Back to Journals » OncoTargets and Therapy » Volume 12
RBPJ inhibits the movability of endometrial carcinoma cells by miR-155/NF-κB/ROS pathway
Authors Xiao Y, Wang X, Dong X, Zhang Y, Liu H
Received 16 April 2019
Accepted for publication 18 July 2019
Published 2 October 2019 Volume 2019:12 Pages 8075—8084
DOI https://doi.org/10.2147/OTT.S212519
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Takuya Aoki
Yufeng Xiao,1,* Xiaoli Wang,2,* Xiping Dong,3 Yan Zhang,1 Haibin Liu4
1Department of Gynecology, Chengwu People’s Hospital, Heze, Shandong Province 274700, People’s Republic of China; 2Department of Gynecology, Liangshan People’s Hospital, Jining, Shandong Province 272699, People’s Republic of China; 3Department of Obstetrics and Gynecology, The First People’s Hospital of Ji’nan, Ji’nan, Shandong Province 250011, People’s Republic of China; 4Department of Gynecology and Obstetrics, Heze Municipal Hospital, Heze, Shandong Province 274000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Haibin Liu
Department of Gynecology and Obstetrics, Heze Municipal Hospital, Heze, Shandong Province 274006, People’s Republic of China
Email [email protected]
Background: Recombination signal-binding protein J (RBPJ) is a crucial downstream effector of Notch signaling, which is involved cell proliferation, differentiation, and apoptosis. It plays an important role in tumorigenesis although the further studies and concrete evidence are still needed. Especially for endometrial carcinoma, the functions and mechanism of RBPJ are still elusive.
Methods: The RNA expressions of RBPJ, miR-155, NF-κB, TNF-α and κB-Ras1 were examined by rt-PCR, and their protein levels were determined by Western Blot. Their expressions were inhibited by transient transfection of related siRNAs. Wound healing and transwell invasion assays were performed in ECC003 cells for measuring the migration and invasion ability, respectively. The ROS levels were detected by flow cytometry with H2DCFDA.
Purpose: This study was designed to investigate biological characteristics and molecular pathway of RBPJ in endometrial carcinoma cells, which may provide a potential therapeutic target for the treatments against endometrial carcinoma.
Results: It was shown in our study that the expression levels of RBPJ were significantly downregulated in different endometrial carcinoma cell lines. And a siRNA-mediated reduction of RBPJ enhanced the migration and invasion ability of ECC003 obviously. Besides, the results showed that the reactive oxygenspecies (ROS) levels increase when inhibiting RBPJ. To investigate the molecular pathway of RBPJ, we examined the expression of nuclear factor-κB (NF-κB), NF-κB inhibitor interacting Ras-like protein 1 (κB-Ras1), tumor necrosis factor-α (TNF-α) and miR-155. The results suggested that the expression of NF-κB and TNF-α significantly was promoted, while κB-Ras1 was inhibited. An upregulated expression was observed with miR-155 as well, which suggested the inhibition of NF-κB signal pathway was mediated by miR-155. Our results of Notch intracellular domain (NICD) knockdown also demonstrated that NICD is required for the inhibition of RBPJ on miR-155. And knockdown of miR-155 could inhibit the mobility of endometrial carcinoma cells.
Conclusion: Our study suggested that RBPJ can inhibit the movability of endometrial carcinoma cells by miR-155/NF-κB/ROS pathway.
Keywords: RBPJ, endometrial carcinoma, ROS, NF-κB signal, miR-155
Introduction
As the transcription factor, recombination signal-binding protein J (RBPJ) belongs to the CSL family, which is essential for Notch signaling.1,2 RBPJ binding Notch intracellular domain (NICD) can modulate cell proliferation, differentiation, and apoptosis by regulating the expression of downstream target genes, which may be related to oncogenesis.3,4 It was reported that the expression of RBPJ was significantly downregulated in cancer cells, and depletion of RBPJ could obviously increase the survival rate of cancer cells and lead to a growth of tumor.5 However, there is no study reported its functions and mechanism in endometrial carcinoma.
