Back to Journals » Journal of Inflammation Research » Volume 15
Reactive Hemophagocytic Lymphohistiocytosis Secondary to Ovarian Adenocarcinoma: A Rare Case Report
Authors Li XY, Zhu SM, Li XY, Dong RS, Zhang AA, Li SJ, Geng YL
Received 7 June 2022
Accepted for publication 9 August 2022
Published 6 September 2022 Volume 2022:15 Pages 5121—5128
DOI https://doi.org/10.2147/JIR.S376756
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Xiao-Yan Li,1,* Shu-Min Zhu,2,* Xin-Yuan Li,2,* Rui-Sheng Dong,3 Ai-Ai Zhang,1 Shu-Jing Li,4 Yu-Lan Geng2
1Department of Laboratory Medicine, Shanxi Province Fenyang Hospital, Fenyang, 032200, People’s Republic of China; 2Department of Laboratory Medicine, The First Hospital of Hebei Medical University, Shijiazhuang, 050031, People’s Republic of China; 3Department of Imaging Medicine, Shanxi Province Fenyang Hospital, Fenyang, 032200, People’s Republic of China; 4Department of Radiology, The First Hospital of Hebei Medical University, Shijiazhuang, 050031, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiao-Yan Li, Department of Laboratory Medicine, Shanxi Province Fenyang Hospital, No. 186 of Shengli Road, Fenyang, Shanxi, 032200, People’s Republic of China, Tel +86 358 7222225, Fax +86 358 7222615, Email [email protected]; Yu-Lan Geng, Department of Laboratory Medicine, The First Hospital of Hebei Medical University, No. 89 of Donggang Road, Shijiazhuang, Hebei, 050031, People’s Republic of China, Tel +86 311 87156567, Fax +86 311 85917029, Email [email protected]
Background: Hemophagocytic lymphohistiocytosis (HLH), a syndrome of immune hyperactivation and abnormal regulation that causes life-threatening inflammation, is mainly characterized by fever, hepatosplenomegaly, cytopenia, and other symptoms. Reactive HLH (rHLH) is typically secondary to immune deregulation caused by underlying rheumatologic, infectious, or malignant conditions. Malignancy-associated HLH (M-HLH) continues to be a critical health problem worldwide. Most malignancies associated with HLH are hematologic tumors, and M-HLH in non-hematologic tumors very rarely occurs.
Case Report: A 34-year-old Chinese woman had a history of persistent fever, acute dizziness, and bicytopenia. She was found to have developed bilateral ovarian cancer. Additional tests showed splenomegaly, hemophagocytes in the bone marrow, low natural killer activity, and hyperferritinemia, which met the diagnostic criteria put forth in the Histiocyte Society HLH-2004. The patient was treated with correcting anemia, increased platelets, and glucocorticoid therapy but showed no response. She progressively deteriorated and died 55 days later.
Conclusion: Hemophagocytic lymphohistiocytosis related to a solid tumor is extremely rare. To the best of the authors’ knowledge, the present case was the first to report rHLH secondary to ovarian adenocarcinoma. It is very significant for a better understanding of the disease mechanisms of HLH and should attract the attention of hematologists and other clinicians as the condition progresses and the cost of treating it increases.
Keywords: ovarian adenocarcinoma, reactive hemophagocytic lymphohistiocytosis, hyperferritinemia, cytopenia, high reticulocyte, natural killer activity
Introduction
Ovarian cancer is the most lethal malignant solid tumor and the leading mortality rate among gynecological malignant tumors,1,2 despite the stable nature of the global incidence of ovarian cancer during the past decades.3 Hemophagocytic lymphohistiocytosis (HLH) is a rare, life-threatening syndrome resulting from an extreme hyperinflammatory condition, subsequent tissue infiltration, and multi-organ failure. Malignancy-associated HLH (M-HLH) is a reactive HLH (rHLH) and also secondary HLH (sHLH), which is mainly associated with hematologic cancers, lymphomas, and multiple myeloma.4–8 To the best of the authors’ knowledge, no published literature has described the association between rHLH and ovarian adenocarcinoma. This paper presents a clinical case to demonstrate this association to raise awareness of the syndrome among hematologists, oncologists, and other clinicians.
