Back to Journals » Research and Reports in Urology » Volume 9
Rationale of combination therapy with antioxidants in medical management of Peyronie’s disease: results of clinical application
Authors Paulis G , Paulis A, Romano G, Barletta D, Fabiani A
Received 13 May 2017
Accepted for publication 21 June 2017
Published 20 July 2017 Volume 2017:9 Pages 129—139
DOI https://doi.org/10.2147/RRU.S141748
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Gianni Paulis,1,2 Andrea Paulis,3 Gennaro Romano,4 Davide Barletta,5 Andrea Fabiani6
1Department of Surgical Sciences, Andrology Center, Regina Apostolorum Hospital, Rome, Italy; 2Department of Uro-Andrology, Peyronie’s Disease Care Center, Rome, Italy; 3Section of Psycho-Sexology, Peyronie’s Disease Care Center, Rome, Italy; 4Department of Urologic Oncology, Section of Avellino, Italian League against Cancer, Avellino, Italy; 5Department of Urology, Andrology Center, San Matteo Hospital, Pavia, Italy; 6Department of Surgery, Section of Urology and Andrology, Macerata, Italy
Abstract: Peyronie’s disease (PD) is a connective tissue disorder involving the tunica albuginea of the corpora cavernosa of the penis. We have published several studies describing a “combined therapy” for PD patients, but the present study aims to clearly demonstrate how the association between various antioxidants in PD treatment can significantly increase the likelihood of therapeutic success. We used the following substances: silymarin, ginkgo biloba, vitamin E, bilberry, topical diclofenac sodium, and pentoxifylline (PTX). We analyzed the therapeutic impact and possible side effects of one or more antioxidants in patients with early-stage PD. To clearly prove that it is possible to achieve better results when combining more than one agent, we designed this study with five treatment groups, corresponding, respectively, to the administration of a single oral antioxidant; two oral antioxidants; three oral antioxidants; five oral antioxidants + local diclofenac; and five oral antioxidants + local diclofenac + PTX by perilesional injection. One hundred and twenty patients were assigned to five groups of treatment designed according to the abovementioned study aim. Outcomes after 6 months of treatment showed that combined antioxidant therapy is effective in treating PD. Statistical analysis showed significant differences between the treatment groups with regard to: improvement and disappearance of penile pain; percentage of reduction in the volume of penile plaque; reduction in penile curvature; recovery of erectile function in patients with erectile dysfunction; increase in the International Index of Erectile Function score; and reduction of psychosexual impact. Furthermore, we observed that the clinical efficacy of combined therapy is greater when topical use of diclofenac gel and perilesional injection of PTX are added to oral treatment with more than one antioxidant. Although several articles have already been published reporting the effectiveness of combined treatment in PD, this is the first study clearly proving how, as the number of substances used in treatment rises, a proportionally greater therapeutic effect is achieved.
Keywords: Peyronie’s disease treatment, combined therapy, penile curvature, penile injections, antioxidant treatment
Introduction
Peyronie’s disease (PD) is a connective tissue disorder involving the tunica albuginea of the corpora cavernosa of the penis. It occurs in 3.2%–13% of adult males.1,2 The disease consists of a thickening of a small area of tissue that gradually turns into an inelastic plaque, which can cause penile deformity (penile curvature, shortening, divot, hourglass deformity, etc), pain during erection, erectile dysfunction [ED], and psychological disorders (psychosexual impact).3 Although the pathogenesis is not completely understood, most authors recognize that genetic predisposition and penile trauma of various degrees greatly favor the onset of PD.4–8 There are two stages in the disease: an initial active inflammatory remodeling phase, lasting ~12–18 months (actually preceded by a brief, acute posttraumatic period, lasting ~2 weeks wherein powerful recruitment of inflammatory cells occurs), and a second stage in which both tissue damage and deformation stabilize.9,10 Medical treatment is mainly indicated in the first stage of PD.11,12 Several options have been suggested, including oral therapy (potaba, colchicine, tamoxifen, vitamin E, carnitine, etc), intralesional injections (verapamil, steroids, interferon, and, recently, collagenase clostridium histolyticum [CCH, Xiaflex]), and physical treatment (iontophoresis, extracorporeal shock wave therapy [ESWT], penile extender).12–15 Recently, partly as a consequence of several important studies demonstrating the decisive role played by oxidative stress in the pathophysiological mechanisms of PD, several authors have published studies proving the efficacy of antioxidants in the treatment of the disease.16–40 A tendency to combine various antioxidants and/or therapy modes in treating PD has thus emerged; recently, various studies have been published confirming the effectiveness of a combination therapy.25,26,34–51 In 1991, Pastorini et al first tested a combination therapy in PD using injectable and oral superoxide dismutase (antioxidant substance) therapy, associated with papaverine intracavernous injection.52 Combination therapy is a treatment strategy in which a number of oral antioxidants are combined with each other and, sometimes, with other drug agents and/or treatment options (including intralesional and physical therapy) to achieve better results as compared to single-drug or single-mode therapy. Of course, this is not new in the medical field, as combined therapy has already been used – for instance, in the treatment of tuberculosis and in chemotherapy for cancer. Although we have already published several studies describing a combined therapy for treatment of patients suffering from PD, the present study aims to clearly demonstrate how the association between various antioxidants in PD treatment can significantly increase the likelihood of therapeutic success.
