Back to Journals » International Journal of General Medicine » Volume 7
Rationale and design of the Miami Healthy Heart Initiative: a randomized controlled study of a community health worker intervention among Latino patients with poorly controlled diabetes
Authors Carrasquillo O, Patberg E, Alonzo Y, Li H, Kenya S
Received 19 October 2013
Accepted for publication 23 November 2013
Published 27 February 2014 Volume 2014:7 Pages 115—126
DOI https://doi.org/10.2147/IJGM.S56250
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Olveen Carrasquillo,1,2 Elizabeth Patberg,1 Yisel Alonzo,1 Hua Li,2 Sonjia Kenya1
1Department of Medicine, 2Public Health Sciences, University of Miami, Miller School of Medicine, Miami, FL, USA
Background: Type 2 diabetes mellitus disproportionately affects the Latino community. Latinos with diabetes are also less likely to have adequate control of cardiovascular risk factors such as cholesterol and blood pressure. Community health workers (CHWs) are increasingly being used to address various health disparity conditions, including diabetes. However, evidence of their effectiveness from randomized controlled trials is limited.
Methods: The Miami Health Heart Initiative is a randomized controlled trial of 300 Latino patients with diabetes. Patients with hemoglobin A1c (HbA1c) ≥8.0% were recruited from Miami-Dade's public hospital system. At baseline, all patients underwent phlebotomy, physical examination, and a structured 90-minute research interview. They were then randomized to either usual care or a CHW intervention called Cariño. For participants in the Cariño arm of the study, CHW services included assistance with nonmedical social services, health education, and patient navigation in which the CHWs serve as a bridge between patients and the health care system. These services were delivered through home visits, phone calls, and group visits. At 12 months, all subjects had a follow-up examination. The primary outcomes at 1 year are changes in systolic blood pressure, low-density lipoprotein, and HbA1c. Secondary outcomes include medication adherence, medication intensification, diabetes self-efficacy, physical activity, and self-reported fruit and vegetable intake.
Discussion: The Miami Healthy Heart Initiative is one of the first rigorously conducted randomized controlled trials to provide evidence on the impact of CHWs on diabetes intermediate outcomes among Latinos. If the data support our primary hypotheses, the study would lend added support to ongoing efforts to incorporate CHWs as part of our national efforts to reduce and ultimately eliminate health disparities.
Keywords: Hispanic, type II diabetes, health care support, community health workers, randomized trial, health care disparities
Background
Type II diabetes affects one in ten adults over the age of 45 years.1 With increasing prevalence of obesity, rates of diabetes among the US population are expected to continue to increase. Control of blood pressure and dyslipidemia in patients with diabetes markedly reduces their risk of cardiovascular complications.2,3 In addition, glycemic control reduces the risk of microvascular complications such as retinopathy and nephropathy.4,5 Achieving optimum management of these conditions often requires coordination of health care service delivery among various providers, lifestyle modifications, and adherence to several concurrent medications, making diabetes a difficult chronic disease to manage.6 For these reasons, less than half of all diabetic patients meet their combined targets for blood pressure, low-density lipoprotein (LDL), and hemoglobin A1c (HbA1c).7,8
Members of racial and ethnic minorities are at increased risk from the burden of diabetes, and often suffer disproportionately from diabetes-related complications. Among Latinos (a term we use interchangeably with Hispanics), the age-adjusted prevalence of diabetes is about twice that of non-Hispanic whites.1,9 Management of diabetes in this higher risk group is often further complicated by additional barriers to care, including lower rates of health insurance coverage, socioeconomic challenges, immigration-related issues, and other cultural, linguistic, and health literacy challenges. Thus, it is increasingly recognized that addressing the excess burden of diabetes in this population requires multifaceted, culturally sensitive interventions.
To date, one of the most promising interventions to improve care of Latino diabetics involves using community health workers (CHWs). CHWs are typically individuals without formalized professional health training who come from the community settings where patients reside.10 Their role is to provide health education in culturally and linguistically appropriate ways, help patients access effective health care by functioning as patient navigators, and assisting with nonmedical barriers such as social issues. Among Latino diabetics, CHWs have been shown to improve diabetes knowledge and healthy behaviors.11–16 However, methodologically rigorous, randomized studies examining physiologic outcomes like blood pressure, lipids, and glycemic control are more limited.
Our review of the peer-reviewed literature found six randomized controlled trials of CHW interventions conducted in the United States among Latinos with Type II diabetes in which HbA1c was as an outcome. (Table 1).17–22 Few included LDL and systolic blood pressure (SBP) as outcomes, and only three showed statistically significant improvements.17,21,22 The interventions in these studies varied, and the population studied was mostly Latinos of Mexican origin. Further, in most of these studies, CHWs were part of a broader health care intervention team. This often included not only CHWs but also licensed health professionals such as nurse practitioners, nurses, and dietitians as part of the intervention team being tested. In many cases, these health professionals played an active role in case management, including medication management. To date, few studies have examined CHWs as a stand-alone intervention.
