species modulator 1 (Romo1) is significantly associated with poor survival outcomes in patients with lung cancer who received surgical resection, conventional fractionated radiotherapy, and chemotherapy. In this study, we investigated whether Romo1 expression is associated with survival outcomes in patients with early-stage lung cancer who were treated with radiosurgery.
Methods: Romo1 protein expression was evaluated and scored in the tumor tissue specimens of 40 patients with non-small cell lung cancer by immunohistochemistry. An optimal cut-off for Romo1 expression was determined and used to allocate patients to low or high Romo1 expression groups. Survival outcomes were compared between the two groups.
Results: Romo1 expression was significantly associated with distant metastasis-free survival. The 1- and 2-year distant metastasis-free survival rates were 96.4% and 92.6% in the low Romo1 expression group and 87.5% and 46.7% in the high Romo1 expression group (P=0.041), respectively. The overall, local recurrence-free, regional recurrence-free, and disease progression-free survival rates were higher in the low Romo1 expression group than the high Romo1 expression group. However, the differences were not statistically significant.
Conclusion: Romo1 overexpression is associated with poor distant metastasis-free survival in patients with non-small cell lung cancer treated with radiosurgery. Further, large-scale prospective studies are required to identify the clinical efficacy of Romo1 as a potential adverse prognostic factor in lung cancer.
Keywords: reactive oxygen species modulator 1, lung cancer, radiosurgery
Introduction
Lung cancer is the leading cause of cancer-related death in Korea as well as many other countries.1–4 Radiation treatment is the primary treatment modality in the management of lung cancer. Definitive radiotherapy with concurrent chemotherapy is the standard treatment for patients with locally advanced non-small cell lung cancer. For patients with early-stage non-small cell lung cancer, stereotactic radiosurgery has been used for patients who are not candidates for or refuse surgical resection. Since a pooled analysis of two randomized trials reported superior overall survival and comparable loco-regional and distant metastasis-free survival of radiosurgery compared with lobectomy in patients with early-stage lung cancer, the clinical use of radiosurgery for patients with resectable lung cancer has increased.5 Therefore, it is important to identify prognostic biomarkers that predict the oncologic outcomes of patients with lung cancer who undergo radiation treatment.
Reactive oxygen species modulator 1 (Romo1) was first discovered in head and neck cancer tissue in 2006. Romo1 is a membrane protein that is located in the mitochondrial outer membrane, where it controls mitochondrial reactive oxygen species (ROS) production.6 Romo1-induced ROS production is essential for the proliferation of cancer and normal cells.7 However, an imbalance between pro-oxidants and anti-oxidants can result in oxidative stress, and oxidative stress is linked with DNA damage, carcinogenesis, and cancer progression.8,9 In addition, it has been reported that Romo1-induced ROS production is associated with chemotherapy (5-fluorouracil) resistance and γ-radiation sensitivity in lung cancer cell lines.10,11
Based on these preclinical studies, clinical studies have been conducted to evaluate the clinical usefulness of Romo1 protein and it was reported that Romo1 overexpression is associated with poor survival in patients with hepatocellular carcinoma and colorectal cancer.12,13 In addition, Romo1 overexpression was significantly associated with poor survival outcomes in patients with non-small cell lung cancer who received surgical resection,14 chemotherapy,15 and definitive radiotherapy,16 in our previous studies. However, the clinical usefulness of Romo1 expression as a prognostic marker in patients with early-stage lung cancer who received radiosurgery has not yet been investigated.
In this study, we investigated the association between Romo1 expression and survival outcomes in patients with early-stage non-small cell lung cancer who were treated with fractionated stereotactic radiosurgery.
Materials and Methods
Patient Selection
The inclusion criteria for this pilot study were histologically confirmed non-small cell lung cancer, underwent radical stereotactic radiosurgery, Eastern Cooperative Oncology Group (ECOG) performance status ≤3, available cancer tissue specimens, no previous or concurrent illness that would compromise the completion of treatment, and available follow-up data. A total of 97 patients received radiosurgery for the treatment of lung cancer at our institution between January 2014 and December 2018. Of the initial 97 patients, 48 patients did not meet the inclusion criteria. Twenty-eight patients had metastatic lung cancer, 11 patients did not have available cancer tissue specimens, and 9 patients had small cell lung cancer. Among the remaining 49 patients, nine patients were excluded from this study because the tissue specimens were not suitable for immunohistochemical staining. In total, 40 patients were finally included in this study. The hospital records, imaging results, and laboratory results of all study participants were retrospectively reviewed. The Institutional Review Board of Kyung Hee University Medical Center approved this study and waived the need for written informed consent. All research was carried out in compliance with the Helsinki Declaration. All patient data accessed complied with relevant patient data protection and privacy regulations.