Materials and methods
In this study, we analyzed the therapeutic impact and possible side effects of one or more antioxidants in patients suffering from PD in its active stage. To clearly prove that it is possible to achieve better results when combining more than one agent, we designed this study with five treatment groups, corresponding, respectively, to the administration of a single oral antioxidant; two oral antioxidants; three oral antioxidants; five oral antioxidants + local diclofenac; and five oral antioxidants + local diclofenac + pentoxifylline (PTX) by perilesional injection. All patients originally enrolled in this study presented to our andrology clinic, from January 2, 2015, to October 31, 2016, for PD in its active stage. We excluded from the study any patient who met one or more of the following exclusion criteria: coexistence of other treatment(s) for other sexual dysfunction before and during the study; stable disease; significant penile curvature preventing complete sexual intercourse; previous therapies for PD; allergy or intolerance to one or more of the substances used in the study; congestive heart failure, ischemic heart disease, peripheral artery disease, and cerebrovascular diseases; concomitant anticoagulant therapy; recent retinal or cerebral hemorrhage or the mere presence of risk factors for hemorrhage; low blood pressure and/or concomitant theophylline therapy; and allergy to plants and pollen.
We, thus, selected and enrolled in the study 141 patients suffering from PD in its active stage and having none of the abovementioned exclusion criteria. All patients were duly informed of any other types of treatment, including surgery, and had refused the option of surgery, if indicated. Patients were casually assigned to groups of treatment duly designed according to the abovementioned study endpoints:
- Group A: silymarin 200 mg/orally/twice daily, for 6 months;
- Group B: silymarin 200 mg/orally/twice daily + ginkgo biloba 250 mg/orally/once daily, for 6 months;
- Group C: silymarin 200 mg/orally/twice daily + ginkgo biloba 250 mg/orally/once daily + vitamin E 400 IU/orally/twice daily, for 6 months;
- Group D: silymarin 200 mg/orally/twice daily + ginkgo biloba 250 mg/orally/once daily + vitamin E 400 IU/orally/twice daily + propolis 600 mg/orally/once daily + bilberry (Vaccinium myrtillus L.) 160 mg/orally/once daily + topical diclofenac sodium 4% spray gel/one application per day (2 pump strokes = 16 mg of diclofenac sodium), for 6 months;
- Group E: silymarin 200 mg/orally/twice daily + ginkgo biloba 250 mg/orally/once daily + vitamin E 400 IU/orally/twice daily + propolis 600 mg/orally/per day + bilberry (Vaccinium myrtillus L.) 160 mg/orally/once daily + topical diclofenac sodium 4% spray gel /one application per day (2 pump strokes = 16 mg of diclofenac sodium) + PTX 100 mg (perilesional injection) twice a month, a total of 12 penile injections in 6 months.
On analysis and consideration of clinical and demographic characteristics of patients in study subgroups, we had to exclude 21 subjects, despite randomization, because of their clinical characteristics (degree of penile curvature, plaque volume, presence of ED or penile pain, age, comorbidity, etc), as their presence in the study would have detracted from the necessary statistical homogeneity among the groups. Statistical analysis of various clinical and demographic characteristics of patients in the analysis dataset confirmed substantial statistical homogeneity between the groups.
The study, thus, included 120 cases, equally distributed into five treatment groups, each of which comprised 24 patients. All patients included in the study underwent a physical examination and accurate review of their medical history, as well as the following tests, both prior to treatment and at the 6-month follow-up: penile color Doppler ultrasound, questionnaire for the assessment of erectile function (International Index of Erectile Function [IIEF]), pain assessment questionnaire (Pain Intensity Numeric Rating Scale [PI-NRS]), weight and height assessment for body mass index (BMI) calculation, and evaluation of the disease’s psychosexual impact using Peyronie’s Disease Questionnaire (PDQ; Symptom Bother Domain).53
Besides assessing the plaque’s echogenicity, color Doppler analysis included three-dimensional study of the plaque’s measurements (length, width, and thickness), with imaging of the penis at maximum erection and photographic poses according to Kelami for goniometric measurement of penile curvature. Plaque volume was measured in cubic centimeters using the ellipsoid formula.54,55
In the IIEF, answers specifically pertaining to penile rigidity were taken into consideration – that is, answers to questions 1–5 and 15. The IIEF total score normally ranges between 26 and 30; therefore, patients with a score <26 were considered to be suffering from ED. The pain intensity questionnaire is based on analogical measurement of pain on a scale with 11 degrees, from 0 to 10 (PI-NRS), with 0 = no pain and 10 = worst pain possible. To evaluate psychosexual impact, we used the PDQ/Symptom Bother Domain Questionnaire, which consists of a set of questions (four scored items and two “yes/no” questions) inquiring about the patient’s degree of preoccupation and distress; scores go from 0 (“not at all bothered”) to 4 (“extremely bothered”); the final score can, therefore, vary from 0 to 16.53 We then recorded the clinical data and analyzed the following comorbidities and risk factors: high blood pressure, dyslipidemia, diabetes mellitus, obesity, chronic prostatitis, benign prostatic hyperplasia, and habitual cigarette smoking. We considered obesity to be present when the BMI was 30 (kg/m2) or higher. All 120 subjects signed a specific informed consent. Our study was conducted according to the principles of the Declaration of Helsinki in 1975, 1983, and subsequent revisions. After the approval of the institutional (Italian League Against Cancer [LILT]) ethics committee, specific written informed consents were obtained from all subjects.