Previous study demonstrated that RBPJ repressed expression of miR-155 by binding to its promoter region. The absence of RBPJ leaded to an upregulation of miR-155.6 As a single-stranded non-coding RNA, miR-155 regulates expressions of many genes, and some of them play key roles in endometrial carcinoma.7,8 The expression of miR-155 also significantly upregulated in many other types of human cancers including thyroid adenoma,9 breast cancer,10–12 colon cancer,10 cervical cancer,13 pancreatic cancer14,15, and lung cancer.16 miR-155 was proved to be involved in the invasion and metastasis of cancer cells. Inhibition of miR-155 expression can significantly reduce the proliferation of colon cancer cells and increase the cell apoptosis.
Nuclear factor-κB (NF-κB) is a major regulator that control cytokines expressions, such as tumor necrosis factor (TNF), by targeting promoter regions.17,18 Besides, NF-κB has been shown to exhibit inhibitory effects on apoptosis by regulating the expression of anti-apoptotic genes.19 For tumorigenesis, NF-κB can promote cancer cell growth and reduce cancer cell apoptosis in various cancers, like pancreatic cancer,20 oral squamous cell carcinoma,21 colorectal cancer22, and breast cancer.23 Reduced activity of NF-κB can promote the apoptotic rate in cancer cells significantly.24 It was reported that the NF-κB inhibitor could inhibit the proliferation of prostate cancer cells and promoted cell apoptosis. Normally, NF-κB remains in inactive bounding to the inhibitor, like κB-Ras1.25 κB-Ras1 is the target gene of miR-155, and miR-155 can activate NF-κB by targeting κB-Ras1.6 The expression of κB-Ras1 obviously downregulated in human tumors. And the deletion of κB-Ras1 leaded to increased tumor growth.26,27
TNF-α is a NF-κB-regulated cytokine that has the ability to promote growth, invasion, and metastasis of cancer cell.6,17 As tumor-promoting factor, TNF-α was involved in the tumorigenesis. Its expression significantly increased in many cancers, including breast cancer, prostatic cancer, bladder cancer, colon cancer, liver cancer, and lymph cancer.28 Previous study demonstrated that TNF-α promoted the tumorigenesis induced by chemical carcinogens, and TNF-α knock-out mice showed tolerance to chemical carcinogens.29,30 Besides, anti-TNF-α can be an effective treatment in colon cancer.31,32
In this study, we examined the expression of RBPJ in different endometrial carcinoma cell lines. And its biological functions and molecular pathway was investigated in endometrial carcinoma cells with RBPJ knockdown, which may provide a novel therapeutic target in the treatment against endometrial cancer.
Materials and methods
Cell culture
Cell lines RL95-2, ECC001, ECC003, Ishikawa, and ECC-E6/E7 were obtained from the ATCC (Manassas, USA). Cell lines were cultured at 5% CO2 and 37 degrees centigrade with DMEM medium containing 1% FBS. Cells were used for experiment during the logarithmic growth phase.
Quantitative real-time polymerase chain reaction
Total RNA was extracted from the cells with TRIzol reagent. Cells were collected into disinfected tubes and washed with 1×PBS buffer. Cells lysis was added for cell fragmentation. Then, phenol/chloroform/isoamyl alcohol was added for removing impurities. After centrifugation, equal volumes isopropanol was added into the supernatant for RNA precipitation. RNA washed by 75% ethanol, and then dissolved by RNase-free ddH2O. And first-strand cDNA synthesis reaction was referred to the manual of Transcriptor First Strand cDNA Synthesis Kit (Roche, Indianapolis, IN, USA). The specific primer sequences were designed for RBPJ (5ʹ-AAG GAG CCC CAC GAG AAA AAT-3ʹ; 5ʹ-ACC GAA CTT GCA TTG ATT CCA G-3ʹ) and NICD (5ʹ-CGC TGA CGG AGT ACA AGT G-3ʹ; 5ʹ- GTA GGA GCC GAC CTC GTT G-3ʹ). cDNA sample, primers, and qPCR Master Mix were used for real-time PCR, referring to the user manual provided with the SYBR@ Green reagent (Roche, Indianapolis, IN, USA).