In order to increase the evidence base with respect to CHWs and diabetes outcomes, the Miami Healthy Heart Initiative (MHHI) is conducting a randomized controlled trial of 300 Latinos with poorly controlled Type II diabetes. The study is examining the impact of a CHW intervention on cardiovascular risk factors of blood pressure and LDL levels as well as glycemic control (HbA1c). The randomized study includes two arms. One is the comparison group, which involves enhanced usual care. The other is the intervention arm of the study, known as Cariño. Unlike other CHW studies, our study is designed to examine the independent impact of a CHW. Thus, Cariño does not include health professionals as a part of the experimental intervention.
Methods
Community setting
Initially, the study was to be conducted in New York City. However, soon after the award was made, the principal investigator moved to Miami. With National Heart, Lung, and Blood Institute (NHLBI) approval, the study site was changed from New York City to this new location. Miami-Dade County provides an ideal laboratory in which to conduct research among diverse Latino subgroups. In the county, 65% of residents are Hispanic.23 Whereas 30 years ago, Cubans made up over 70% of Miami Hispanics,24 over the last 20 years a rapid influx of Latinos from most regions of Latin America now make Miami one of the most diverse Latino communities in the US. The 2010 Census showed only half the Latinos in the county are Cuban, 17% are South American, 15% Central American, and 7% Puerto Rican.25 Accordingly, Miami’s large multiethnic Latino population is well suited to testing interventions targeting our nation’s increasingly diverse and rapidly growing Latino population.26
Health care setting
All of the patients in the MHHI were recruited from the Jackson Health Care System (JHS). JHS is Miami-Dade County’s public hospital system, which provides health care for over a million medically indigent patients, the majority of whom are uninsured. The system has a US$1.8 billion budget, of which more than US$700 million is spent on charity care.27 Study participants were recruited from two outpatient primary care clinics, both affiliated with JHS. At these two clinics, care is provided by internal medicine or family medicine trainees (residents and fellows) under the supervision of faculty from the University of Miami.
Inclusion criteria
To qualify for MHHI, study patients had to be between 35–70 years of age, self-identify as Hispanic/Latino, have visited the clinic twice within the last year for diabetes care, and had their most recent HbA1c measuring ≥8.0%.
Exclusion criteria
Patients were excluded from the study if they were diagnosed with diabetes at younger than 25 years of age, self-reported being a type 1 diabetic, had been diagnosed with diabetes less than 1 year prior, or had a life-threatening comorbidity such as an active cancer diagnosis. Additionally, subjects could not be participating in another intervention study (or live with someone who is), plan to be away from the country for more than 3 months in the next year, plan to move away from the county within the next year, or be unable to communicate in English or Spanish.
Recruitment
Our recruitment strategy was twofold. First, using JHS’s electronic medical records (EMR; Cerner, North Kansas City, MO, USA), we obtained a list of age-eligible patients having had at least two visits to the JHS ambulatory clinics with a diagnosis of diabetes and the most recent laboratory result for HbA1c being greater than or equal to 8.0%. From this list, patients having Hispanic surnames were selected. Using contact information in the EMR, we then sent a recruitment letter to potential participants explaining the nature of the study. We also provided them with a phone number they could call if they did not wish to participate in the study (patient opt-out). Two weeks after the letters were mailed, the study research assistant (RA) made up to ten attempts at different times and days to contact potentially eligible subjects via telephone. At the call we confirmed receipt of the letter and described the study in more detail, including time commitment, compensation, and obligations. If the patient was interested, the RA conducted a brief inclusion/exclusion screen to determine if the subject met eligibility criteria, including Latino ethnicity. If the patient was eligible, an appointment was scheduled for an in-person enrollment visit at the University’s Clinical Research Center (CRC).
The second recruitment strategy consisted of sending letters to providers and clinic medical directors informing them of the study and seeking direct referrals. In addition, through presentations at medical school faculty meetings and university conferences, we also informed providers and medical staff about MHHI. We also placed study flyers and brochures in the clinic with information on MHHI and how providers could refer patients. If the RA was referred a patient by the provider while they were still in the clinic, the RA performed an inclusion screen on site. Otherwise the RA followed-up with the patient through phone calls using the above protocols.
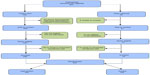
The final recruitment breakdown is depicted in Figure 1. We attempted to contact 863 patients, of whom 794 were identified through the EMR electronic search and 69 were referred to us from providers. Of the 794, no patients called to notify us that they did not wish to be part of the study. We were not able to establish contact with 230 of the 794 patients. Of the 564 patients whom we were able to speak with, 354 met study criteria; 266 of these were randomized into the study. Of the 69 subjects referred to us by providers, we were able to contact 61, of whom 34 were randomized. Overall, among the 395 eligible study subjects, we were able to conduct a complete baseline assessment at the CRC and randomize 76%. Recruitment for the study was completed in November 2012 when we had enrolled our planned 300 patients.