The following antioxidants were used in the study: PTX, propolis, bilberry (Vaccinium myrtillus L.), vitamin E, silymarin, and ginkgo biloba. In addition, in groups D and E, diclofenac – an active substance already widely known for its anti-inflammatory properties – was used topically. PTX was used only via perilesional injection, in consideration of the possibility of side effects secondary to oral therapy.40
Statistical analysis
Statistical comparison between baseline and follow-up categorical variables was calculated both cumulatively and individually between the groups by using the chi-square test (χ2 test). Comparative analysis between baseline and continuous follow-up parameters was undertaken cumulatively using analysis of variance (one-way ANOVA) and individually between the groups using Student’s t-test. A value of p < 0.05 was considered statistically significant. IBM SPSS 22.0 was used for the statistical analysis.
Results
One hundred and twenty patients (median age [SD], 51.2 [±10.2] years; range 27–72 years) participated in the present study.
In 83.3% of cases (100 out of 120), penile curvature varied between 5 and 50 degrees, with an average (SD) of 25.4 (±11.3) degrees. At the beginning of treatment, the mean (SD) time since PD onset was 8.6 (±2.1) months. ED was present in 32.5% of cases (39 out of 120); the IIEF score was between 11 and 25, with an average (SD) of 22.30 (±3.28). Penile pain was present in 50.0% of cases (60 out of 120), and the mean PI-NRS score was 4.6 (±1.9). The psychological impact of the disease, recorded with the PDQ/Symptom Bother Domain questionnaire, had a mean score of 7.9 (±3.64).
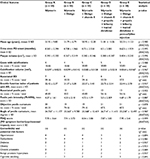
In cases where calcification was present (23 out of 120 cases, 19.1% of cases), it was very minimal (mean volume = 0.031± 0.017 cm3) and situated within the plaque, confirming that PD was in a progressive, active stage in all cases. Table 1 shows the substantial statistical homogeneity of the baseline clinical and demographic characteristics and comorbidities of the groups (with p-values between 0.878 and 0.999).
Analysis of results after 6 months of treatment
Statistical analysis revealed statistically significant differences between the treatment groups with regard to: improvement and disappearance of penile pain; percentage of reduction in the volume of penile plaque; reduction in penile curvature; recovery of erectile function in patients with ED; increase in the IIEF score (both in patients with and without ED); and reduction of psychosexual impact (Table 2).
After 6 months of treatment, a reduction in plaque volume was obtained in all treatment groups, although more in-depth statistical analysis made it possible to identify substantial differences between certain therapy groups (Table 3). These results prove that as the number of substances employed grows (combined therapy), the efficacy of treatment grows proportionally. Furthermore, statistical analysis of all other outcomes yields the same conceptual conclusion. Notably, no disease progression took place in any of the treatment groups, and – in particular – none of the following occurred: increase in penile plaque volume; onset of penile pain in patients without this symptom; increase in the intensity of penile pain in patients with this symptom; worsening in penile curvature; onset of ED in patients with normal penile rigidity; or increase of psychosexual impact caused by PD.
Evaluation of the tolerability of the substances
Table 4 lists the side effects by the therapy administered. In general, tolerability was satisfactory after individual (silymarin) or multimodal therapy. No side effect occurred after intake of propolis and/or bilberry. For all therapy arms, recorded side effects (Table 4) were mild and transient and disappeared gradually in the course of treatment. In no case was it necessary to suspend treatment. Onset of penile tumescence after perilesional infiltration of PTX occurred in a single patient and at almost every injection (10 times out of 12), without being associated with other side effects. We did not consider this occurrence as an adverse effect, but merely an effect of the well-known non-specific phosphodiesterase (PDE)-inhibiting action of PTX; therefore, we decided not to interrupt the cycle of local injections. This patient (30 years old) was the youngest in group E, his baseline IEEF score was 26 (normal IIEF scores: 26–30), and his score increased to 29 after multimodal treatment. In our study, no marked or severe adverse effect occurred.
  | Table 4 Side effects for each substance Note: Data presented as % cases / total cases (n/N). |
Discussion
The properties and mechanism of action of the substances used in this study are already known, but we provide a brief overview of this topic.