Transient transfection
Before transfection, cell density was reached up to 60–80%. Cells were transfected with 100 nm siRNA against RBPJ and NICD through Lipofectamine 2000. The specific siRNA sequences were designed against RBPJ (SiRBPJ-1: 5ʹ- CCA GAU UUC UGC CUU AAU UGU −3ʹ; SiRBPJ-2: 5ʹ- GAU UUC UGC CUU AAU UGU UCU −3ʹ; SiRBPJ-3: 5ʹ-GAA GCU AUG CGA AAU UAU UUA-3ʹ) and NICD (SiNICD-1: 5ʹ- CUA CUA UUU UUC GAU UUG AAU −3ʹ; SiNICD-2: 5ʹ- CUA UUU UUC GAU UUG AAU AGA −3ʹ; SiNICD-3: 5ʹ- ACC AAA ACC GAC UCU AUU CAA −3ʹ).
Wound healing assay
ECC003 cells, ECC003 cells transfected with SiRBPJ, and ECC-E6/E7 cells (5×105 cells) were cultured at six-well tissue culture plates and wounded with a sterile 10-μL pipette tip. The loosely attached cells were removed by PBS buffer. The cells were cultured at serum-free medium and photographed.
Transwell invasion assay
ECC003 cells, ECC003 cells transfected with SiRBPJ and ECC-E6/E7 cells were suspended in serum-free medium containing BSA after washed by PBS buffer. Cell suspension was added to 24-transwell Boyden chamber with extracellular matrix-coated membrane inserts (8-mm pore size) (BD Falcon, Corning-Costar, New York, NY, USA). 24-transwell chamber was incubated overnight in incubator at 37 degrees centigrade and 5% CO2. Invaded cells were fixed by formaldehyde for 10 mins and stained by crystal violet. The average number of cells per field was expressed as the percentage of the control after normalizing for cell number.
Detection of reactive oxygen species (ROS) level
Cell in logarithmic phase was cultured and seeded in six-well plate at a density of 4×105/mL. Cell was washed by PBS buffer. After centrifugation, the cell was resuspended by PBS buffer. The probe H2DCFDA was added and the mixture was incubated at 37°C for 30 mins. ROS level was measured by flow cytometry.
Western blot
Total proteins were extracted with cell lysate. After SDS-PAGE, proteins were transferred to polyvinylidene difluoride membrane. The membrane was stained with 1×Ponceau S for 5 mins and then was closed by TBS solution for 1 hr. Primary antibody diluted with TBST was added for 2 hrs at RT. Then, the membrane was washed by TBST three times for 10 mins. Secondary antibody diluted with TBST and the membrane was incubated for 2 hrs. The result was analyzed by ECL detection system.
Statistical analysis
At least three repeated experiments have been performed. The data were processed by SPSS 25.0 statistical software. Two-tailedt-test and one-way ANOVA was applied in the analysis, in which *P<0.05 and **P<0.01.
Results
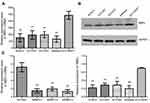
The expression of RBPJ decrease in endometrial carcinoma cell lines
We detected the expression level of RBPJ in different endometrial carcinoma cell lines with real-time PCR. The results showed that the expressions of RBPJ were significantly downregulated in endometrial carcinoma cell lines compared to the normal cell line ECC-E6/E7 (Figure 1A and B). The mRNA and protein expression level of RBPJ varied as well among different endometrial carcinoma cell lines. According to this result, the expression of RBPJ was highest in ECC003, while the lowest expression of RBPJ was detected in Ishikawa.
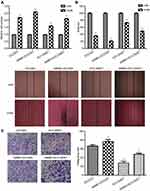
RBPJ inhibits the proliferation, migration, and invasion capacities of endometrial carcinoma cell
To investigate the biological functions of RBPJ in endometrial carcinoma, the expression of RBPJ was knockeddown by siRNAs in ECC003 and ECC-E6/E7. Three different siRNAs were designed based on the sequence of RBPJ mRNA and tested for their interference abilities. Expressions of RBPJ mRNA were examined after transient transfections and incubation. As a result, all siRNAs can suppress RBPJ expression effectively, especially SiRBPJ-3 (Figure 1C). Thus, we selected SiRBPJ-3 for suppressing RBPJ expressions in further experiments.