Case Report
A 34-year-old Chinese woman presented with a permanent fever over 20 days, was acutely dizzy for one day and had low red blood cell (RBC), hemoglobin (Hgb), and platelet (Plt) counts. She was admitted to the Department of Hematology at Shanxi Province Fenyang Hospital. She claimed intermittent fever with a maximum temperature of 38.0℃, mainly at noon and at night. Thirteen days prior, the patient had been hospitalized for three days due to an infection, and at discharge, her complete blood cell (CBC) count was within the reference ranges. One day prior, she had suddenly felt dizzy and sweaty, which ceased after approximately 5 minutes. The outpatient examination revealed an abnormal CBC count.
At the initial physical examination, the patient had a temperature of 36.7℃, a pulse of 140 beats per minute, a respiration rate of 20 incursions per minute, and a blood pressure of 109/85 mmHg. She had an anemic appearance and positive tenderness on the sternum. Her breathing and heart sounds were essentially normal. Her mind was clear, and no other obvious abnormalities were detected during the rest of the physical examination.
Initial laboratory findings showed noticeably elevated counts related to white blood cells, neutrophils, monocytes, a decreased RBC count, a decreased Hgb concentration, and lower Plt count. Both the count and percentage of reticulocytes were markedly higher than the reference ranges. Biochemistry assay, serum alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, gamma-glutamyl transpeptidase, lactate dehydrogenase (LDH), alpha-hydroxybutyrate dehydrogenase, creatine kinase isoenzyme MB, glucose, C-reactive protein, and procalcitonin levels were noticeably higher. A chemiluminescent immunoassay revealed mildly elevated serum carcinoembryonic antigen, an extremely high carbohydrate antigen 125 and ferritin levels. In addition, the case initially also revealed a prolonged prothrombin time, activated partial thromboplastin time, and elevated D-dimer levels (Table 1). Viral respiratory pathogen panels, an autoimmune diseases workup, human immunodeficiency virus, syphilis, and viral hepatitis panels were also sent, all of which came back negative.
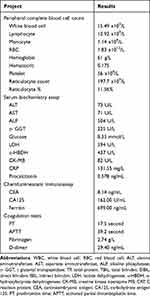 |
Table 1 Laboratory Finding on Admission |
An abdominal ultrasound showed an increased liver parenchyma echo, splenomegaly, a small amount of effusion in the right iliac fossa, and a bilateral pelvic-cyst solid mass (Figure 1). Computed tomography scanning showed an effusion of bilateral thoracic cavities (Figure 2). Furthermore, magnetic resonance imaging showed abnormal signals of the bilateral appendages, abnormal lymph node shadows of the lesser curvature of the stomach in the abdominal cavity, multiple small nodule shadows in the greater omentum, lesser omentum, and mesentery, abnormal signal shadows in the pelvis, sacrum, thoracic vertebrae, and lumbar vertebrae, and effusion in the abdominal and pelvic cavities (Figure 3).
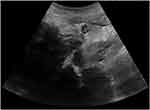 |
Figure 1 Left pelvic cyst-solid mass with ultrasound (size 16.3×9.7x7.6 cm). Criss-cross: left pelvic cyst-solid mass. |
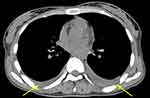 |
Figure 2 CT showed effusion of bilateral thoracic cavities (yellow arrow). |
Based on the above observations, this case was diagnosed as ovarian cancer with a high suspicion of HLH. To start a specific treatment, it was necessary to affirm the occurrence of HLH, for which external laboratory tests were required. The patient’s serum triglyceride level was 1.46 mmol/L, which was within the reference range. Epstein–Barr virus deoxyribonucleic acid (DNA) and cytomegalovirus DNA were negative. Peripheral lymphocyte subsets revealed that percentages of CD4+T (helper T, Th), CD8+T (cytotoxicity T, Tc) and B cells were slightly higher, natural killer (NK) percentages and counts were noticeably decreased, and the remaining lymphocyte subsets and CD4/CD8 were within the reference ranges (Table 2). Bone marrow cytomorphology revealed clustered abnormal cells and hemophagocytes, mainly RBC and hemosiderin in the cytoplasm (Figure 4). The histopathological findings of ultrasound-guided puncture biopsy specimens on Hematoxylin and Eosin staining showed ovarian adenocarcinoma. Over the following five days, her ferritin level increased, and her RBC and platelet counts and fibrinogen levels decreased. Her body temperature remained between 36.5℃ and 36.9℃.