PTX is a hemorrheologic agent that was initially used to treat peripheral vascular diseases. PTX has antioxidant, anti-inflammatory, and antifibrotic activity56–61 and is also a non-specific PDE inhibitor.62
Propolis is a plant-based substance, processed by bees (Apis mellifera L.), which consists of a mixture of compounds mainly comprising flavonoids (acacetin, apigenin, catechin, chrysin, galangin, kaempferol, luteolin, myricetin, naringenin, pinocembrin, quercetin, and rutin), as well as caffeic acid and caffeic acid phenethyl ester (CAPE). Because of its many components, propolis has antioxidant, anti-inflammatory, and antifibrotic properties.63–66
Bilberry (Vaccinium myrtillus L.) contains several substances, including anthocyanins (cyanidin, delphinidin, petunidin, malvidin, and peonidin) as well as quercetin, tannins, ellagitannins, catechin, epicatechin, gallocatechin, and epigallocatechin, as well as small quantities of vitamin C. Thanks to its various components, bilberry has antioxidant, anti-inflammatory, and antifibrotic activity.66–68
Vitamin E has various properties: it is an antioxidant, anti-inflammatory, and antifibrotic agent, and acts against cell proliferation via a mechanism of protein kinase C (PKC) inhibition.17,69–71 A recent study by these authors showed the effectiveness of vitamin E when associated with other substances.72
Silymarin, from milk thistle, has long been used as a hepatoprotective agent; it also has antifibrotic, anti-inflammatory, and antioxidant properties.73–75
Ginkgo biloba, through its extract, has mainly been used to treat memory and concentration problems, but various studies have shown it has antioxidant, antifibrotic, and anti-inflammatory properties.76–80
Diclofenac has anti-inflammatory, analgesic, and antioxidant activity, but its antifibrotic activity has also been ascertained, as it has been shown to inhibit fibroblast proliferation.81–84
As a topical spray gel, diclofenac has excellent penetration capacity. Studies have identified the presence of high concentrations of diclofenac in the synovial tissue of the knee joint after topical administration of diclofenac sodium 4% spray gel.85 With the proven efficacy of diclofenac in the treatment of large-joint inflammation where the joint-capsule thickness is, on average 1–5 mm thick, and – in particular – in knee-joint inflammation (joint-capsule thickness ~2 mm), it was inferred that topical application of diclofenac in its 4% spray gel formulation could easily allow drug penetration into the corpora cavernosa as the thickness of the tunica albuginea varies from 0.5 to 2 mm.86 Combining a nonsteroidal anti-inflammatory drug (NSAID) with antioxidants means taking a combined – or multimodal – treatment approach. It is possible to clearly infer from the results of our study that the combination of several active substances in PD treatment leads to a proportionally greater therapeutic effect.87 The results show that the higher the number of substances used in treatment, the higher the reduction in pain score, and this is also true for all other study endpoints: the number of patients in whom a reduction in the degree of penile curvature was observed was greater when a higher number of treatment substances was used. The same was true with regard to the recovery of normal penile rigidity, for instance. Table 3 shows that, whereas in general there are no statistically significant differences in results between certain contiguous groups (A vs B, B vs C, and C vs D), an extremely relevant statistically significant difference was observed between groups D and E (p < 0.0001), proving that perilesional PTX injections significantly improve the global effectiveness of treatment. In group E, the mean reduction in penile plaque volume was 50.9%, versus 30.5% in group D, where no perilesional PTX injection was administered. The same occurred with regard to the degree of penile curvature: in group E, after 6 months of treatment, the degree of penile curvature dropped, on average, by 58%, compared to a 30.5% reduction obtained in group D (p < 0.0001), where no perilesional PTX injection was administered. It must be mentioned that total disappearance of penile curvature was observed only in group E, albeit in two patients out of 21.
Another important consideration is that in this study, regardless of the number of substances used, antioxidants always proved capable of arresting disease progression. This is most likely due to the fact that oxidative stress plays an essential role in the pathogenesis of PD.16–23
Conclusion
The results of the present study show that combined therapy with antioxidants is effective in treating PD. Furthermore, the study allows us to observe that the clinical efficacy of combined therapy is greater when topical use of diclofenac gel and perilesional injection of PTX are added to oral treatment with more than one antioxidant. Although several articles have already been published reporting the effectiveness of combined therapy in PD, this is the first study clearly proving how, as the number of substances used in treatment rises, a proportionally greater therapeutic effect is achieved.25,26,34–52,72,87
Though the treatment results we achieved in this study are statistically very significant, further randomized controlled trials are necessary to confirm the efficacy of combined therapy with antioxidants in the treatment of PD.
Disclosure
The authors report no conflicts of interest in this work.
Andrea Paulis (Psychologist) is the son of Gianni Paulis. Andrea collaborates in his father’s studies as in other past articles.