The capacity of cell proliferation was investigated in ECC003 and ECC-E6/E7 with SiRBPJ-3. The results showed that the knockdown of RBPJ promoted the cell proliferation (Figure 2A). We also examined the migration and invasion of endometrial carcinoma cells by the wound healing and transwell invasion assays, respectively. It was suggested in the wound healing assays that the downregulation of RBPJ expression could promote the migration ability of ECC003 and ECC-E6/E7 cell line, in which the width of wound in SiRBPJ was significantly reduced compared to the control (Figure 2B). Similarly, in transwell invasion assays, the knockdown of RBPJ obviously improved the ability of ECC003 and ECC-E6/E7 cell line in invasion (Figure 2C). These results demonstrated that the RBPJ plays an important role in the proliferation and movability of endometrial carcinoma cells.
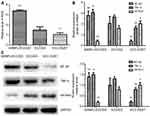
RBPJ suppresses endometrial carcinoma cells via reducing ROS level
It has been reported that ROS enhanced the ability of tumor movability. By measuring intracellular ROS levels, we found the ROS level in SiRBPJ-ECC003 was significantly higher than that in ECC003 and ECC-E6/E7 (Figure 3A). These results may suggest that the reduction of RBPJ could lead to an accumulation of ROS in endometrial carcinoma cell, which may enhance the movability of endometrial carcinoma cells.
RBPJ inhibits NF-κB signal pathway in endometrial carcinoma
Since ROS was involved in NF-κB signal pathway and inflammation via the release of TNF-α, we next detected the expression of NF-κB, TNF-α, and κB-Ras1, the up-stream inhibitor of NF-κB, by real-time PCR and Western blot. It was shown in our study that the expression of NF-κB and TNF-α significantly upregulated when RBPJ was suppressed (Figure 3B and C). Besides, the expression of κB-Ras1 in SiRBPJ-ECC003 was inhibited simultaneously, which suggested RBPJ may inhibit NF-κB signal pathway by regulating κB-Ras1 in endometrial carcinoma cells (Figure 3B and C). These results above demonstrated that RBPJ can inhibit NF-κB signal pathway, which may be involved in the growth of endometrial carcinoma.
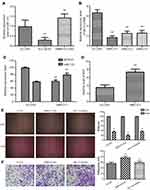
RBPJ binding NICD reduces the expression of miR-155 in endometrial carcinoma
Previous study reported that κB-Ras1 is a target gene of miR-155.6 Thus, we examined the expression of miR-155 by real-time PCR to investigate if RBPJ regulates the expression of miR-155 in endometrial carcinoma cells. The results showed that the expression of miR-155 was significantly raised in SiRBPJ-ECC003, and miR-155 expression was relatively lower in ECC-E6/E7 (Figure 4A), which was consistent with our assuming.
To further confirm it, we interfered with the expression of NICD by siRNA, which can bind with RBPJ to activate its inhibitory ability. As we described before, three kinds of siRNA were designed to target NICD mRNA in ECC003, and we select SiNICD-1, the most efficient one, for next tests (Figure 4B). We detected the mRNA transcription level of κB-Ras1 and miR-155 in ECC003 and SiNICD-ECC003 (NICD knockdown in ECC003) by real-time PCR. The results showed that the mRNA level of miR-155 was markedly upregulated, while the mRNA level of κB-Ras1 was significantly downregulated in SiNICD-ECC003 (Figure 4C). Thus, we can conclude that the inhibitory effect of RPBJ on miR-155 was mediated by NICD in endometrial carcinoma cells. To further confirm it, the level of ROS was measured as well. As expected, it significantly increased in SiNICD-ECC003 (Figure 4D). Moreover, we examined the migration and invasion of ECC003 treated with siRBPJ and miR-155 inhibitor, which suggested that the knockdown of miR-155 significantly inhibited the migration and invasion of SiRBPJ-ECC003 (Figure 4E and F). These results above indicated that the knockdown of RBPJ or NICD had a significant influence on the expression of miR-155 in endometrial carcinoma cells, and then abolished the inhibition on NF-κB signal pathway and intracellular ROS level.