Consent and enrollment
Qualifying patients were formally enrolled in the study at the CRC during an in-person visit with an RA who was blinded to participant treatment status. Participants were instructed to fast for 12 hours prior to the study interview, withhold diabetes medications in the morning but take all other medications as usual and bring in bottles containing all of their prescribed medications. At the CRC, the study was again explained in detail to participants and informed consent was obtained. The study protocol was approved by the University of Miami Institutional Review Board (Protocol #20090751). The study was also registered in the ClinicalTrials.gov registry.
Primary outcomes
After informed consent, data on the three primary outcomes, SBP, LDL, and HbA1c, were collected. For blood pressure, three readings were taken according to American Heart Association guidelines28 using the Omron HEM -705CP automated oscillometric device (Lake Forest, IL, USA). For LDL and HbA1c, a certified phlebotomist obtained 30 cc of blood. Of this, 10 cc was spun and delivered to the University of Miami Diabetes Research Institute for lipid profile and HbA1c analysis. HbA1c determination was performed using latex agglutination (D-10; Bio-Rad, Hercules, CA, USA) and lipid profile using the Cobas c501 from Roche (Indianapolis, IN, USA). LDL was estimated using the Friedewald equation.29 For patients with triglycerides >400 mg/dL, direct LDL was measured using the ultracentrifugation beta quantification method.
The other 20 cc of blood was labeled and processed for long-term storage at the University of Miami freezer farm (−80°C) for up to 5 years. All 300 patients in the study agreed to the storage of these specimens using identifiers that could not be linked back to individual subjects. A separate consent form was used to obtain permission allowing these samples to be used for future nongenetic studies. After phlebotomy, participants were allowed to eat a snack and take their diabetes medications. They then underwent a 90-minute structured research interview in Spanish or English. The RA also reviewed the medications brought in by the participants and wrote their names, dose, and frequency on a participant medication sheet. Insulin type, dosage, and frequency were recorded. At 12 months, participants returned for similar evaluation (see Table 2 for list of items included in initial and 12-month survey). For their time, effort, and transportation expenses, participants receive US$40 compensation at each of the two visits.
Secondary outcomes
In selecting which measures to include as part of our baseline intake survey, a primary goal was obtaining data that may help elucidate the impact and pathways of the various components of our intervention. Given existing concepts of factors that contribute to successful blood pressure, cholesterol, and glycemic control among patients with diabetes,6 we theorized that the CHW intervention would be successful in improving these physiologic measures through two primary pathways.
One potential pathway was through medications. This could be achieved by having patients increase their adherence to prescribed medication. Improved blood pressure, cholesterol, and glycemic control could also be achieved if a patient’s provider increased the dosage or number of medications being used to treat one of these conditions (medication intensification). Thus, we included measures of medication adherence and medication intensification as secondary outcomes. Self-reported medication adherence was based on the four item Morisky Medication Adherence Scale (MMAS), which solicits information regarding situational factors that interfere with adherence (eg, forgetfulness, not remembering to bring medications along when out of town).30 Medication intensification was assessed by having two physicians, blinded to study allocation, independently review the medication form listing all medications each participant was taking at baseline and then at 1 year. The physician reviewers used implicit criteria to decide if there was evidence of medication intensification for blood pressure, cholesterol, or diabetes and recorded these in a spreadsheet. Results were then compared and when discrepancies occurred, both physicians discussed these cases to reach consensus.
The second pathway through which we theorized blood pressure, cholesterol, and diabetes could be better controlled was through lifestyle changes, primarily diet and exercise. For diet, several detailed instruments exist that allow for precise assessments of changes in dietary composition, such as 24-hour food frequency questionnaires. However, using these lengthy instruments would have considerably increased the length of our interview. As our goal was to detect any major changes in diet, for this secondary outcome we chose to use the Centers for Disease Control (CDC)’s Behavioral Risk Factor Surveillance System (BRFSS) Fruit and Vegetable Questionnaire.31 The BRFSS instrument has been used in many diverse population groups, is concise, and would not substantially add to respondent burden. For exercise, we measured self-reported physical activity. We used the International Physical Activity Questionnaire, which is a widely used instrument that has been validated in Latino populations.32
Potential confounding variables
In the survey, we also included variables that, if not equally balanced across both groups through randomization, may be potential confounders of primary and secondary outcomes. These included baseline sociodemographics such as age, sex, income, educational attainment, and citizenship (we did not ask about undocumented status). We expect that these variables will be balanced between study arms through the randomization process. However, if that does not occur, we may need to adjust for these confounders when performing our analysis. Other potential confounders we included were depression, acculturation, and health literacy. We measured depression using the EURO-D scale,33 which has been validated in Latinos. For acculturation, we used a questionnaire based on the modified Marin–Marin scale that is used in the National Health Interview Survey.34 Health literacy was assessed using the Short Assessment of Health Literacy– Spanish and English (SAHL- S&L) 18 word recognition instrument, which has comparable psychometrics in both Spanish and English.35 Body mass index could also be a potential confounder. To assess body mass index, the RA measured participant weight and height with no shoes and light clothing using a Genentech Stadiometer and Platform Scale (South San Francisco, CA, USA) at the baseline and exit interviews. A summary of the outcomes and covariates measured in this MHHI is shown in Table 2.