References
Schwarzer U, Sommer F, Klotz T, Braun M, Reifenrath B, Engelmann U. The prevalence of Peyronie’s disease: results of a large survey. BJU Int. 2001;88(7):727–730. | ||
Dibenedetti DB, Nguyen D, Zografos L, Ziemiecki R, Zhou X. A population-based study of Peyronie’s disease: prevalence and treatment patterns in the United States. Adv Urol. 2011;2011:282503. | ||
Paulis G, Romano G, Paulis A. Prevalence, psychological impact, and risk factors of erectile dysfunction in patients with Peyronie’s disease: a retrospective analysis of 309 cases. Res Rep Urol. 2016;8:95–103. | ||
Rompel R, Mueller-Eckhardt G, Schroeder-Printzen I, Weidner W. HLA antigens in Peyronie’s disease. Urol Int. 1994;52(1):34–37. | ||
Dolmans GH, Werker PM, de Jong IJ, et al; LifeLines Cohort Study. WNT2 locus is involved in genetic susceptibility of Peyronie’s disease. J Sex Med. 2012;9(5):1430–1434. | ||
Jarow JP, Lowe FC. Penile trauma: an etiologic factor in Peyronie’s disease and erectile dysfunction. J Urol. 1997;158(4):1388–1390. | ||
Devine CJ Jr, Somers KD, Jordan SG, Schlossberg SM. Proposal: trauma as a cause of Peyronie’s lesion. J Urol. 1997;157(1):285–290. | ||
Campbell J, Alzubaidi R. Understanding the cellular basis and pathophysiology of Peyronie’s disease to optimize treatment for erectile dysfunction. Transl Androl Urol. 2017;6(1):46–59. | ||
Garaffa G, Trost LW, Serefoglu EC, Ralph D, Hellstrom WJ. Understanding the course of Peyronie’s disease. Int J Clin Pract. 2013;67(8):781–788. | ||
Levine LA, Larsen S. Diagnosis and management of Peyronie disease. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 11th ed. Philadelphia: Elsevier Saunders; 2015:722–748. | ||
Hatzimouratidis K, Giuliano F, Moncada I, et al; European Association of Urology]. EAU Guidelines on Erectile Dysfunction, Premature Ejaculation, Penile Curvature and Priapism (Peyronie’s Disease Management, non-operative treatment, paragraph 3.3.2.3.1 p.34); 2016. Available from: https://uroweb.org/wp-content/uploads/EAU-Extended-Guidelines-2016-Edn.pdf. Accessed February 26, 2017. | ||
Hellstrom WJ, Bivalacqua TJ. Peyronie’s disease: etiology, medical, and surgical therapy. J Androl. 2000;21(3):347–354. | ||
Hauck EW, Diemer T, Schmelz HU, Weidner W. A critical analysis of nonsurgical treatment of Peyronie’s disease. Eur Urol. 2006;49(6):987–997. | ||
Gelbard M, Goldstein I, Hellstrom WJ, et al. Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of Peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol. 2013;190(1):199–207. | ||
Martínez-Salamanca JI, Egui A, Moncada I, et al. Acute phase Peyronie’s disease management with traction device: a nonrandomized prospective controlled trial with ultrasound correlation. J Sex Med. 2014;11(2):506–515. | ||
Rajasekaran M, Hellstrom WJ, Sikka SC. Nitric oxide induces oxidative stress and mediates cytotoxicity to human cavernosal cells in culture. J Androl. 2001;22(1):34–39. | ||
Sikka SC, Hellstrom WJ. Role of oxidative stress and antioxidants in Peyronie’s disease. Int J Impot Res. 2002;14(5):353–360. | ||
Gonzalez-Cadavid NF, Magee TR, Ferrini M, Qian A, Vernet D, Rajfer J. Gene expression in Peyronie’s disease. Int J Impot Res. 2002;14(5):361–374. | ||
Moreland RB, Nehra A. Pathophysiology of Peyronie’s disease. Int J Impot Res. 2002;14(5):406–410. | ||
Bivalacqua TJ, Champion HC, Hellstrom WJ. Implications of nitric oxide synthase isoforms in the pathophysiology of Peyronie’s disease. Int J Impot Res. 2002;14(5):345–352. | ||
Davila HH, Magee TR, Vernet D, Rajfer J, Gonzalez-Cadavid NF. Gene transfer of inducible nitric oxide synthase complementary DNA regresses the fibrotic plaque in an animal model of Peyronie’s disease. Biol Reprod. 2004;71(5):1568–1577. | ||
Gonzalez-Cadavid NF, Rajfer J. Mechanisms of disease: new insights into the cellular and molecular pathology of Peyronie’s disease. Nat Clin Pract Urol. 2005;2(6):291–297. | ||
El-Sakka AI, Salabas E, Dinçer M, Kadioglu A. The pathophysiology of Peyronie’s disease. Arab J Urol. 2013;11(3):272–277. | ||
Lemourt Oliva M, Filgueiras López E, Rodríguez Barroso A, González Oramas E, Bordonado R. Clinical evaluation of the use of propoleum in Peyronie’s disease. Arch Esp Urol. 1998;51(2):171–176. | ||
