Back to Journals » Clinical Epidemiology » Volume 8
Rate of bleeding-related episodes in adult patients with primary immune thrombocytopenia: a retrospective cohort study using a large administrative medical claims database in the US
Authors Altomare I, Cetin K, Wetten S, Wasser J
Received 5 February 2016
Accepted for publication 17 April 2016
Published 20 June 2016 Volume 2016:8 Pages 231—239
DOI https://doi.org/10.2147/CLEP.S105888
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Henrik Sørensen
Ivy Altomare,1 Karynsa Cetin,2 Sally Wetten,3 Jeffrey S Wasser4
1Department of Medicine, Duke University School of Medicine, Durham, NC, 2Center for Observational Research, Amgen Inc., Cambridge, MA, USA; 3Centre for Observational Research, Amgen Ltd., Uxbridge, UK; 4Carole and Ray Neag Comprehensive Cancer Center, University of Connecticut School of Medicine, Farmington, CT, USA
Background: Immune thrombocytopenia (ITP) is a rare disorder characterized by low platelet counts and an increased tendency to bleed. The goal of ITP therapy is to treat or prevent bleeding. Actual rates of bleeding are unknown. Clinical trial data may not reflect real-world bleeding rates because of the inclusion of highly refractory patients and more frequent use of rescue therapy.
Methods: We used administrative medical claims data in the US to examine the occurrence of bleeding-related episodes (BREs) – a composite end point including bleeding and/or rescue therapy use – in adults diagnosed with primary ITP (2008–2012). BRE rates were calculated overall and by ITP phase and splenectomy status. Patients were followed from ITP diagnosis until death, disenrollment from the health plan, or June 30, 2013, whichever came first.
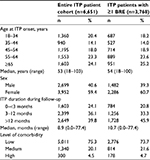
Results: We identified 6,651 adults diagnosed with primary ITP over the study period (median age: 53 years; 59% female). During 13,064 patient-years of follow-up, 3,768 patients (57%) experienced ≥1 BRE (1.08 BREs per patient-year; 95% confidence interval: 1.06–1.10). The majority (58%) of BREs consisted of rescue therapy use only. Common bleeding types were gastrointestinal hemorrhage, hematuria, ecchymosis, and epistaxis. Intracranial hemorrhage was reported in 74 patients (1%). Just over 7% of patients underwent splenectomy. Newly diagnosed and splenectomized patients had elevated BRE rates.
Conclusion: We provide current real-world estimates of BRE rates in adults with primary ITP. The majority of ITP patients experienced ≥1 BRE, and over half were defined by rescue therapy use alone. This demonstrates the importance of examining both bleeding and rescue therapy use to fully assess disease burden.
Keywords: immune thrombocytopenia, bleeding, cohort study, epidemiology
Introduction
Immune thrombocytopenia (ITP) is a rare autoimmune disorder characterized by isolated thrombocytopenia leading to an increased risk of clinically significant bleeding. Although published reports of ITP incidence vary, a recent critical review of the literature suggested that the incidence of ITP in adults is between 16 and 39 cases per million per year.1 This translates into roughly 3,900–9,500 adults being diagnosed with ITP in the US each year.
Low platelet counts, increasing patient age, use of concurrent medications, and male sex are all associated with an increased risk of bleeding in patients with ITP.2 Symptoms range in severity from no bleeding to relatively mild petechiae or purpura to more serious internal bleeding problems such as gastrointestinal or even intracranial hemorrhage. The goal of ITP therapy is to treat or prevent bleeding, and patients with platelet counts >30×109/L usually do not require treatment and can safely be observed.3 On the other hand, patients determined to be at imminent risk of bleeding or those with active bleeding can be treated with a therapy to quickly, even if temporarily, reduce this risk. This is termed “rescue therapy”.