Randomization
Once the initial intake was completed, a blinded statistician randomized all newly enrolled subjects in a 1:1 ratio to the Cariño arm or Enhanced Usual Care (EUC). Participants were stratified by sex during randomization to ensure that males and females were equally distributed across the two arms of the intervention. The study research coordinator kept track of participant assignment and informed the CHW coordinator when new participants had been assigned to the intervention arm. At the 12-month follow-up, the RA doing the assessment was instructed she could not ask patients to which group they belonged. Participants were also instructed not to inform the RA of their allocation status at the 12-month follow-up visit.
Data management
Data were collected using a paper survey instrument. Initially, two RAs independently entered the data, which was cross-checked for consistency. Data were entered using the Velos data management system (Fremont, CA, USA).36 After adequate consistency was verified, data entry was done by one RA and cross-checking was reduced to every tenth entry. Later, 2 years after the study began, data entry was switched from paper to direct computer-assisted data entry.
Comparison group
The EUC arm of the study included periodic mailings and retention phone calls. At months 1, 3, 6, and 9 EUC participants were sent health education brochures from the National Institutes of Health (NIH) and CDC on different aspects of diabetes self-care and management.37–40 Approximately 2 weeks after each mailing, the research coordinator made up to ten attempts to contact the participant to ensure receipt of delivery and verify continued participation in the study. Finally, at month 11, an RA sent a reminder postcard to all participants in order to facilitate scheduling of an exit interview at month 12.
Cariño
Conceptual framework guiding Cariño
Our intervention was informed by contemporary models of successful interventions aimed at improving health among racially and ethnically diverse communities.41 Within this framework are important elements of the chronic care model, including self-management support, clinical information systems, delivery system redesign, decision support, health care organization, and community resources.42,43 Our approach was also guided by the fact that as people spend almost all of their time residing within their communities, interventions that focus solely on care delivered within traditional health care systems may have a limited impact. In our framework, the patient’s community and environment, encompassing everything from governmental policies to community and health care organizations, are considered as levers to be addressed as part of a comprehensive intervention. For this reason, Cariño is primarily focused on community-level interventions in which the CHWs successfully link persons in underserved settings with approaches that can effectively improve their overall health.
Selection and training of CHWs
In selecting CHWs for Cariño, priority was given to candidates who possessed in-depth knowledge of, and respect for, diverse Latino populations in Miami-Dade. Whenever possible, CHWs were chosen who were familiar with, or came from, similar communities in which they would be working. All three Cariño CHWs had a bachelor’s degree, but none had any advanced health professions training or licensure to practice in a health care field. These three CHWs were employed through the University of Miami’s Jay Weiss Center for Social Medicine and Health Equity. They were not part of JHS. Before working with clients, CHWs completed a 75-hour training curriculum. This course includes basic research training on clinical practice and human subjects (20 hours), diabetes clinical and behavioral interventions (20 hours), and CHW-specific skills (35 hours). An important training resource was the NHLBI CHW training manual.44 Another was the CDC CHW Training Manual for Heart Disease and Stroke.45 CHW trainees also attended five client home visits with another CHW before receiving their initial caseload. Ongoing periodic CHW training on issues such as CHW skills, clinic and insurance navigation, cardiovascular disease and diabetes care, and human subjects occurred on average about every 2 months.
Service delivery
In Cariño, the CHWs were supervised by a CHW coordinator who was a master’s level health professional. The coordinator assigned newly randomized patients to a CHW based on his/her current caseload and geographic service area. Throughout the intervention period, the CHW coordinator monitored study fidelity and service intensity with individual meetings with CHWs on a weekly basis to review their daily activity logs and pathway action plans, and to help the CHWs with issues affecting individual patients. The coordinator also organized monthly continuing education meetings among all the CHWs, helped troubleshoot issues of the CHW program, and arranged for educational sessions by the MMHI investigators as needed.