Discussion
As a DNA-binding protein, RBPJ can recognize the promoter-specific sequence of target genes.2 Particularly, RBPJ plays an important role in the Notch signaling pathway.33–35 As the downstream signal molecule of Notch, RBPJ binding NICD regulates the expression of target genes related to cell cycle or apoptosis, like Cyclin D1, Cyclin-dependent kinase 2, p21, and B-cell CLL/lymphoma 2 (Bcl-2).36,37 It has been proved that RBPJ is involved in tumorigenesis. Depletion of RBPJ in breast cancer and lung cancer could accelerate the growth of tumor.5 And RBPJ inhibition significantly reduced cell proliferation of small cell lung cancer.38 However, there are no studies reported the functions of RBPJ in endometrial carcinoma, and its mechanism has not been clear.
In this study, we investigated the biological functions of RBPJ in endometrial cancer cells and revealed its molecular pathway. It was shown that RBPJ expression was reduced in endometrial carcinoma cell lines. In order to investigate the biological functions of RBPJ in endometrial carcinoma, the siRNAs targeting RBPJ were designed to reduce the cellular expression of RBPJ. The results of real-time PCR showed that the expression of RBPJ is reduced to about one fifth. Besides, we found knockdown of RBPJ significantly enhanced the cell proliferation and mobility. Considering the expression of RBPJ decreased gradually as well in these three lines, it suggested that RBPJ could effectively reduce the mobility of endometrial carcinoma cell, which may be involved in the metastasis of endometrial carcinoma.
ROS is the product of aerobic metabolism.39,40 ROS at a high level could cause DNA and protein damage and tumorigenesis. It was reported that, in various cancer cells, the level of ROS was elevated compared to normal cell.41 ROS is reported that it contributed to the metastasis of tumor cells and resistance to apoptosis.42–46 To investigate whether the inhibitory effect of RBPJ on endometrial carcinoma cell was mediated by ROS, we measured the ROS content. The results showed that the RBPJ gene expression and the ROS level were negatively related. This may because RBPJ directly inhibits the production of ROS or enhance the ability of scavenging ROS. Since ROS is associated with inflammation, the inflammation signal pathway was examined as well. The results showed that the expressions of inflammatory factors TNF-α and NF-κB are significantly upregulated, which indicated the enhancement of inflammatory reaction. Moreover, the expression of κB-Ras1, inhibitor of NF-κB, was detected. The result showed that κB-Ras1 expression was inhibited obviously, which suggested that RBPJ is involved in inflammation response by regulating the expression of κB-Ras1.
Our study demonstrated that RBPJ inhibits the expression of miR-155 in endometrial carcinoma. Since previous study on bone marrow niche indicated miR-155 could inhibit the expression of κB-Ras1 by targeting the sequences at its 3ʹ-UTR,6,47 we assumed that the expression of κB-Ras1 might be promoted by RBPJ. It may be due to the miR-155’s promoter targeting of RBPJ-NICD complex. To further investigate it, the expression of NICD was knockeddown in endometrial carcinoma. The result indicated that NICD is required for the inhibition of RBPJ on miR-155. This may be due to the interaction between RBPJ and NICD, which activate the inhibitory activity of RBPJ. To investigate whether RBPJ inhibits the movability of endometrial carcinoma cells by miR-155/NF-κB/ROS pathway, we inhibited the expression of RBPJ and miR-155 in ECC003. By wound healing assay and transwell invasion assay, we found knockdown of miR-155 significantly decreased the mobility of SiRBPJ-ECC003. It suggested that RBPJ inhibits the mobility of endometrial carcinoma cells by inhibiting miR-155.
In summary, our study investigated the biological functions and molecular mechanism of RBPJ in endometrial carcinoma, which may provide a potential therapeutic target for endometrial carcinoma treatments.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kojika S, Griffin JD. Notch receptors and hematopoiesis. Exp Hematol. 2001;29(9):1041–1052.