Clinical Evaluation
The initial diagnosis was pathologically confirmed in all patients based on either a percutaneous needle or endoscopic bronchial biopsy. The pretreatment evaluation consisted of a complete history and physical examination, chest radiography, pulmonary function test, basic laboratory studies, trans-thoracic echocardiogram, electrocardiogram, chest computed tomography (CT) scan, positron emission tomography (PET), and brain magnetic resonance imaging. The cancer stage was determined according to the 8th edition of the American Joint Committee on Cancer staging system.
Quantification of Romo1 Expression
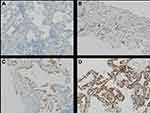
Formalin-fixed paraffin-embedded tumor tissue specimens were used for evaluation of Romo1 protein expression, and Romo1 expression was measured by immunohistochemical staining. The immunohistochemical staining methods used for evaluating Romo1 protein expression were described in detail in our previous study.16 A pathologist (JY Sung) who was blinded to the clinical data examined all sections and detailed methods were also described in our previous study.16 Representative examples of immunohistochemical staining for Romo1 in tumor tissue section were shown in Figure 1. The final H scores ranged from 0 to 300.
Radiosurgery
All patients underwent a four-dimensional CT simulation to track the movement of the targets along the respiratory cycle. The gross tumor volume (GTV) encompassed the primary tumors visualized on chest CT and PET. The GTV was created using maximal intensity projection images. The clinical target volume (CTV) included the GTV plus a 3–5 mm margin, and the planning target volume was created by adding a 5 mm margin to the CTV. All patients received fractionated stereotactic radiosurgery. The prescription dose was determined by a radiation oncologist (M Kong) based on the size of the target lesion, the patient’s general condition, and the probability of treatment-related toxicity. A total of 44–60 Gy was delivered in 2–4 fractions with the daily course. Radiation oncologists carried out the whole process of radiosurgery. If there was a movement of the patient’s trunk during radiosurgery, irradiation was temporarily suspended. After the correction of the patient’s position and checking of the on-board CT images, radiosurgery was started again. Radiosurgery was performed with a TomoTherapy (Accuray Inc., Madison, WI, USA) or Clinac iX (Varian Medical System Inc., Palo Alto, CA, USA).
Outcome Evaluation and Statistical Analyses
Follow-up visits were scheduled 1 month after the completion of radiosurgery and every 2–3 months after that. Visits were more frequent for those who experienced treatment-related toxicities. During the follow-up visits, basic laboratory studies, chest radiographs, and chest CT scans were conducted. PET was also performed as needed. The primary endpoint of this study was overall survival. The secondary endpoints were local recurrence-free survival, regional recurrence-free survival, distant metastasis-free survival, and disease progression-free survival. The definitions of local, regional, and distant metastasis were described in our previous study.16 Radiation pneumonitis was diagnosed based on characteristic clinical symptoms and imaging findings within the radiosurgery field and graded according to the Radiation Therapy Oncology Group toxicity criteria. Other radiosurgery-related toxicities were evaluated using the Common Toxicity Criteria for Adverse Events version 4.0. Baseline characteristics between groups were compared using a Wilcoxon rank-sum test for discrete variables and a Mann–Whitney U-test for continuous variables. Actuarial survival rates between groups were compared using the Kaplan-Meier methods and Log rank tests. Survival times were calculated from the radiosurgery start to the event or final follow-up visit. A multivariate analysis was conducted using Cox’s proportional hazard regression test with a backward method. All tests were two-sided, and a P-value of <0.05 was considered statistically significant. All analyses were performed using SPSS version 21.0 (IBM corporation, Armonk, NY, USA).
Results
Romo1 H scores were normally distributed with a median of 125 (range, 60–300). The optimal cut-off value of the H score to determine low and high Romo1 expression groups was defined as the point with the lowest P-value by the Log rank test for all possible H scores. The cut-off value was determined to be 190. Using this cut-off value, 31 and 9 patients were allocated to the low and high Romo1 expression groups, respectively. Patient and tumor characteristics of both groups are summarized in Table 1. The most common fractionation schedule of radiosurgery was a total of 50 Gy in 4 fractions, with 13 patients (32.5%) treated with this fractionation schedule. Eight patients (20%) were treated with a total of 54 Gy in 3 fractions, five patients (12.5%) were treated with a total of 51 Gy in 3 fractions, 4 patients (10%) were treated with a total of 48 Gy in 3 fractions, and 3 patients (7.5%) were treated with a total of 52 Gy in 4 fractions. Almost all patients had several underlying diseases. The most common underlying diseases were hypertension, chronic obstructive pulmonary disease, and ischemic heart disease. Two patients in the low Romo1 expression group and one patient in the high Romo1 expression group did not have any underlying disease. The median follow-up duration of all patients was 20 months (range, 3.5–48). There was no significant difference in the patient and tumor characteristics between the low and high Romo1 expression groups.