Effective treatment of ITP, and therefore reduction in bleeding risk, has been the objective of several recent randomized clinical trials for newer ITP therapies.4–7 Although these trials offer important insights regarding the burden of bleeding in ITP patients, the true background risk of bleeding in this patient population is not well characterized. Rates of bleeding reported in the clinical trial setting may not reflect real-world bleeding rates in ITP for a number of reasons. Importantly, patients participating in trials are a highly selected group of ITP patients, often refractory with extremely low platelet counts, and are perhaps at higher risk of bleeding than the average asymptomatic ITP patient. Additionally, bleeding rates measured in ITP clinical trials are affected by both the assigned treatment and the required use of rescue therapies. Since rescue therapies are employed to rapidly increase platelet counts in patients actively bleeding or at imminent risk of bleeding, ethical considerations demand that these therapies be used in clinical trials perhaps more often than they are utilized in routine clinical practice.
We undertook this study to better understand the rates of bleeding and the extent of rescue therapy use outside the clinical trial setting. We examined the occurrence of bleeding-related episodes (BREs) – a composite end point encompassing both actual bleeding and the use of rescue therapies – in adult patients with primary ITP using administrative medical claims data in the US.
Methods
Study population
We conducted this retrospective cohort study using two large administrative medical claims databases in the US – the MarketScan® Commercial Claims and Encounters Database and the MarketScan Medicare Supplemental and Coordination of Benefits Database. The commercial database represents employees and their spouses and dependents covered by employer-sponsored private health insurance, and the Medicare Supplemental database covers individuals with Medicare (primary payer) and supplemental insurance, including both employees aged ≥65 years and retirees with employer-sponsored supplemental coverage. All data analyzed for this study were de-identified and fully compliant with the Health Insurance Portability and Accountability Act (HIPAA) of 1996.
ITP patients
We included adults (≥18 years) with ≥2 outpatient claims separated by ≥30 days (but ≤365 days) or ≥1 inpatient claim carrying a diagnosis code for ITP (Table S1) between January 1, 2008, and December 31, 2012. Patients were required to have ≥12 months of continuous coverage before the onset of ITP. Because ITP is a diagnosis made by excluding other related causes, we searched for claims carrying general thrombocytopenia diagnosis codes (Table S1) in the 12 months before the first ITP code. For patients who had at least one such claim, the first of these claims was used to define the onset of ITP. For all other patients, the first claim with an ITP code defined the onset of ITP.
In an effort to include only patients with primary ITP, individuals were excluded if they had any claims carrying a diagnosis code for another medical condition known to cause thrombocytopenia (Table S2) in the 12 months before the onset of ITP. Additionally, to minimize the inclusion of prevalent ITP patients, we excluded patients with any general thrombocytopenia codes that extended beyond 12 months prior to the first claim with an ITP code.
Bleeding-related episodes
A BRE was defined as an actual bleeding event and/or use of rescue therapy (intravenous [IV] immunoglobulin administration, IV steroid administration, or platelet transfusion) using a combination of diagnosis and procedure codes (Table S3). BREs were constructed using the same published algorithm that was developed to retrospectively analyze BREs in clinical trials of ITP patients.8
ITP phase, splenectomy, and comorbidity
ITP was classified into phases based on disease duration as outlined by Rodeghiero et al:3 newly diagnosed (0 month to <3 months), persistent (3–12 months), and chronic (>12 months). Duration of ITP was estimated as the time between ITP onset and the date of the last claim carrying an ITP diagnosis code during follow-up. Splenectomies were captured based on the presence of any claim carrying a relevant code for splenectomy (Table S4). The level of comorbidity was also assessed, using a version of the Charlson Comorbidity Index adapted for use with administrative claims9–12 based on relevant diagnosis codes (Table S5) recorded in claims within the 12-month period prior to ITP diagnosis. Scores were categorized as low (0), medium (1–2), or high (≥3).