Within 48 hours of being assigned a new client, the CHW contacted the participant to schedule the first of four intake visits. These visits comprised the “enactment phase” of Cariño which took place during months 1–2. Ideally, the first home visit needed to occur within 1 month of randomization. Through open-ended questions, the CHW conducted an assessment of the participant’s social context, current knowledge about diabetes, and its management, and views on medication adherence, including barriers or facilitators to adherence and pertinent lifestyle and health behaviors. Information was also sought on barriers to communication with providers and health care staff, and in interacting with the health care system. The second to fourth intake visits addressed goal setting and dealing with barriers. During these visits, the CHW’s efforts included: stimulating self-management by teaching problem-solving skills, facilitating navigation of the health care system, providing referrals to or assistance in accessing both social and medical community-based resources, and giving counseling and coaching aimed at the improvement of lifestyle behaviors.
During this enactment phase of Cariño, the CHW met face-to-face with the client ideally at least twice. He/she helped clients who were uninsured, undocumented, and/or financially challenged to obtain health care and assisted with access to low-cost medications and medical services at reduced cost. The CHWs were also encouraged to attend medical appointments during this phase to focus on developing communication and advocacy skills between patient and provider. Also during this period, the CHW provided tailored information to the participant using various cardiovascular disease and diabetes education curricula. These included resources from the NIH and CDC.37–40 The educational materials and service referrals were tailored to each client; CHWs were encouraged to modify standardized protocols in order to best meet individual needs, while staying within the guidelines of service intensity.
After the 2-month Cariño enactment phase, participants entered the “maintenance phase” (months 3–12). During this period, fewer face-to-face contacts were envisioned, although adjustments were allowed to meet participants’ needs. In this period, the bulk of CHW support was shifted to telephone communication, with weekly or biweekly calls to the client, and group sessions. Diabetes education classes, led by CHWs, met every month, while group exercise classes, such as walking groups, met every 2 weeks. CHWs were responsible for communicating with their assigned participants before each group session to encourage them to attend.
During months 10–12, CHW focus was on educating clients about community resources that could be accessed after program participation was complete, ensuring participants had access to ongoing diabetes medical assistance, and reiterating diabetes education based on clients’ individual needs. At the end of month 12, CHWs conducted a final face-to-face visit and exit interview to review accomplishments clients had made during the intervention. Group graduation ceremonies were scheduled annually, in which intervention participants received a certificate of participation and gift bag. An overview of the phases of the 12 month Cariño is provided in Figure 2.
  | Figure 2 Overview of the phases of the 1-year Cariño CHW Intervention. |
Sample size and statistical power
In the grant proposal that was originally submitted to the NHLBI, we had proposed that the Framingham Risk Factor Score (FRFS) would be our primary outcome. For adequate power, this would have required a sample size of 800 persons. However, we received funding for 4 years instead of 5 years. This substantially reduced the amount of time we would have to recruit participants. Given this, we decided to change our primary outcome from the FRFS to individual modifiable components of FRFS, namely blood pressure and LDL. As these are linear continuous measures, even with a smaller sample of 300 participants, we would still have adequate power for these outcomes, as shown below. In addition, as our study was being conducted among diabetics, we also included HbA1c as a third primary outcome.
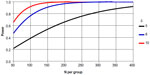
Our primary hypothesis was that patients randomized to the Cariño intervention would have greater reduction in SBP, LDL, and HbA1c than those in the comparison group. Our sample size consideration and power analyses were based on testing these primary outcomes. In reviewing studies on blood pressure among Latino diabetics46,47 as well as other groups,48 variance estimates for blood pressure have been in the magnitude of 15–20 mmHg. As Figure 3 illustrates, using a variance estimate of standard deviation =21 mmHg and a type I error rate (alpha) of 0.05 for a two-sided Student’s t test, with 300 subjects in each group, we would have over 95% power to detect a minimum difference (δ) of 8 mmHg between two groups, in mean SBP. This corresponds to a medium Cohen effect size (balanced design) of 0.48.49,50 Even with an expected attrition rate of 25%, we would still have over 90% power to detect a difference of 8 mmHg. These are smaller than the 10 mmHg threshold cited in the American Heart Association guidelines for secondary prevention of cardiovascular disease.51 This ensured that with a sample size of 300 participants, we would have more than sufficient statistical power to detect clinical benefits in blood pressure.
For LDL, using data from Latino with diabetes,47 we assumed a variance estimate of 33 mg/dL. Using a two-sided Student’s t test at alpha =0.05, we will have over 80% power to detect a minimum LDL difference of 10 mg/dL (Cohen effect size =0.26). For HbA1c, with a standard deviation of 1.3647 and a type I error of 0.05, even with 113 patients in each group (after attrition), we could achieve over 80% power to detect a mean pre- and post-intervention difference of 0.51% in HbA1c between the two groups (see Table 3).