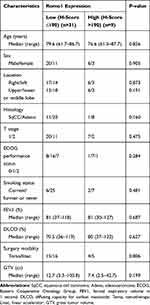 |
Table 1 Patient and Tumor Characteristics |
The survival outcomes of all patients are summarized in Table 2. During the follow-up period, 29 patients (72.5%) remained alive (twenty-two in low and seven patients in high Romo1 expression group). Five and two patients died due to bacterial pneumonia and cancer progression, respectively. The remaining four patients died due to aggravation of chronic obstructive pulmonary disease, subdural hemorrhage, chronic renal failure, and coronary heart disease, respectively. During the follow-up period, a total of nine patients (22.5%) experienced recurrences (six in low and three patients in high Romo1 expression group); two, one, and four patients experienced a local, regional, and distant recurrence, respectively. The remaining two patients experienced local and regional recurrences, and local, regional, and distant metastasis, respectively. Among the 2 patients who experienced local recurrence alone, 1 patient received salvage radiosurgery, and the other refused salvage treatment. The patient who experienced regional recurrence alone received salvage conventional fractionated radiotherapy and chemotherapy. The patient who experienced local and regional recurrences also received salvage conventional fractionated radiotherapy and chemotherapy. The patients who experienced distant metastasis alone received palliative chemotherapy. Furthermore, the patient who experienced local, regional, and distant metastasis also received palliative chemotherapy.
 |
Table 2 Survival Outcomes of the Whole Cohort |
There was no grade ≥4 toxicity. The most common toxicity was radiation pneumonitis. Grade 1 radiation pneumonitis developed in 5 patients (12.5%), grade 2 in 26 patients (65%), and grade 3 in 9 patients (22.5%). Among the 9 patients who developed grade 3 radiation pneumonitis, 3 patients experienced grade 3 dyspnea. In addition, 1 patient experienced a rib fracture. All patients with radiosurgery-related toxicities were successfully treated with conservative management, and some of the patients with grade 3 radiation pneumonitis were treated with steroid agents.
We analyzed the survival outcomes of whole patients according to Romo1 expression, and the results are summarized in Table 3. Although Romo1 expression was not associated with overall, local recurrence-free, regional recurrence-free, and disease progression-free survival, Romo1 expression was significantly associated with distant metastasis-free survival. The 1- and 2-year distant metastasis-free survival rates were 96.4% and 92.6% in the low Romo1 expression group and 87.5% and 46.7% in the high Romo1 expression group (P=0.041) (Figure 2), respectively. To identify whether Romo1 expression is a significant prognostic factor for distant metastasis in patients with lung cancer treated with radiosurgery, a multivariate analysis, including other variables, was conducted (Table 4). Romo1 expression was found to be weakly associated with distant metastasis-free survival in the multivariate analysis (Hazard Ratio, 0.190; 95% Confidence Interval, 0.032–1.141; P=0.059).
 |
Table 3 Analysis of Survival According to Romo1 Expression |
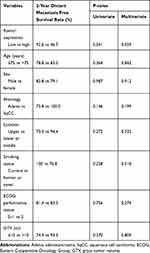 |
Table 4 Analysis of the Prognostic Factors for Distant Metastasis-Free Survival |
Discussion
The current study found that Romo1 overexpression is associated with poor distant metastasis-free survival in patients with non-small cell lung cancer treated with fractionated stereotactic radiosurgery. To the best of our knowledge, this is the first study that has evaluated the relationship between Romo1 expression and survival outcomes in patients with lung cancer treated with radiosurgery.
It has been suggested that Romo1 could be used as a prognostic marker for patients with non-small lung cancer. In 2015, Lee et al retrospectively evaluated Romo1 protein expression and the oncologic outcomes of 110 patients with non-small cell lung cancer who received surgical resection, and found that Romo1 overexpression in tumor tissues was significantly associated with early disease recurrence and poor overall survival.14 In 2017, Lee et al conducted a similar retrospective study in patients with lung cancer who received chemotherapy. The clinical data of 88 patients with non-small cell lung cancer who received chemotherapy were evaluated, and Romo1 overexpression was found to be associated with poor response to treatment as well as poor disease-free and overall survival.15 In 2019, Kong et al reported that the overall, loco-regional recurrence-free and disease progression-free survival rates of the Romo1 overexpression group were significantly lower than the low Romo1 expression group in patients with stage III non-small cell lung cancer who were treated with conventional fractionated radiotherapy.