Statistical analysis
Patients were followed from the date of ITP onset until the date of death, date of disenrollment from the health plan, or June 30, 2013, whichever came first. The overall rate of BREs (and associated 95% confidence interval [CI; Poisson]) in the patient cohort was estimated by summing the number of unique BREs during the time at risk divided by the total number of patient-years at risk. Time at risk was defined as the time between the date of ITP onset and the end of follow-up. Additionally, rates for BREs that contained only bleeding events, those that contained only rescue therapy use, and those that contained both were estimated separately.
For BRE rates by ITP phase, patients only contributed time at risk according to the definitions of each ITP phase. Similarly for BRE rates by splenectomy status, patients only contributed time at risk until the date of splenectomy or from the date of splenectomy until end of follow-up to the respective nonsplenectomized and splenectomized groups.
All analyses were performed using SAS®, Version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
In a database of ~67 million adults, we identified 6,651 patients who met our claims-based criteria for a diagnosis of ITP during the study period. Over half (57%) experienced at least one BRE during 13,046 patient-years of follow-up. Table 1 summarizes the demographic and clinical characteristics for the overall study cohort and for the subset that experienced a BRE. Among all ITP patients, the median age was 53 years at ITP onset (range: 18–103 years), and 59% were female. During follow-up, the median duration of ITP was 9 months (range: 0–77 months), and ~40% met the criteria for chronic ITP (duration >12 months). Over 75% had a low level of comorbidity at ITP diagnosis. Characteristics in the subset that experienced a BRE were similar except that the median duration of ITP during follow-up was longer (11 months), and ~46% met the criteria for chronic ITP.
Rates of BREs among the entire ITP patient cohort
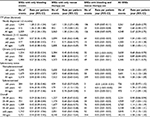
A total of 14,115 BREs occurred during 13,046 patient-years, resulting in a rate of 1.08 BREs per patient-year in the ITP patient cohort (95% CI: 1.06–1.10; Table 2). The majority (58%) of BREs were defined solely by the use of rescue therapies, and the remaining 41% and 2% contained only bleeding events or use of both rescue therapies and bleeding events, respectively (Figure 1). The rate of BREs with only rescue therapy use was 0.62 per patient-year (95% CI: 0.61–0.64), and the rate of BREs with only bleeding events was 0.44 per patient-year (95% CI: 0.43–0.45; Table 2). IV steroid treatment was the most frequently administered rescue therapy (0.51/patient-year [95% CI: 0.50–0.52]). The most common types of bleeding events (ie, events occurring in ≥5% of patients) were gastrointestinal hemorrhage, hematuria, spontaneous ecchymosis, and epistaxis (Table 3). A total of 126 intracranial hemorrhagic events were reported in 74 patients (1% of the cohort) corresponding to a rate of <0.01 per patient-year (95% CI: 0.008–0.011).
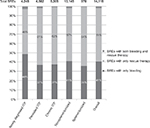  | Figure 1 Type of BREs in the study cohort overall and by phase of ITP and splenectomy status. Abbreviations: BREs, bleeding-related episodes; ITP, immune thrombocytopenia. |
  | Table 3 Bleeding events occurring in at least 5% of patients in the study cohort Abbreviations: GI, gastrointestinal; ICD-9, International Classification of Diseases, Ninth Revision. |
Rates of BREs by ITP duration
The rate of BREs was highest in newly diagnosed ITP (<3 months after ITP onset) with a rate of 2.67 per patient-year (95% CI: 2.59–2.75) but was still elevated in the persistent (disease duration of 3–12 months) compared with chronic (disease duration of >12 months) phase (1.08 per patient-year [95% CI: 1.05–1.11] compared with 0.73 per patient-year [95% CI: 0.71–0.75], respectively; Table 2). The proportion of BREs with only bleeding events was also highest in the newly diagnosed ITP phase (Figure 1). In newly diagnosed ITP patients, 48% of BREs contained only bleeding and 49% of BREs contained only use of rescue therapies. In both persistent and chronic ITP phases, 37% of BREs were defined by bleeding alone and 61% and 62% contained only rescue therapy use.