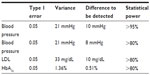  | Table 3 Statistical power for each of our primary outcomes endpoints with 300 subjects enrolled |
Statistical analysis
The intervention effects on outcomes (SBP, LDL, and HbA1c) will be evaluated using linear mixed models for continuous variables (primary outcomes) with categorical classification variables to index time intervals pre- and post-intervention as well as interventional groups. This methodology is appropriate to the paired nature of the data, and can appropriately estimate test variances taking into account the correlation that arises with the measures taken on the same subject in this pre/post-experimental design. We will use statistical tests of the estimates of the regression parameters from this model to compare the major outcomes before and after the different interventions in the entire cohort as well as between each intervention group. We will additionally incorporate an interaction term to enable us to investigate the differential effect of the intervention between the groups as well as the changes in the estimated differences between the intervention groups comparing pre- and post-intervention time intervals. Following intention-to-treat principles, we will analyze participant data as part of their original random group assignment. For each analysis, we will include all available participant data for the particular outcome at baseline and at 12 months.
Prior to analyses, we will examine baseline values of all variables from each arm; however, no P-values will be provided and covariates (other than baseline values) are not proposed for inclusion in the main analyses of treatment effects. Examination of baseline differences on key variables between subjects remaining and those lost to follow-up will also be conducted. The first set of analyses will not adjust for dropout. Only cases with complete data are included; however, as stated, these analyses will include those who did not complete the CHW intervention but who returned to provide the follow-up interview, under an intent-to-treat design. The intent-to-treat analysis of the total group will then be repeated using baseline values carried forward to account for cases lost to follow-up (using SAS PROC MIXED, SAS version 9.2; SAS Institute, Cary, NC, USA). However, baseline values carried over may not always be the best method, depending on the type of variable studied. For example, blood pressure may increase over time due to aging. Thus, other methods of examining missing data will be considered (eg, propensity scores, Expectation-Maximization algorithm, and multiple imputation sensitivity analyses). We use SAS version 9.2 (SAS Institute) for all analyses with an α of 0.05 to test for significance.
Limitations
In Cariño, our goal was to determine whether community-based CHWs provide added value to an existing health care system. Thus, we did not use health care professionals nor medication management as part of the intervention. This latter approach creates a new system rather than simply improving on systems which already exist. Another potential limitation is the sustainability of the CHW program after the study is completed. Even if our CHW is proven effective, it is still not clear if health care systems will choose to include CHWs as part of their health care delivery teams or whether payers would provide reimbursement for CHWs as they do for other programs such as home nurse visitation. We will also be collecting data on health care utilization outcomes allowing us to carry out future cost analysis which would help inform payers and policymakers in such decisions. In fact, some payers are already moving in that direction. For example, in July 2013, the Centers for Medicare and Medicaid Services created a new rule which allows state Medicaid agencies to reimburse for preventive services provided by professionals that may fall outside of a state’s clinical licensure system, such as CHWs, as long as the services are recommended by a physician or other licensed practitioner. This new rule offers state Medicaid agencies the option to reimburse, for the first time, community-based CHW preventive services.
Summary
The MHHI is a randomized controlled trial of 300 Latino patients with uncontrolled type II diabetes who are assigned to either a CHW intervention or usual care and followed for 1 year. The primary outcomes are blood pressure, LDL, and glycemic control. Secondary outcomes include medication adherence, physical activity, and fruit and vegetable intake. MHHI is one of the first large-scale randomized control trials involving Latinos with diabetes having at least a year of follow-up and similar methodological rigor as a pharmaceutical trial.
Acknowledgments
Our study was sponsored by the National Heart, Lung, and Blood Institute under an investigator-initiated grant (R01 HL083857).
Disclosure
The authors report no conflicts of interest in this work.