Lemourt Oliva M, Fragas Valdés R, Bordonado Ramírez R, Santana JL, González Oramas E, Merino A. Peyronie’s disease. Evaluation of 3 therapeutic modalities: propoleum, laser and simultaneous propoleum-laser. Arch Esp Urol. 2005;58(9):931–935. | ||
Cavallini G, Biagiotti G, Koverech A, Vitali G. Oral propionyl-l-carnitine and intraplaque verapamil in the therapy of advanced and resistant Peyronie’s disease. BJU Int. 2002;89(9):895–900. | ||
Biagiotti G, Cavallini G. Acetyl-L-carnitine vs tamoxifen in the oral therapy of Peyronie’s disease: a preliminary report. BJU Int. 2001;88(1):63–67. | ||
Lin G, Shindel AW, Banie L, et al. Pentoxifylline attenuates transforming growth factor-beta1-stimulated elastogenesis in human tunica albuginea-derived fibroblasts part 2: interference in a TGF-beta1/Smad-dependent mechanism and downregulation of AAT1. J Sex Med. 2010;7(5):1787–1797. | ||
Shindel AW, Lin G, Ning H, et al. Pentoxifylline attenuates transforming growth factor-β1-stimulated collagen deposition and elastogenesis in human tunica albuginea-derived fibroblasts part 1: impact on extracellular matrix. J Sex Med. 2010;7(6):2077–2085. | ||
Safarinejad MR. Safety and efficacy of coenzyme Q10 supplementation in early chronic Peyronie’s disease: a double-blind, placebo-controlled randomized study. Int J Impot Res. 2010;22(5):298–309. | ||
Brant WO, Dean RC, Lue TF. Treatment of Peyronie’s disease with oral pentoxifylline. Nat Clin Pract Urol. 2006;3(2):111–115. | ||
Smith JF, Shindel AW, Huang YC, et al. Pentoxifylline treatment and penile calcifications in men with Peyronie’s disease. Asian J Androl. 2011;13(2):322–325. | ||
Dell’Atti L, Ughi G. Efficacy of pentoxifylline in Peyronie’s disease: clinical case of a young man. Arch Ital Urol Androl. 2014;86(3):237–238. | ||
Ciociola F, Colpi GM. Peyronie’s disease: a “triple oxygenant therapy”. Arch Ital Urol Androl. 2013;85(1):36–40. | ||
Alizadeh M, Karimi F, Fallah MR. Evaluation of verapamil efficacy in Peyronie’s disease comparing with pentoxifylline. Glob J Health Sci. 2014;6(7 Spec No):23–30. | ||
Favilla V, Russo GI, Privitera S, et al. Combination of intralesional verapamil and oral antioxidants for Peyronie’s disease: a prospective, randomised controlled study. Andrologia. 2014;46(8):936–942. | ||
Paulis G, D’Ascenzo R, Nupieri P, et al. Effectiveness of antioxidants (propolis, blueberry, vitamin E) associated with verapamil in the medical management of Peyronie’s disease: a study of 151 cases. Int J Androl. 2012;35(4):521–527. | ||
Paulis G, Cavallini G, Brancato T, Alvaro R. Peironimev-Plus® in the treatment of chronic inflammation of tunica albuginea (Peyronie’s disease). Results of a controlled study. Inflamm Allergy Drug Targets. 2013;12(1):61–67. | ||
Paulis G, Cavallini G, Giorgio GD, Quattrocchi S, Brancato T, Alvaro R. Long-term multimodal therapy (verapamil associated with propolis, blueberry, vitamin E and local diclofenac) on patients with Peyronie’s disease (chronic inflammation of the tunica albuginea). Results of a controlled study. Inflamm Allergy Drug Targets. 2013;12(6):403–409. | ||
Paulis G, Barletta D, Turchi P, et al. Efficacy and safety evaluation of pentoxifylline associated with other antioxidants in medical treatment of Peyronie’s disease: a case-control study. Res Rep Urol. 2015;8:1–10. | ||
Abern MR, Larsen S, Levine LA. Combination of penile traction, intralesional verapamil, and oral therapies for Peyronie’s disease. J Sex Med. 2012;9(1):288–295. | ||
Lamprakopoulos A, Zorzos I, Lykourinas M. The use of betamethasone and yaluronidase injections in the treatment of Peyronie’s disease. Scand J Urol Nephrol. 2000;34(6):355–360. | ||
Prieto Castro RM, Leva Vallejo ME, Regueiro Lopez JC, Anglada Curado FJ, Alvarez Kindelan J, Requena Tapia MJ. Combined treatment with vitamin E and colchicine in the early stages of Peyronie’s disease. BJU Int. 2003;91(6):522–524. | ||
Candebat Montero LH, Miranda Reyes PL, Díaz García F, González Ferro I, Barbosa Ramos F, Codorniu Furet J. Peyronie’s disease: treatment with interferon and laser. Arch Esp Urol. 2008;61(3):413–423. Spanish. | ||