Rates of BREs by splenectomy status
Among the ITP patient cohort, 492 patients (7%) underwent splenectomy during follow-up. In these patients, the rate of BREs was 1.17 per patient-year (95% CI: 1.09–1.24), which was slightly higher than that observed in nonsplenectomized patients (1.08 per patient-year [95% CI: 1.06–1.09]; Table 2). Compared with nonsplenectomized patients, a higher proportion of BREs in splenectomized patients was defined by use of rescue therapy alone (64% versus 57%; Figure 1).
Discussion
We identified 6,651 patients diagnosed with primary ITP during a 5-year period from an administrative medical claims database of roughly 67 million adults. Although our study was not designed to measure the incidence of ITP, this corresponds to ~20 cases of ITP per million persons per year in the US, which is in the range of previously reported estimates.1 Consistent with the phenotype of a typical ITP patient,13 our cohort of ITP patients was ~60% female, the median age at diagnosis was 53 years, and there was a low comorbid burden. Additionally, just under a quarter of our study cohort did not go beyond the newly diagnosed phase of ITP during follow-up, over one-third reached the persistent phase, and ~40% reached the chronic phase. Our classification of patients into these phases must be interpreted with caution given its reliance on a claims-based definition of ITP duration and length of follow-up, but our study could lend insight into the natural history of ITP. The rate of spontaneous remissions in ITP is a topic of great debate and not known with certainty. According to one recent review, spontaneous remissions occur in 5–11% of patients,13 a slightly lower estimate than our data set reflects. Other reports cite remission rates of ~30%.14,15 Lastly, only 7% of the ITP patients in this cohort underwent splenectomy at some point during the 5.5-year follow-up. Given that 40% reached the chronic phase of the disease, and assuming splenectomy was only undertaken for patients in this phase, our data suggest that approximately one of five US patients with chronic ITP undergoes splenectomy in current clinical practice.
Our study is unique in that our ITP patient population is not limited to patients with the lowest platelet counts and/or the most advanced disease. Instead, our study cohort reflects all-comers and therefore potentially offers valuable information regarding the real-world burden of this disease. ITP patients in our cohort experienced ~14,000 BREs over 13,000 patient-years of follow-up, reflecting a rate of just over one BRE per patient per year. Importantly, the majority of patients experienced at least one BRE, and over half of all BREs were defined by rescue therapy use alone.
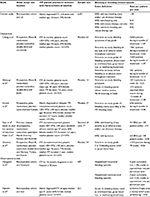
Previous reports on bleeding in ITP patients primarily stem from clinical trials of ITP therapies that provide rates of first-time, on-study actual bleeding events in highly selected patient populations.5,7,8,16–20 Examples from recent studies are summarized in Table 4, including descriptions of the ITP patients included and the definition of bleeding employed. To our knowledge, only one other study reported on rates of the measure we utilized in our study – the BRE composite end point. Stasi et al8 conducted a post hoc analysis of data from a randomized, 52-week, open-label study evaluating the efficacy and tolerability of romiplostim versus standard of care in patients with ITP.7 Patients receiving standard of care had a rate of 9.4 BREs per 100 patient-weeks, translating into ~4.9 BREs per patient per year, and over half of the BREs involved rescue with IV immunoglobulin.8 We observed a markedly lower rate of BREs, but the observation that roughly half of the BREs involved rescue therapy is consistent with our findings. Importantly, subjects in the trial were nonsplenectomized and were required to have a history of at least one prior ITP therapy and a platelet count of <50×109/L at baseline. Other recent clinical trials of ITP therapies have also provided data on bleeding of various degrees of severity in ITP patients who have had the disease for a relatively long duration (especially if they were postsplenectomy), were refractory to previous treatments, and/or had a platelet count below a certain threshold (Table 4).5,16–18 Because our study followed all newly diagnosed patients, regardless of initial platelet count or other disease and patient characteristics, for a maximum of 5.5 years, patients with highly refractory and/or long-standing chronic ITP were, for the most part, excluded. Therefore, it is not surprising that we generally observed lower rates of BREs and bleeding events in our cohort of ITP patients compared with those previously recorded in the clinical trial setting.