References
Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the US population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29(6):1263–1268. | |
Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes (UKPDS 38). UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703–713. | |
Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events (CARE) trial. The Care Investigators. Circulation. 1998;98(23):2513–2519. | |
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. | |
Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–865. | |
American Diabetes Association. Standards of medical care in diabetes – 2013. Diabetes Care. 2013;36 Suppl 1:S11–S66. | |
Brown AF, Gregg EW, Stevens MR, et al. Race, ethnicity, socioeconomic position, and quality of care for adults with diabetes enrolled in managed care: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care. 2005;28(12):2864–2870. | |
Trivedi AN, Zaslavsky AM, Schneider EC, Ayanian JZ. Trends in the quality of care and racial disparities in Medicare managed care. N Engl J Med. 2005;353(7):692–700. | |
Shai I, Jiang R, Manson JE, et al. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care. 2006;29(7):1585–1590. | |
Witmer A, Seifer SD, Finocchio L, Leslie J, O’Neil EH. Community health workers: integral members of the health care work force. Am J Public Health. 1995;85(8 Pt 1):1055–1058. | |
Brown SA, Hanis CL. A community-based, culturally sensitive education and group-support intervention for Mexican Americans with NIDDM: a pilot study of efficacy. Diabetes Educ. 1995;21(3):203–210. | |
Philis-Tsimikas A, Walker C. Improved care for diabetes in underserved populations. J Ambul Care Manage. 2001;24(1):39–43. | |
Philis-Tsimikas A, Walker C, Rivard L, et al; Project Dulce. Improvement in diabetes care of underinsured patients enrolled in project dulce: a community-based, culturally appropriate, nurse case management and peer education diabetes care model. Diabetes Care. 2004;27(1):110–115. | |
Teufel-Shone NI, Drummond R, Rawiel U. Developing and adapting a family-based diabetes program at the US-Mexico border. Prev Chronic Dis. 2005;2(1):A20. | |
Community health Workers/Promotores de salud: Critical connections in communities. Centers for Disease Control and Prevention website. Available from: http://www.cdc.gov/diabetes/projects/comm.htm. Accessed July 1, 2013. | |
Corkery E, Palmer C, Foley ME, Schechter CB, Frisher L, Roman SH. Effect of a bicultural community health worker on completion of diabetes education in a Hispanic population. Diabetes Care. 1997;20(3):254–257. | |
Spencer MS, Rosland AM, Kieffer EC, et al. Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: a randomized controlled trial. Am J Public Health. 2011;101(12):2253–2260. | |
Balcázar HG, de Heer H, Rosenthal L, et al. A promotores de salud intervention to reduce cardiovascular disease risk in a high-risk Hispanic border population, 2005–2008. Prev Chronic Dis. 2010;7(2):A28. | |
Babamoto KS, Sey KA, Camilleri AJ, Karlan VJ, Catalasan J, Morisky DE. Improving diabetes care and health measures among hispanics using community health workers: results from a randomized controlled trial. Health Educ Behav. 2009;36(1):113–126. | |
Sixta CS, Ostwald S. Texas-Mexico border intervention by promotores for patients with type 2 diabetes. Diabetes Educ. 2008;34(2):299–309. | |
Lujan J, Ostwald SK, Ortiz M. Promotora diabetes intervention for Mexican Americans. Diabetes Educ. 2007;33(4):660–670. | |
Brown SA, Garcia AA, Kouzekanani K, Hanis CL. Culturally competent diabetes self-management education for Mexican Americans: the Starr County border health initiative. Diabetes Care. 2002;25(2):259–268. | |
State and county QuickFacts: Miami-Dade County, Florida. Miami-Dade County – US Census Bureau website. http://quickfacts.census.gov/qfd/states/12/12086.html. Accessed July 7, 2013. | |
Boswell TD. Racial and ethnic change and Hispanic residential segregation patterns in metropolitan Miami, 1980 (Dialogue #81). LACC Occasional Papers Series. Dialogues (1980–1994). 1987:Paper 58. | |
Miami-Dade County, Department of Planning and Zoning 2011. Hispanics by country of origin in Miami-Dade. Data Flash. 2011;16. Available from: http://www.miamidade.gov/planning/library/reports/data-flash/2011-hispanics-by-origin.pdf. Accessed July 7, 2013. | |
Ennis SR, Ríos-Vargas M, Albert NG. Hispanic Population 2010. US Census Bureau 2010 Census Briefs # C2010BR-04. Available from: http://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf. Accessed December 6, 2013. | |
Jackson Health System. Public Health Trust. Financial Recovery Board Public Hearing, August 16, 2012. Available from: https://www.jhsmiami.org/workfiles/wmApps/publicDocs/docLib/PHT_Financial%20Recovery%20Board%20Meetings%20-%20Prior/08-16-2012%20-%20PHT%20FRB%20Public%20Budget%20Hearing.pdf. Accessed July 7, 2013. | |
Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88 (5 Pt 1):2460–2470. | |
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentrations of low-density lipoprotein cholesterol in plasma, without the use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. | |
Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. | |