Cakan M, Demirel F, Aldemir M, Altug U. Does smoking change the efficacy of combination therapy with vitamin E and colchicines in patients with early-stage Peyronie’s disease? Arch Androl. 2006;52(1):21–27. | ||
Park TY, Jeong HG, Park JJ, et al. The efficacy of medical treatment of Peyronie’s disease: potassium para-aminobenzoate monotherapy vs. combination therapy with tamoxifen, L-carnitine, and phosphodiesterase type 5 inhibitor. World J Mens Health. 2016;34(1):40–46. | ||
Taylor FL, Levine LA. Non-surgical therapy of Peyronie’s disease. Asian J Androl. 2008;10(1):79–87. | ||
Kuehhas FE, Weibl P, Georgi T, Djakovic N, Herwig R. Peyronie’s disease: nonsurgical therapy options. Rev Urol. 2011;13(3):139–146. | ||
Larsen SM, Levine LA. Review of non-surgical treatment options for Peyronie’s disease. Int J Impot Res. 2012;24(1):1–10. | ||
Halal AA, Geavlete P, Ceban E. Pharmacological therapy in patients diagnosed with Peyronie’s disease. J Med Life. 2012;5(2):192–195. | ||
Levine LA. Peyronie’s disease: contemporary review of non-surgical treatment. Transl Androl Urol. 2013;2(1):39–44. | ||
Pastorini S, Marino G, Brigato R, Griffa D, Cocimano V. The therapy of plastic penile induration using superoxide dismutase per os and injection combined with vasoactive intracavernous pharmacotherapy. Minerva Urol Nefrol. 1991;43(2):75–78. Italian. | ||
Hellstrom WJ, Feldman R, Rosen RC, Smith T, Kaufman G, Tursi J. Bother and distress associated with Peyronie’s disease: validation of the Peyronie’s disease questionnaire. J Urol. 2013;190(2):627–634. | ||
Behre HM, Zitzmann M. Imaging diagnostics. In: Nieschlag E, Behre HM, Nieschlag S, editors. Andrology: Male Reproductive Health and Dysfunction. Berlin: Springer-Verlag; 2010:101–108. | ||
Kim CK, Cho JY. Prostate. In: Kim SH, editor. Radiology Illustrated: Uroradiology. Berlin: Springer-Verlag; 2012:826. | ||
Costantini TW, Deree J, Peterson CY, et al. Pentoxifylline modulates p47phox activation and downregulates neutrophil oxidative burst through PKA-dependent and -independent mechanisms. Immunopharmacol Immunotoxicol. 2010;32(1):82–91. | ||
Freitas JP, Filipe PM. Pentoxifylline. A hydroxyl radical scavenger. Biol Trace Elem Res. 1995;47(1–3):307–311. | ||
Sunil VR, Vayas KN, Cervelli JA, et al. Pentoxifylline attenuates nitrogen mustard-induced acute lung injury, oxidative stress and inflammation. Exp Mol Pathol. 2014;97(1):89–98. | ||
Crouch SP, Fletcher J. Effect of ingested pentoxifylline on neutrophil superoxide anion production. Infect Immun. 1992;60(11):4504–4509. | ||
Gavella M, Lipovac V. Pentoxifylline-mediated reduction of superoxide anion production by human spermatozoa. Andrologia. 1992;24(1):37–39. | ||
Ji Q, Zhang L, Jia H, Xu J. Pentoxifylline inhibits endotoxin-induced NF-kappa B activation and associated production of proinflammatory cytokines. Ann Clin Lab Sci. 2004;34(4):427–436. | ||
Anaya JM, Espinoza LR. Phosphodiesterase inhibitor pentoxifylline: an antiinflammatory/immunomodulatory drug potentially useful in some rheumatic diseases. J Rheumatol. 1995;22(4):595–599. | ||
Russo A, Longo R, Vanella A. Antioxidant activity of propolis: role of caffeic acid phenethyl ester and galangin. Fitoterapia. 2002;73(Suppl 1): S21–S29. | ||
Wang X, Gong G, Yang W, Li Y, Jiang M, Li L. Antifibrotic activity of galangin, a novel function evaluated in animal liver fibrosis model. Environ Toxicol Pharmacol. 2013;36(2):288–295. | ||
Min YD, Choi CH, Bark H, et al. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-kappaB and p38 MAPK in HMC-1 human mast cell line. Inflamm Res. 2007;56(5):210–215. | ||
Nakamura T, Matsushima M, Hayashi Y, et al. Attenuation of transforming growth factor-β-stimulated collagen production in fibroblasts by quercetin-induced heme oxygenase-1. Am J Respir Cell Mol Biol. 2011;44(5):614–620. | ||
Rahman MM, Ichiyanagi T, Komiyama T, Hatano Y, Konishi T. Superoxide radical- and peroxynitrite-scavenging activity of anthocyanins; structure-activity relationship and their synergism. Free Radic Res. 2006;40(9):993–1002. | ||