There is a paucity of data on the risk of bleeding in ITP patients outside of clinical trial populations (Table 4).19,20 In a Danish population-based cohort study of 507 adults with a hospital-based diagnosis of ITP for ≥6 months, Nørgaard et al19 reported a 5-year cumulative incidence of 1.4% (95% CI: 0.5–3.9) for hospitalized intracranial hemorrhage and 3.6% (95% CI: 2.1–5.8) for all other hospitalized bleeding episodes, treating death as a competing event. More recently, Palandri et al20 conducted a study of 557 consecutive patients of all ages diagnosed with ITP and followed up for a median of 6.9 years at a single institution in Italy. During follow-up, 63 grade ≥2 hemorrhages occurred in 48 patients, translating roughly into a rate of 0.02 grade ≥2 hemorrhages per patient per year. In our larger US study of 6,651 ITP patients followed up for an average of 2 years, we observed 5,975 BREs that were not defined by rescue therapy use alone (ie, involved bleeding only or bleeding plus rescue therapy use), which suggests a slightly higher bleeding rate (~0.4 bleeding episodes per patient per year) than previous observational studies. This likely reflects differences in study setting (geographical and setting of ITP care), duration of follow-up after ITP diagnosis, method of ITP patient identification, bleeding definitions, or other important aspects of study design (eg, inclusion/exclusion of prevalent ITP patients at the start of follow-up).
In our study, patients were at highest risk for BREs during the first 3 months after ITP onset; the rate of BREs in this newly diagnosed phase was ~2.5 times higher than the rate in the persistent phase and 3.5 times higher than the rate in the chronic phase. BRE rates were lowest in patients with chronic ITP, where a higher proportion of patients experienced use of rescue therapy as opposed to actual bleeding. The reason why fewer events were observed in chronic ITP patients could not be determined from our analysis; perhaps it represents an inherent lessening of bleeding risk in all-comers with the chronic phase of the disease but more likely reflects response to appropriate therapy and preventative measures. It is also at least partly a result of the fact that patients who have acute ITP often present with bleeding-related symptoms at initial diagnosis, particularly for those captured in a claims research setting.
We also found that rates of BREs were higher in splenectomized versus nonsplenectomized patients in our cohort of ITP patients, but estimates were less precise in the splenectomized group because of the relatively small sample size (only 7% of patients underwent splenectomy during follow-up). However, our findings are consistent with previous research that has demonstrated a risk of major postoperative bleeding in splenectomized patients.21 Given that patients in our study were only followed up for an average of 2 years, we were unable to observe any longer term risk of BREs postsplenectomy.
The most common types of bleeding observed in our cohort of ITP patients, listed in Table 3, are as expected. Other, more rarely reported types of bleeding not listed included menorrhagia, hemoptysis, and postmenopausal bleeding. By nature of this being a claims analysis, in order for us to identify a bleeding event, it must have been properly recorded as bleeding according to the International Classification of Diseases (9th Revision) (ICD-9) during the patient encounter. Therefore, minor bleeding (such as petechiae or oral mucosal bleeding) and perhaps even more significant bleeding may be underreported in our data. Our methodology most likely allows for a conservative estimate for the overall rate of bleeding and tends to identify the most severe and therefore clinically relevant bleeding events with reasonable accuracy.