Centers for Disease Control. State-Specific Trends in Fruit and Vegetable Consumption Among Adults – United States, 2000–2009. MMWR Morb Mortal Wkly Rep. 2010;59(35):1125–1130. | |
Guidelines for the data processing and analysis of the international physical activity questionnaire. http://www.ipaq.ki.se/scoring.pdf. Updated 2005. Accessed July 1, 2013. | |
Prince MJ, Reischies F, Beekman AT, et al. Development of the EURO-D scale – a European, Union initiative to compare symptoms of depression in 14 European centres. Br J Psychiatry. 1999;174:330–338. | |
Marin G, Sabogal F, Marin BV. Development of a short acculturation scale for Hispanics. Hisp J Behav Sci. 1987;9:183–205. | |
Lee DD, Stucky BD, Lee JY, Rozier RG, Bender DE. Short Assessment of Health Literacy – Spanish and English: A comparable test of health literacy for Spanish and English speakers. Health Serv Res. 2010;45(4):1105–1120. | |
Velos eResearch website. http://velos.com/solutions/by-product/velos-eresearch-2/. Accessed July 7, 2013. | |
Four steps to manage your diabetes for life. Available from http://www.ndep.nih.gov/publications/PublicationDetail.aspx? PubId=4&redirect=true. Accessed July 7, 2013. | |
National Institute for Aging. Conversando con su medico. NIH No 04-3452S. 2004. Available from: http://www.nia.nih.gov/espanol/publicaciones/conversando-con-su-medico. Accessed July 7, 2013. | |
Platillos latinos ¡Sabrosos y saludables! Delicious heart healthy Latino recipes. http://www.nhlbi.nih.gov/health/public/heart/other/sp_recip.pdf. Accessed July 7, 2013. | |
Latino cardiovascular health resources. http://www.nhlbi.nih.gov/health/prof/heart/latino/latin_pg.htm. Accessed July 1, 2013. | |
Chin MH, Walters AE, Cook SC, Huang ES. Interventions to Reduce Racial and Ethnic Disparities in Health Care. Med Care Res Rev. 2007; 64(Suppl 5):7S–28S. | |
Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA. 2002;288(15):1909–1914. | |
Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood). 2001;20(6):64–78. | |
National Heart, Lung, and Blood Institute. Your Heart, Your Life: A Lay Health Educator’s Manual for the Hispanic Community. 2008. NHLBI Item No: 08-3674. | |
Centers for Disease Control. The Community Health Worker’s Sourcebook: A Training Manual for Preventing Heart Disease and Stroke. Updated July 31, 2013. Available from: http://www.cdc.gov/dhdsp/programs/nhdsp_program/chw_sourcebook/pdfs/sourcebook.pdf. Accessed December 6, 2013. | |
Sacco RL, Boden-Albala B, Abel G, et al. Race-ethnic disparities in the impact of stroke risk factors: the northern Manhattan stroke study. Stroke. 2001;32(8):1725–1731. | |
Shea S, Weinstock RS, Starren J, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus. J Am Med Inform Assoc. 2006;13(1):40–51. | |
Cheng EM, Cunningham WE, Towfighi A, et al. Randomized, controlled trial of an intervention to enable stroke survivors throughout the Los Angeles County safety net to “stay with the guidelines”. Circ Cardiovasc Qual Outcomes. 2011;4(2):229–234. | |
Machin D, Campbell M, Fayers PM, Pinol A. Sample Size Tables for Clinical Studies. 2nd ed. Oxford: Blackwell Science; 1997. | |
Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale (NJ): Lawrence Earlbaum Associates; 1988. | |
Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362(9395):1527–1535. | |
Piette JD, Schillinger D, Potter MB, Heisler M. Dimensions of patient-provider communication and diabetes self-care in an ethnically diverse population. J Gen Intern Med. 2003;18(8):624–633. | |
Lorig K, Stewart A, Ritter P, González V, Laurent D, Lynch J. Outcome Measures for Health Education and Other Health Care Interventions. Thousand Oaks (CA): Sage Publications; 1996:24–40. | |
Lorig KR, Ritter PL, Jacquez A. Outcomes of border health Spanish/English chronic disease self-management programs. Diabetes Educ. 2005;31(3):401–409. | |
Peterson KA, Hughes M. Readiness to change and clinical success in a diabetes educational program. J Am Board Fam Pract. 2002;15(4):266–271. | |
Samuel-Hodge CD, DeVellis RF, Ammerman A, Keyserling TC, Elasy TA. Reliability and validity of a measure of perceived diabetes and dietary competence in African American women with type 2 diabetes. Diabetes Educ. 2002;28(6):979–988. | |
Oemar M, Oppe M. EQ-5D-3L User’s Guide. Version 5.0. 2013. Euro-Qual Group, Netherlands. Available from: http://www.euroqol.org/about-eq-5d/publications/user-guide.html. Accessed Jan 9, 2014. | |
Cohen J. Design and Methods of the Medical Expenditure Panel Survey Household Component. AHCPR Pub No 97-0026. 1997: Rockville (MD): Agency for Health Care Policy and Research. Available from: http://www.meps.ahrq.gov/data_files/publications/mr1/mr1.shtml. Accessed December 6, 2013. | |
Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test Guidelines for Use in Primary Care. 2nd ed. World Health Organization Department of Mental Health and Substance Dependence. 2001. Available from: http://whqlibdoc.who.int/hq/2001/WHO_MSD_MSB_01.6a.pdf. | |
Gurland B, Kuriansky J, Sharpe L, Simon R, Stiller P, Birkett P. The Comprehensive assessment and Referral Evaluation (CARE) – rationale, development and reliability. Int J Aging Hum Dev. 1977;8(1):9–42. | |
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.