 |
Table 2 Lymphocyte Subsets Results |
 |
Figure 4 The bone marrow showed clustered abnormal cells (A) and haemophagocytes (B) (Wright-Giemsa staining, original magnification x 1000). |
The case met 6 of the 8 HLH-2004 diagnostic criteria (fever, splenomegaly, bicytopenia, hemophagocytes in bone marrow, low NK cell activity, and hyperferritinemia)9 and the presentation was compatible with rHLH, despite an HScore10 score of only 131. She was diagnosed with rHLH (sHLH) secondary to ovarian adenocarcinoma and treated to correct anemia and increase platelets; glucocorticoid therapy was also included. Unfortunately, no obvious improvement occurred, and her condition worsened. On day 15 of admission, she was discharged voluntarily. The patient died 55 days later.
All procedures that were performed for the current case were in accordance with the ethical standards of the institutional and national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent for data publication was obtained from the patient’s relative because she had died 55 days following discharge from the hospital.
Discussion
The incidence of HLH in adults is not precisely known. However, a Japanese observational study reported that up to 40% of HLH cases occurred in adults.11,12 Among the precipitating factors for adult HLH, malignancy was related to 44% of cases.13 And 0.9% of adult patients were hematologic cancers, mostly acute myeloid leukemia/myelodysplastic syndrome and T-lymphoma, and some diffuse large B-cell lymphoma, Hodgkin’s lymphoma, chronic lymphocytic leukemia, follicular lymphoma, and splenic marginal B-cell lymphoma.14,15 Among neoplasia cases in intensive care units (ICUs), non-Hodgkin’s lymphoma was predominant (23%).16
The association of rHLH with malignant hematopathy has been well established. Other malignancies that may cause HLH are very rare. A 25-year-long follow-up study revealed that only one case in 1206 multiple myeloma patients occurred HLH. Sakai et al found that a 51-year-old man with hepatocellular carcinoma incurred HLH.17 Similarly, only three cases with prostate cancer have been described as having rHLH in the literature.18–20 One HLH case involving ovarian cancer was reported in 2016 and was associated with ovarian dysgerminoma.21 The authors report in the current study an ovarian adenocarcinoma case that presented an early and unusual complication that was characterized by HLH. To the best of the authors’ knowledge, this is the first time the relationship between rHLH and ovarian adenocarcinoma has been described in the literature. However, the novelty of this case in the association of rHLH and ovarian adenocarcinoma will raise questions for future hematology and oncology contexts.
Existing published literature diagnosed HLH according to biological findings (bicytopenia, cytolysis, elevated LDH, and ferritin levels), as well as using cytological evidence of macrophages in the bone marrow.8,17–20 Fever, splenomegaly, cytopenia, and hyperferritinaemia22 are common features for indicating the presence of cancer or HLH. The present study case was diagnosed with ovarian cancer, based on the available information when the patient first presented to Shanxi Province Fenyang Hospital. Whether she had sHLH (or rHLH) required additional confirmation. The patient met 6 of the 8 HLH-2004 diagnostic criteria for HLH,9 despite having had a low HScore (131).10 Low HScores have also been observed in other cases with rHLH (sHLH).23–25 Making an HLH diagnosis is very difficult, particularly for critically ill adult patients (including those with neoplasm) because clinical findings must exclude a number of differential diagnoses, while laboratory abnormalities lack specificity. In adults, HLH is characterized by severe immune activation and a sepsis-like syndrome, leading to high numbers of undiagnosed cases and mortality rates of up to 68%.16 In the ICU, undiagnosed rates of HLH were as high as 77.8%.26 Early diagnosis and specific immunosuppressive therapy can help to avoid poor outcomes; however, the diagnostic criteria (HLH-2004) are adopted from pediatric HLH. The HScore, also adopted from pediatrics, can be used to estimate an rHLH risk; however, this may cause missing diagnosis for some rHLH cases less than HScore 146.10 Currently, there is a lack of specific diagnostic tests and no established valid diagnostic criteria for detecting HLH in adults.