There are some limitations that should be kept in mind when interpreting these results, many of which are inherent to observational studies using claims data. Identification of ITP patients and BREs relied on ICD-9 diagnosis codes from an administrative medical claims database and not on actual clinical data. To our knowledge, there have been no validation studies on the specific code for primary ITP (287.31), which was introduced in 2005. However, prior to this, a study was conducted to assess the performance of the code 287.3 (the more general code for primary thrombocytopenia, which includes ITP) for accurately identifying patients with ITP using medical records as the gold standard, and it performed well.22 For example, among inpatients, the sensitivity of the code for indicating a diagnosis of ITP was 100% (95% CI: 94–100) and the specificity was 89% (95% CI: 84–94). We expect that the performance of the more specific code for ITP would be similar to or better than the more general code, since the other non-ITP forms of primary thrombocytopenia now have separate codes as well. Regarding identification of BREs, inpatient data in particular can be extremely limited because coding practices in this setting do not require a detailed accounting of services provided. It is likely that we undercounted the use of therapies and transfusions received in this setting. However, since these services take place during the hospitalization itself, we were unlikely to miss the episode. It is also possible that some BREs we classified as “rescue therapy use only” involved actual bleeding as well, which could explain the relatively high proportion of BREs that were identified only through rescue therapy use. Importantly, this would not have caused us to miss the episode itself and therefore would not have influenced our estimates of the overall rates of BRE.
Another major limitation is that oral steroid use and steroid dose changes were excluded in our definition of a BRE. This may have led to an underestimation of BRE rates but was done in part because of data limitations and in part to purposefully minimize the inclusion of false-positive events. There is significant variation in the purpose of oral steroids or dose changes in ITP patients (treatment of an adverse event, planned dose adjustments for preventing bleeding, confirmation of an ITP diagnosis, or to induce remission).
More generally, outside of the BRE definition, we did not describe use of ITP medications (eg, oral corticosteroids, azathioprine, rituximab, thrombopoietin-receptor agonists) in this patient population. We also lacked data on platelet counts, which precluded us from using platelet counts as a marker of disease severity and/or response to treatments received and correlating platelet counts to the BREs in ITP patients overall and by phase of ITP. Importantly, the goal of this study was to measure real-world rates of BREs and not to tie the occurrence of a BRE to severity of disease, response (or lack of response) to a specific therapy, or relapse after a response to a particular treatment based on predefined platelet count thresholds. Future research could stratify BRE rates on receipt of the ITP medications most frequently used in the first- and second-line settings and more formally explore the real-world effectiveness of these medications in raising platelet counts and thus lowering the risk of bleeding or need for rescue therapy.
A primary strength of our study is that the MarketScan databases represent a powerful source of data in terms of its representation of the entire US. According to the Henry J Kaiser Family Foundation, in 2014, roughly 49% of the US population had employer-sponsored insurance and ~13% had Medicare.23 Among those with Medicare, employer-sponsored plans provide supplemental coverage to about 4 in 10 beneficiaries.24 This means that findings from these two databases can be generalized to roughly 154 million Americans who have insurance through their employers and roughly 17 million Medicare beneficiaries who have employer-sponsored supplemental insurance (of the total 42 million of Medicare beneficiaries in the US).
Conclusion
Despite any limitations, our study provides valuable clinical data on ITP patients. This information cannot be generated by clinical trials and is absent from the existing published literature. Though our primary objective was to analyze real-world rates of bleeding and use of rescue therapy among adults with ITP, our analysis yielded additional important information. We offer insight into the incidence of ITP in the US, the proportion of patients meeting the criteria for each phase of ITP (newly diagnosed, persistent, and chronic), the current use of splenectomy in the modern era of anti-CD20 monoclonal antibodies and thrombopoietin mimetics, and the occurrence of rarer but serious types of bleeding such as intracranial hemorrhage. In our study, the majority of ITP patients experienced at least one BRE, and over half of all BREs were defined by rescue therapy use alone. This demonstrates the importance of examining both bleeding and rescue therapy use to fully assess disease burden and ultimately help determine the relative success of different ITP therapies.