In this study, we investigated whether Romo1 expression is associated with survival outcomes in patients with lung cancer who were treated with fractionated radiosurgery. The results of this study also showed that Romo1 overexpression was associated with poor survival outcomes in patients with lung cancer treated with radiosurgery. However, Romo1 overexpression was significantly associated only with distant metastasis-free survival, and not with overall or loco-regional recurrence-free survival. At present, we are unsure why Romo1 overexpression was not associated with loco-regional recurrence-free survival in patients with lung cancer treated with radiosurgery. A possible reason is the small sample size of the current study. This is a pilot study that includes only 40 patients. In addition, this study included patients with early-stage lung cancer treated with radiosurgery. According to several previous studies, the loco-regional recurrence rates were 5–15% in patients with early-stage lung cancer who were treated with radiosurgery,5,17–19 and only 5 patients (12.5%) experienced loco-regional recurrences in this study. The small sample size and rare events might have resulted in this study being underpowered to detect existing differences in the statistical analysis. Another possible reason is that Romo1 overexpression might be mainly correlated with distant metastasis rather than loco-regional recurrence. In 2017, Kim et al evaluated Romo1 protein expression and survival outcome of 190 patients with colorectal cancer who received surgical resection followed by adjuvant chemotherapy, and suggested that Romo1 acts as a key regulator of lymphatic metastasis by increasing the invasive activity of cancer cells.13 In that study, although Romo1 overexpression was significantly associated with lymphatic invasion and lymph node ratio, Romo1 expression was not related with primary tumor size and/or T staging. In our current study, there was also no significant correlation between Romo1 expression and T stage, and which was also found in previous study of hepatocellular carcinoma patients.12 To investigate the effects of Romo1 expression on the metastatic ability, Kim et al conducted in vitro study that revealed the association between Romo1 overexpression and high lymphatic metastatic tendency.13 Therefore, we can hypothesize that because Romo1 mainly induces lymphatic invasion of primary tumor and subsequent lymphatic metastasis, Romo1 overexpression was associated only with distant metastasis-free survival in our current study.
Although the exact role of Romo1 in cancer cells should be confirmed in further studies, this is a pilot study and statistical significance is not an essential assessment criterion because the results are expected to form the basis for the power calculation for further large sample size studies. Although the results of this study should be confirmed through further investigations, this and our previous studies have consistently shown that Romo1 overexpression is significantly associated with poor survival outcomes in patients with non-small cell lung cancer regardless of which treatment modality was used.14–16 These studies provide a strong basis for further large-scale prospective studies evaluating the clinical usefulness of Romo1 protein as a prognostic marker in non-small cell lung cancer. Notably, another report showed that Romo1 overexpression is associated with poor overall outcomes in patients with hepatocellular carcinoma12 and colorectal cancer.13 Therefore, it will be necessary to conduct further studies evaluating the effect of Romo1 expression in other cancers.
There are several limitations in this study. First, this study was retrospective and may, therefore, have inherent biases. For example, fractionation schedules of radiosurgery were not decided by a pre-determined clear protocol. These biases may make it challenging to interpret the obtained results. Second, the sample size was small, so minor differences in the statistical analyses might not be detected. However, because we only included patients with early-stage non-small cell lung cancer who received radiosurgery in our study, the patient characteristics were homogeneous. Finally, the follow-up duration was not long. Despite these limitations, we believe that this study still provides valuable information for further large-scale prospective studies.
Conclusion
In conclusion, Romo1 overexpression is associated with poor distant metastasis-free survival in patients with non-small cell lung cancer treated with fractionated radiosurgery. Further large-scale prospective studies are required to identify the clinical usefulness of Romo1 as a potential adverse prognostic factor in lung cancer. If large-scale prospective studies confirm our results, Romo1 can be used as a novel biomarker to classify high-risk patients who may require close follow-up.
Abbreviations
Romo1, reactive oxygen species modulator 1; ROS, reactive oxygen species; ECOG, Eastern Cooperative Oncology Group; CT, computed tomography; PET, positron emission tomography; GTV, gross tumor volume; CTV, clinical target volume; H score, histologic scores; SqCC, squamous cell carcinoma; Adeno, adenocarcinoma; FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity for carbon monoxide; Tomo, tomotherapy; Linac, linear accelerator.