Adult HLH is very different from the disease in children. Lachmann et al established one protocol and tried to investigate biomarkers for the diagnoses of critical HLH in adults.27 These diagnostic biomarkers for critically ill patients in the ICU included11 laboratory panels, which may be useful for diagnosing critically ill adult patients, such as the current case, in the future.
The pathophysiology of HLH includes the activities of macrophages, NK cells, cytotoxic T cells (Tc) cells, and altered numbers of CD4 and CD8 cells.28 Investigators have found that NK cells from adults with HLH exhibited an activated phenotype and normal cytotoxic capacity. In contrast, NK cell numbers and interferon-γ production are greatly diminished.29 Sustained over-activated Tc cells and macrophages, and the resultant inflammatory cytokine release, are core pathogeneses that usually cause tissue damage, vital clinical features (fever, hepatosplenomegaly, cytopenia, elevated ALT, AST and ferritin levels, and coagulopathy), and even organ failure.30 This may have been the case for the patient in the current study. Tc or NK cells cannot remove activated macrophages, giving rise to enhanced macrophage activity and raising various cytokines levels.31 The present case had normal Tc cells and abnormally low NK cell activity/count. As a subset of whole innate lymphoid cells, NK cells are currently defined as effector cells that are similar to Tc cells and exert natural cytotoxicity against primary tumor cells and metastasis by inhibiting proliferation, migration, and colonization to distant tissues.32 Natural killer cells have dual roles, ie, controlling cancer development and progression and promoting the onset of immune-suppressant tumor microenvironments.33 The number of and low activity of NK cells in the present case may have resulted from ovarian cancer-associated exhaustion and the enhanced macrophage activation associated with HLH.
Reticulocytes are the youngest erythrocytes in the peripheral blood released from the bone marrow, which reflects the erythropoietic activity of bone marrow. The reticulocyte count distinguishes disorders resulting from the rapid destruction or loss of RBCs caused by bone marrow failure, high being considered as hemolysis and low as bone marrow depression.34 Being the key HLH feature, histiocytes or macrophages (hemophagocytes) engulfing RBCs cause hemolysis and severe anemia, while markedly increased reticulocyte count was also found in some studies35,36 just as in the present case, which suggested that reticulocytosis might be a HLH characteristic.
Conclusion
In conclusion, HLH is related to solid tumor types that very rarely occur. To the authors’ knowledge, this case is the first HLH secondary to ovarian adenocarcinoma and the second to ovarian cancer. It is very significant for a better understanding of the disease mechanisms of HLH. Furthermore, in the context of malignancies, including ovarian cancer, HLH must be diagnosed promptly by hematologists, oncologists, or other clinicians so that specific therapy can be started promptly to control the disease, interrupt its progression, and reduce the cost of treating it.
Abbreviations
HLH, haemophagocytic lymphohistiocytosis; rHLH, reactive haemophagocytic lymphohistiocytosis HLH; M-HLH, malignancy-associated haemophagocytic lymphohistiocytosis; NK, nature killer; MOF, multi-organ failure; sHLH, secondary haemophagocytic lymphohistiocytosis; RBC, red blood cell; Hgb, hemoglobin; Plt, platelet; CBC, complete blood cells; WBC, white blood cell; ALT, alanine transaminase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; γ-GGT, γ- glutamyl transpeptidase; LDH, lactate dehydrogenase; α-HBDH, α-hydroxybutyrate dehydrogenase; CK-MB, creatine kinase isoenzyme MB; Glu, glucose; CRP, C reaction protein; CEA, carcinoembryonic antigen; CA, carbohydrate antigen; PT, prothrombin time; APTT, activated partial thromboplastic time; US, abdominal ultrasound; CT, computed tomography; MRI, magnetic resonance imaging; EBV, Epstein Barr virus; Th, helper T; Tc, cytotoxicity T; ICU, Intensive Care Units; IFN-γ, interferon-γ.
Data Sharing Statement
All data during the study are included within the article.