Acknowledgments
This study was funded by Amgen Inc. The abstract of this paper was presented at the American Society of Hematology annual meeting, December 6–9, 2014, San Francisco, CA, USA, as an oral presentation: http://www.bloodjournal.org/content/124/21/202. The actual paper, however, has never been published.
Disclosure
IA is a consultant for Amgen and Genentech and an advisory board member for Incyte and Novartis. KC and SW are employees of Amgen. JSW receives research support from Amgen and is a consultant and advisory board member for Amgen. JSW has also consulted for Novartis. The authors report no other conflicts of interest in this work.
References
Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85(3):174–180. | ||
Cines DB, Bussel JB. How I treat idiopathic thrombocytopenic purpura (ITP). Blood. 2005;106(7):2244–2251. | ||
Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393. | ||
Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357(22):2237–2247. | ||
Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377(9763):393–402. | ||
Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371(9610):395–403. | ||
Kuter DJ, Rummel M, Boccia R, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363(20):1889–1899. | ||
Stasi R, Murali M, Michel M, et al. Evaluation of bleeding-related episodes in patients with immune thrombocytopenia (ITP) receiving romiplostim or medical standard of care. Int J Hematol. 2012;96(1):26–33. | ||
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. | ||
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. | ||
Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. | ||
Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584–590. | ||
Fogarty PF, Segal JB. The epidemiology of immune thrombocytopenic purpura. Curr Opin Hematol. 2007;14(5):515–519. | ||
Moulis G, Palmaro A, Montastruc JL, Godeau B, Lapeyre-Mestre M, Sailler L. Epidemiology of incident immune thrombocytopenia: a nationwide population-based study in France. Blood. 2014;124(22):3308–3315. | ||
Schiavotto C, Rodeghiero F. Twenty years experience with treatment of idiopathic thrombocytopenic purpura in a single department: results in 490 cases. Haematologica. 1993;78(6 suppl 2):22–28. | ||
Shirasugi Y, Ando K, Miyazaki K, et al. Romiplostim for the treatment of chronic immune thrombocytopenia in adult Japanese patients: a double-blind, randomized Phase III clinical trial. Int J Hematol. 2011;94(1):71–80. | ||
Arnold DM, Heddle NM, Carruthers J, et al. A pilot randomized trial of adjuvant rituximab or placebo for nonsplenectomized patients with immune thrombocytopenia. Blood. 2012;119(6):1356–1362. | ||
Ghanima W, Khelif A, Waage A, et al. Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9978):1653–1661. | ||
Nørgaard M, Jensen AØ, Engebjerg MC, et al. Long-term clinical outcomes of patients with primary chronic immune thrombocytopenia: a Danish population-based cohort study. Blood. 2011;117(13):3514–3520. | ||
Palandri F, Polverelli N, Sollazzo D, et al. Have splenectomy rate and main outcomes of ITP changed after the introduction of new treatments? A monocentric study in the outpatient setting during 35 years. Am J Hematol. 2016;91(4):E267–E272. | ||
Gonzalez-Porras JR, Escalante F, Pardal E, et al. Safety and efficacy of splenectomy in over 65-yrs-old patients with immune thrombocytopenia. Eur J Haematol. 2013;91(3):236–241. | ||
Segal JB, Powe NR. Accuracy of identification of patients with immune thrombocytopenic purpura through administrative records: a data validation study. Am J Hematol. 2004;75(1):12–17. | ||
Henry J. Kaiser Family Foundation. Health Insurance Coverage of the Total Population. Available from: http://kff.org/other/state-indicator/total-population/. Updated 2016. Accessed May 7,2015. | ||
Henry J. Kaiser Family Foundation. An Overview of Medicare. Available from: http://kff.org/medicare/issue-brief/an-overview-of-medicare/. Updated April 1, 2016. Accessed April 29, 2016. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.