Acknowledgments
Authors would like to thank to Jeenyung Kong for writing assistance and Editage for English language editing.
Disclosure
Prof. Dr. Moonkyoo Kong reports grants from the Ministry of Health & Welfare, Republic of Korea and the Ministry of Science and ICT, Republic of Korea, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Jung KW, Won YJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015;47(2):127–141. doi:10.4143/crt.2015.060
2. Jemal A. Global burden of cancer: opportunities for prevention. Lancet. 2012;380(9856):1797–1799. doi:10.1016/S0140-6736(12)61688-2
3. Kim HC, Jung CY, Cho DG, et al. Clinical characteristics and prognostic factors of lung cancer in korea: a pilot study of data from the korean nationwide lung cancer registry. Tuberc Respir Dis. 2019;82(2):118–125. doi:10.4046/trd.2017.0128
4. Park JY, Jang SH. Epidemiology of lung cancer in Korea: recent trends. Tuberc Respir Dis. 2016;79(2):58–69. doi:10.4046/trd.2016.79.2.58
5. Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630–637. doi:10.1016/S1470-2045(15)70168-3
6. Chung YM, Kim JS, Yoo YD. A novel protein, Romo1, induces ROS production in the mitochondria. Biochem Biophys Res Commun. 2006;347(3):649–655. doi:10.1016/j.bbrc.2006.06.140
7. Na AR, Chung YM, Lee SB, Park SH, Lee MS, Yoo YD. A critical role for Romo1-derived ROS in cell proliferation. Biochem Biophys Res Commun. 2008;369(2):672–678. doi:10.1016/j.bbrc.2008.02.098
8. Sosa V, Moline T, Somoza R, Paciucci R, Kondoh H, LL ME. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013;12(1):376–390. doi:10.1016/j.arr.2012.10.004
9. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi:10.1038/35041687
10. Kim IG, Kim SY, Kim HA, et al. Disturbance of DKK1 level is partly involved in survival of lung cancer cells via regulation of ROMO1 and gamma-radiation sensitivity. Biochem Biophys Res Commun. 2014;443(1):49–55. doi:10.1016/j.bbrc.2013.11.038
11. Hwang IT, Chung YM, Kim JJ, et al. Drug resistance to 5-FU linked to reactive oxygen species modulator 1. Biochem Biophys Res Commun. 2007;359(2):304–310. doi:10.1016/j.bbrc.2007.05.088
12. Chung JS, Park S, Park SH, et al. Overexpression of Romo1 promotes production of reactive oxygen species and invasiveness of hepatic tumor cells. Gastroenterology. 2012;143(4):1084–1094 e1087. doi:10.1053/j.gastro.2012.06.038
13. Kim HJ, Jo MJ, Kim BR, et al. Reactive oxygen species modulator-1 (Romo1) predicts unfavorable prognosis in colorectal cancer patients. PLoS One. 2017;12(5):e0176834. doi:10.1371/journal.pone.0176834
14. Lee SH, Min JW, Lee JS, et al. Reactive oxygen species modulator 1 (Romo1) overexpression is an independent predictor of poor survival in NSCLC patients who undergo surgical resection. Lung Cancer. 2015;87(1):45–52. doi:10.1016/j.lungcan.2014.11.004
15. Lee SH, Choi SI, Lee JS, et al. Reactive oxygen species modulator 1 (romo1) predicts poor outcomes in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy. Cancer Res Treat. 2017;49(1):141–149. doi:10.4143/crt.2016.133
16. Kong M, Sung JY, Lee SH. Reactive oxygen species modulator 1 as an adverse prognostic marker in stage III non-small cell lung cancer treated with radiotherapy: a retrospective pilot study. Onco Targets Ther. 2019;12:8263–8273. doi:10.2147/OTT.S217514
17. Giuliani ME, Hope A, Mangona V, et al. Predictors and patterns of regional recurrence following lung SBRT: a report from the elekta lung research group. Clin Lung Cancer. 2017;18(2):162–168. doi:10.1016/j.cllc.2016.10.006
18. Nakamura M, Nishikawa R, Mayahara H, et al. Pattern of recurrence after CyberKnife stereotactic body radiotherapy for peripheral early non-small cell lung cancer. J Thorac Dis. 2019;11(1):214–221. doi:10.21037/jtd.2018.12.115
19. Spratt DE, Wu AJ, Adeseye V, et al. Recurrence patterns and second primary lung cancers after stereotactic body radiation therapy for early-stage non-small-cell lung cancer: implications for surveillance. Clin Lung Cancer. 2016;17(3):177–183 e172. doi:10.1016/j.cllc.2015.09.006
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.