Ethics Approval and Consent to Participate
Written informed consent was obtained from the patient’s relative because she died prior to submission of the case report, and approved by Shanxi Province Fenyang Hospital Ethics Committee (No. 2021051). Additional informed consent was obtained from the patient’s relative for whom identifying information is included in this article.
Consent for Publication
The patient’s relative has given the consent for the case report to be published. The written informed consent to publish this information was obtained from this patient’s relative.
Funding
No external funding was received to conduct this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
2. Lheureux S, Gourley C, Vergote I, et al. Epithelial ovarian cancer. Lancet. 2019;393(10177):1240–1253. doi:10.1016/S0140-6736(18)32552-2
3. Gaona-Luviano P, Medina-Gaona LA, Magaña-Pérez K. Epidemiology of ovarian cancer. Chin Clin Oncol. 2020;9(4):47. doi:10.21037/cco-20-34
4. Astigarraga I, Gonzalez-Granado LI, Allende LM, et al. Haemophagocytic syndromes: the importance of early diagnosis and treatment. Pediatr. 2018;89(2):
5. Chahine Z, Jayakrishnan T, Samhouri Y, et al. Haemophagocytic lymphohistiocytosis that spontaneously resolved: a case of EBV. BMJ Case Rep. 2020;13(11):e235544. doi:10.1136/bcr-2020-235544
6. Risma KA, Marsh RA. Hemophagocytic lymphohistiocytosis: clinical presentations and diagnosis. J Allergy Clin Immunol Pract. 2019;7(3):824–832. doi:10.1016/j.jaip.2018.11.050
7. Seo JJ. Hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis: recent advances and controversies. Blood Res. 2015;50(3):131–139. doi:10.5045/br.2015.50.3.131
8. Mendes FR, Sobral KM, Culler HF, et al. Acquired hemophagocytic lymphohistiocytosis as initial manifestation of multiple myeloma: a case report and literature review. Medicine. 2020;99(39):e22299. doi:10.1097/MD.0000000000022299
9. Henter JI, Horne A, Arico M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. doi:10.1002/pbc.21039
10. Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613–2620. doi:10.1002/art.38690
11. Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503–1516. doi:10.1016/S0140-6736(13)61048-X
12. Ishii E, Ohga S, Imashuku S, et al. Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int J Hematol. 2007;86(1):58–65. doi:10.1532/IJH97.07012
13. Kapoor S, Morgan CK, Siddique MA, et al. Intensive care unit complications and outcomes of adult patients with hemophagocytic lymphohistiocytosis: a retrospective study of 16 cases. World J Crit Care Med. 2018;7(6):73–83. doi:10.5492/wjccm.v7.i6.73
14. Machaczka M, Vaktnas J, Klimkowska M, et al. Malignancy-associated hemophagocytic lymphohistiocytosis in adults: a retrospective population-based analysis from a single center. Leuk Lymphoma. 2011;52(4):613–619. doi:10.3109/10428194.2010.551153
15. Tamamyan GN, Kantarjian HM, Ning J, et al. Malignancy-associated hemophagocytic lymphohistiocytosis in adults: relation to hemophagocytosis, characteristics, and outcomes. Cancer. 2016;122(18):2857–2866. doi:10.1002/cncr.30084
16. Barba T, Maucort-Boulch D, Iwaz J, et al. Hemophagocytic lymphohistiocytosis in intensive care unit: a 71-compliant retrospective study. Medicine. 2015;94(51):e2318. doi:10.1097/MD.0000000000002318
17. Sakai T, Shiraki K, Deguchi M, et al. Hepatocellular carcinoma associated with hemophagocytic syndrome. Hepatogastroenterology. 2001;48(41):1464–1466.