2. Hsieh JJ, Hayward SD. Masking of the CBF1/RBPJ kappa transcriptional repression domain by epstein-barr virus EBNA2. Science. 1995;268(5210):560–563. doi:10.1126/science.7725102
3. Borggrefe T, Oswald F. The notch signaling pathway: transcriptional regulation at notch target genes. Cell Mol Life Sci. 2009;66(10):1631–1646. doi:10.1007/s00018-009-8668-7
4. Lv Q, Shen R, Wang J. RBPJ inhibition impairs the growth of lung cancer. Tumour Biol. 2015;36(5):3751–3756. doi:10.1007/s13277-014-3015-5
5. Kulic I, Robertson G, Chang L, et al. Loss of the notch effector RBPJ promotes tumorigenesis. J Exp Med. 2015;212(1):37–52. doi:10.1084/jem.20121192
6. Wang L, Zhang H, Rodriguez S, et al. Notch-dependent repression of miR-155 in the bone marrow niche regulates hematopoiesis in an NF-kappaB-dependent manner. Cell Stem Cell. 2014;15(1):51–65. doi:10.1016/j.stem.2014.04.021
7. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi:10.1016/s0092-8674(04)00045-5
8. Faraoni I, Antonetti FR, Cardone J, Bonmassar E. miR-155 gene: a typical multifunctional microRNA. Biochim Biophys Acta. 2009;1792(6):497–505. doi:10.1016/j.bbadis.2009.02.013
9. Nikiforova MN, Tseng GC, Steward D, Diorio D, Nikiforov YE. MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. J Clin Endocrinol Metab. 2008;93(5):1600–1608. doi:10.1210/jc.2007-2696
10. Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103(7):2257–2261. doi:10.1073/pnas.0510565103
11. Iorio MV, Ferracin M, Liu CG, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65(16):7065–7070. doi:10.1158/0008-5472.CAN-05-1783
12. Yan LX, Huang XF, Shao Q, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14(11):2348–2360. doi:10.1261/rna.1034808
13. Wang X, Tang S, Le SY, et al. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS One. 2008;3(7):e2557. doi:10.1371/journal.pone.0002557
14. Lee EJ, Gusev Y, Jiang J, et al. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007;120(5):1046–1054. doi:10.1002/ijc.22394
15. Szafranska AE, Davison TS, John J, et al. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene. 2007;26(30):4442–4452. doi:10.1038/sj.onc.1210228
16. Esquela-Kerscher A, Slack FJ. Oncomirs – microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–269. doi:10.1038/nrc1840
17. Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17(1):3–9. doi:10.1165/ajrcmb.17.1.f132
18. Sethi G, Sung B, Aggarwal BB. Nuclear factor-kappaB activation: from bench to bedside. Exp Biol Med (Maywood). 2008;233(1):21–31. doi:10.3181/0707-MR-196
19. Jo H, Zhang R, Zhang H, et al. NF-kappa B is required for H-ras oncogene induced abnormal cell proliferation and tumorigenesis. Oncogene. 2000;19(7):841–849. doi:10.1038/sj.onc.1203392
20. Sebens S, Arlt A, Schäfer H. NF-κ B as a molecular target in the therapy of pancreatic carcinoma. Pancreatic Cancer. 2008;177:151–164. doi:10.1007/978-3-540-71279-4_17
21. Takamune Y, Ikebe T, Nagano O, Shinohara M. Involvement of NF-kappaB-mediated maturation of ADAM-17 in the invasion of oral squamous cell carcinoma. Biochem Biophys Res Commun. 2008;365(2):393–398. doi:10.1016/j.bbrc.2007.11.010
22. Charalambous MP, Lightfoot T, Speirs V, Horgan K, Gooderham NJ. Expression of COX-2, NF-kappaB-p65, NF-kappaB-p50 and IKKalpha in malignant and adjacent normal human colorectal tissue. Br J Cancer. 2009;101(1):106–115. doi:10.1038/sj.bjc.6605120
23. Frasor J, Weaver A, Pradhan M, et al. Positive cross-talk between estrogen receptor and NF-kappaB in breast cancer. Cancer Res. 2009;69(23):8918–8925. doi:10.1158/0008-5472.CAN-09-2608
24. Lee HY, Youn SW, Kim JY, et al. FOXO3a turns the tumor necrosis factor receptor signaling towards apoptosis through reciprocal regulation of c-Jun N-terminal kinase and NF-kappaB. Arterioscler Thromb Vasc Biol. 2008;28(1):112–120. doi:10.1161/ATVBAHA07.153304
25. Fenwick C, Na SY, Voll RE, et al. A subclass of ras proteins that regulate the degradation of IB. Science. 2000;287(5454):869–873. doi:10.1126/science.287.5454.869
26. Lin H, Wang Y, Zhang X, Liu B, Zhang W, Cheng J. Prognostic significance of kappaB-Ras1 expression in gliomas. Med Oncol. 2011;29(2):1272–1279. doi:10.1007/s12032-011-9835-x
27. Oeckinghaus A, Postler TS, Rao P, et al. kappaB-Ras proteins regulate both NF-kappaB-dependent inflammation and Ral-dependent proliferation. Cell Rep. 2014;8(6):1793–1807. doi:10.1016/j.celrep.2014.08.015
28. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi:10.1038/nature01322
29. Moore RJ, Owens DM, Stamp G, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5(7):828–831. doi:10.1038/10552
30. Suganuma M, Okabe S, Marino MW, Sakai A, Sueoka E, Fujiki H. Essential role of tumor necrosis factor alpha (TNP-alpha) in tumor promotion as revealed by TNF-alpha-deficient mice. Cancer Res. 1999;59(18):4516–4518.
31. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–2476. doi:10.1056/NEJMoa050516
32. Popivanova BK, Kitamura K, Wu Y, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118(2):560–570. doi:10.1172/JCI32453
33. Castel D, Mourikis P, Bartels SJJ, Brinkman AB, Tajbakhsh S, Stunnenberg HG. Dynamic binding of RBPJ is determined by notch signaling status. Genes Dev. 2013;27(9):1059–1071. doi:10.1101/gad.211912.112
34. Hori K, Cholewa-Waclaw J, Nakada Y, et al. A nonclassical bHLH Rbpj transcription factor complex is required for specification of GABAergic neurons independent of notch signaling. Genes Dev. 2008;22(2):166–178. doi:10.1101/gad.1628008
35. Shi C, Qian J, Ma M, Zhang Y, Han B. Notch 3 protein, not its gene polymorphism, is associated with the chemotherapy response and prognosis of advanced NSCLC patients. Cell Physiol Biochem. 2014;34(3):743–752. doi:10.1159/000363039
36. Guo D, Ye J, Dai J, et al. Notch-1 regulates Akt signaling pathway and the expression of cell cycle regulatory proteins cyclin D1, CDK2 and p21 in T-ALL cell lines. Leuk Res. 2009;33(5):678–685. doi:10.1016/j.leukres.2008.10.026
37. Gao F, Yao M, Shi Y, et al. Notch pathway is involved in high glucose-induced apoptosis in podocytes via Bcl-2 and p53 pathways. J Cell Biochem. 2013;114(5):1029–1038. doi:10.1002/jcb.24442
38. Onishi H, Ichimiya S, Yanai K, et al. RBPJ and MAML3: potential therapeutic targets for small cell lung cancer. Anticancer Res. 2018;38(8):4543–4547. doi:10.21873/anticanres.12758
39. Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol. 2007;8(9):722–728. doi:10.1038/nrm2240
40. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94(3):909–950. doi:10.1152/physrev.00026.2013
41. Panieri E, Santoro MM. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis. 2016;7(6):e2253. doi:10.1038/cddis.2016.105
42. Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44(5):479–496. doi:10.3109/10715761003667554
43. Roy K, Wu Y, Meitzler JL, et al. NADPH oxidases and cancer. Clin Sci (Lond). 2015;128(12):863–875. doi:10.1042/CS20140542
44. Stanicka J, Russell EG, Woolley JF, Cotter TG. NADPH oxidase-generated hydrogen peroxide induces DNA damage in mutant FLT3-expressing leukemia cells. J Biol Chem. 2015;290(15):9348–9361. doi:10.1074/jbc.M113.510495
45. Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90(17):7915–7922. doi:10.1073/pnas.90.17.7915
46. Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51(3):794–798.
47. Miranda KC, Huynh T, Tay Y, et al. A pattern-based method for the identification of microRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126(6):1203–1217. doi:10.1016/j.cell.2006.07.031
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.