Hou DX, Yanagita T, Uto T, Masuzaki S, Fujii M. Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: structure-activity relationship and molecular mechanisms involved. Biochem Pharmacol. 2005;70(3):417–425. | ||
Godbout JP, Berg BM, Krzyszton C, Johnson RW. Alpha-tocopherol attenuates NFkappaB activation and pro-inflammatory cytokine production in brain and improves recovery from lipopolysaccharide-induced sickness behavior. J Neuroimmunol. 2005;169(1–2):97–105. | ||
Haas AL, Boscoboinik D, Mojon DS, Böhnke M, Azzi A. Vitamin E inhibits proliferation of human Tenon’s capsule fibroblasts in vitro. Ophthalmic Res. 1996;28(3):171–175. | ||
Fazzio A, Marilley D, Azzi A. The effect of alpha-tocopherol and beta-tocopherol on proliferation, protein kinase C activity and gene expression in different cell lines. Biochem Mol Biol Int. 1997;41(1):93–101. | ||
Paulis G, Brancato T, D’Ascenzo R, et al. Efficacy of vitamin E in the conservative treatment of Peyronie’s disease: legend or reality? A controlled study of 70 cases. Andrology. 2013;1(1):120–128. | ||
Cho JW, Il KJ, Lee KS. Downregulation of type I collagen expression in silibinin-treated human skin fibroblasts by blocking the activation of Smad2/3-dependent signaling pathways: potential therapeutic use in the chemoprevention of keloids. Int J Mol Med. 2013;31(5):1148–1152. | ||
Surai PF. Silymarin as a natural antioxidant: an overview of the current evidence and perspectives. Antioxidants (Basel). 2015;4(1):204–247. | ||
Kim BR, Seo HS, Ku JM, et al. Silibinin inhibits the production of pro-inflammatory cytokines through inhibition of NF-κB signaling pathway in HMC-1 human mast cells. Inflamm Res. 2013;62(11):941–950. | ||
Rong Y, Geng Z, Lau BH. Ginkgo biloba attenuates oxidative stress in macrophages and endothelial cells. Free Radic Biol Med. 1996;20(1):121–127. | ||
Huang CH, Yang ML, Tsai CH, Li YC, Lin YJ, Kuan YH. Ginkgo biloba leaves extract (EGb 761) attenuates lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and NF-κB-dependent matrix metalloproteinase-9 pathway. Phytomedicine. 2013;20(3–4):303–309. | ||
Yao X, Chen N, Ma CH, et al. Ginkgo biloba extracts attenuate lipopolysaccharide-induced inflammatory responses in acute lung injury by inhibiting the COX-2 and NF-κB pathways. Chin J Nat Med. 2015;13(1):52–58. | ||
Liu SQ, Yu JP, He L, Yu HG, Luo HS. Effects of nuclear factor kappaB and transforming growth factor beta1 in the anti-liver fibrosis process using Ginkgo biloba extract. Zhonghua Gan Zang Bing Za Zhi. 2005;13(12):903–907. Chinese. | ||
Li EG, Tian J, Xu ZH. Effects of Gingko biloba extract (EGb 761) on vascular smooth muscle cell calcification induced by β-glycerophosphate. Ren Fail. 2016;38(4):552–557. | ||
Maffei Facino R, Carini M, Aldini G, Saibene L, Macciocchi A. Antioxidant profile of nimesulide, indomethacin and diclofenac in phosphatidylcholine liposomes (PCL) as membrane model. Int J Tissue React. 1993;15(6):225–234. | ||
Rojo C, Álvarez-Figueroa MJ, Soto M, Cañete A, Pessoa-Mahana D, López-Alarcón C. Scavenging activity of diclofenac. Interaction with ABTS radical cation and peroxyl radicals. J. Chil Chem Soc. 2009; 54(1):58–62. | ||
Tang YZ, Liu ZQ. Evaluation of the free-radical-scavenging activity of diclofenac acid on the free-radical-induced haemolysis of human erythrocytes. J Pharm Pharmacol. 2006;58(5):625–631. | ||
Nguyen KD, Lee DA. In vitro evaluation of antiproliferative potential of topical cyclooxygenase inhibitors in human Tenon’s fibroblasts. Exp Eye Res. 1993;57(1):97–105. | ||
Efe T, Sagnak E, Roessler PP, et al. Penetration of topical diclofenac sodium 4% spray gel into the synovial tissue and synovial fluid of the knee: a randomised clinical trial. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):345–350. | ||
Wadsworth LT, Kent JD, Holt RJ. Efficacy and safety of diclofenac sodium 2% topical solution for osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled, 4 week study. Curr Med Res Opin. 2016;32(2):241–250. | ||
Paulis G. Combination therapy (in the treatment of Peyronie’s disease). In: Cavallini G, Paulis G, editors. Peyronie’s Disease. A Comprehensive Guide. Switzerland: Springer International Publishing; 2015:97–105. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.