18. Dumont L, Salaroli A, Dal Lago L, et al. Prostate cancer and reactive haemophagocytic lymphohistiocytosis. Eur J Case Rep Intern Med. 2021;8(4):002425. doi:10.12890/2021_002425
19. Koizumi K, Haseyama Y, Machino R, et al. The hemophagocytic syndrome in prostate cancer revealed by disseminated carcinomatosis of the bone marrow. J Urol. 2002;168(3):1101–1102. doi:10.1016/S0022-5347(05)64588-0
20. Declerck L, Adenis C, Amela E, et al. Prostate cancer related haemophagocytic syndrome: successful treatment with chemotherapy. Acta Oncol. 2012;51(2):268–269. doi:10.3109/0284186X.2011.608377
21. Nosratian-Baskovic M, Tan B, Folkins A, et al. Hemophagocytic lymphohistiocytosis as a paraneoplastic syndrome associated with ovarian dysgerminoma. Gynecol Oncol Rep. 2016;17:38–41. doi:10.1016/j.gore.2016.05.013
22. Sandnes M, Ulvik RJ, Vorland M, et al. Hyperferritinemia-A clinical overview. J Clin Med. 2021;10(9):2008. doi:10.3390/jcm10092008
23. Knaak C, Nyvlt P, Schuster FS, et al. Hemophagocytic lymphohistiocytosis in critically ill patients: diagnostic reliability of HLH-2004 criteria and HScore. Crit Care. 2020;24(1):244. doi:10.1186/s13054-020-02941-3
24. Mottaghipisheh H, Kalantar K, Amanati A, et al. Comparison of the clinical features and outcome of children with hemophagocytic lymphohistiocytosis (HLH) secondary to visceral leishmaniasis and primary HLH: a single-center study. BMC Infect Dis. 2021;21(1):732. doi:10.1186/s12879-021-06408-w
25. Merrill SA, Naik R, Streiff MB, et al. A prospective quality improvement initiative in adult hemophagocytic lymphohistiocytosis to improve testing and a framework to facilitate trigger identification and mitigate hemorrhage from retrospective analysis. Medicine. 2018;97(31):e11579. doi:10.1097/MD.0000000000011579
26. Lachmann G, Spies C, Schenk T, et al. Hemophagocytic lymphohistiocytosis: potentially underdiagnosed in intensive care units. Shock. 2018;50(2):149–155. doi:10.1097/SHK.0000000000001048
27. Lachmann G, Knaak C, von Haefen C, et al. Diagnostic biomarkers for adult haemophagocytic lymphohistiocytosis in critically ill pse Strobe-Coatients (HEMICU): a prospective observational study protocol. BMJ Open. 2019;9(10):e032695. doi:10.1136/bmjopen-2019-032695
28. Dalal BI, Vakil AP, Khare NS, et al. Abnormalities of the lymphocyte subsets and their immunophenotype, and their prognostic significance in adult patients with hemophagocytic lymphohistiocytosis. Ann Hematol. 2015;94(7):1111–1117. doi:10.1007/s00277-015-2350-y
29. Carvelli J, Piperoglou C, Farnarier C, et al. Functional and genetic testing in adults with HLH reveals an inflammatory profile rather than a cytotoxicity defect. Blood. 2020;136(5):542–552. doi:10.1182/blood.2019003664
30. Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: an update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. 2020;34(4):101515. doi:10.1016/j.berh.2020.101515
31. Merli P, Quintarelli C, Strocchio L, et al. The role of interferon-gamma and its signaling pathway in pediatric hematological disorders. Pediatr Blood Cancer. 2021;68(4):e28900. doi:10.1002/pbc.28900
32. Chiossone L, Dumas PY, Vienne M, et al. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018;18(11):671–688. doi:10.1038/s41577-018-0061-z
33. Di Vito C, Mikulak J, Zaghi E, et al. NK cells to cure cancer. Semin Immunol. 2019;41:101272. doi:10.1016/j.smim.2019.03.004
34. Parodi E, Romano F, Ramenghi U. How we use reticulocyte parameters in workup and management of pediatric hematologic diseases. Front Pediatr. 2020;8:588617. doi:10.3389/fped.2020.588617
35. Yoshihara S, Li Y, Xia J, et al. Posttransplant hemophagocytic lymphohistiocytosis driven by myeloid cytokines and vicious cycles of T-Cell and macrophage activation in humanized mice. Front Immunol. 2019;10:186. doi:10.3389/fimmu.2019.00186
36. Ru YX, Bao ST, Dong SX, et al. Analysis of monocyte and histiocytic cell populations in bone marrow of patients with confirmed and suspected cases of reactive histocytosis. Ultrastruct Pathol. 2013;37(2):93–101. doi:10.3109/01913123.2012.742174
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.