Back to Journals » Risk Management and Healthcare Policy » Volume 13
Rate and Treatment of Retinopathy of Prematurity in Extremely Low Birth Weight Infants with Gestational Age ≤28 Weeks in Eastern China
Authors Zhang M , Xu G, Wang X, Ni Y , Huang X
Received 15 September 2020
Accepted for publication 17 November 2020
Published 7 December 2020 Volume 2020:13 Pages 2867—2873
DOI https://doi.org/10.2147/RMHP.S282102
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Marco Carotenuto
Meng Zhang,1,2 Gezhi Xu,1,2 Xin Wang,1,2 Yingqin Ni,1,2 Xin Huang1,2
1Department of Ophthalmology, Eye and ENT Hospital of Fudan University, Shanghai 200031, People’s Republic of China; 2Institute of Eye Research, Eye and ENT Hospital of Fudan University, Shanghai 200031, People’s Republic of China
Correspondence: Yingqin Ni; Xin Huang
Department of Ophthalmology, Eye and ENT Hospital of Fudan University, 83 Fenyang Road, Shanghai 200031, People’s Republic of China
Tel +86-21-64377134
Fax +86-21-64377151
Email [email protected]; [email protected]
Purpose: To investigate the incidence and clinical characteristics of ROP in extremely preterm (EP) and extremely low birth weight (ELBW) infants in eastern China.
Patients and Methods: This retrospective study included 104 infants with a birth weight (BW) ≤ 1000 g and gestational age (GA) ≤ 28 weeks, who were admitted to the Eye and ENT Hospital of Fudan University over 10 years. The infants were examined for ROP with RetCam. Infants with type 1 ROP and aggressive posterior ROP (AP-ROP) were treated. The risk factors evaluated were GA and BW.
Results: Mean GA was 26.63 ± 0.88 weeks and mean BW was 892.39 ± 108.06 g. Of the 104 infants, 83 (79.8%) developed ROP, three (2.9%) had AP-ROP, 14 (13.5%) had type 1 ROP, and 10 (9.6%) had type 2 ROP. The proportions of infants with BW ≤ 750 g and 751– 1000 g were 8.7% and 91.3%, respectively, and the incidences of severe ROP in these infants were 22.2% and 15.8%, respectively. The infants with severe ROP had a mean GA of 26.56 ± 0.68 weeks and mean BW of 860.00 ± 163.48 g, and 47.1% of severe ROP occurred in infants with a GA of 26 weeks. However, multivariate logistic regression showed that the severity of ROP was not directly inversely related to GA or BW in this study population.
Conclusion: In EP and ELBW Chinese infants, who were admitted to the Eye and ENT Hospital of Fudan University, the development of ROP was more frequent and the incidence of severe ROP that progressed to the stage that required treatment was high.
Keywords: retinopathy of prematurity, extremely low birth weight, extremely preterm, risk factors
Introduction
Retinopathy of prematurity (ROP) is a complication of prematurity that is characterized by abnormal retinal vascular development and may lead to blindness. Patients with a history of ROP presented a higher risk of myopia and retinal disease when they grown up.1 Recently, with the development of neonatal care in developing countries, the survival rate of extremely low birth weight (ELBW) infants has increased (ELBW: birth weight ≤1000 g).2 As a result, the incidence of severe ROP has increased.3 Most mild or moderate ROP regresses spontaneously, but severe ROP may result in blindness. Therefore, it is crucial to detect and treat severe ROP as early as possible.4,5 Worldwide data suggest that the incidence and clinical characteristics of ROP vary significantly from country to country.6–8 In some lower- and middle-income countries, severe ROP is still an important cause of blindness in premature infants. In the previous work, our research team has evaluated the natural involution of acute retinopathy of prematurity not requiring treatment.9 However, there have been limited studies of the incidence and clinical characteristics of ROP among extremely preterm (EP:gestational age≤ 28 weeks) and ELBW infants in the Chinese population.
In this research, we studied the rate of any ROP and ROP treatment, in ELBW infants with GA ≤28 weeks based on two risk factors: GA and BW in eastern China.
Patients and Methods
Patients
This was a retrospective study. We reviewed the medical records of EP and ELBW infants with BW ≤ 1000 g and GA ≤ 28 weeks who were admitted to the Eye and ENT Hospital of Fudan University over a 10-year period between January 2010 and January 2020. Infants with a major congenital malformation or chromosomal anomalies or who died before the end of ROP screening were excluded.
The demographic data collected included GA, BW, gender, ROP stage, postmenstrual age (PMA:gestational age at birth plus chronological age in weeks) at the onset of ROP, treatment, PMA at treatment intervention, and time to regression. The study was approved by the Institutional Review Board of the Eye and ENT Hospital of Fudan University, and all the patients’ guardians provided written informed consent. We confirm that this study was conducted in accordance with the Declaration of Helsinki.
ROP Screening Methods and Treatment
Tropicamide 0.5% eye drops were instilled four times in 1 hour before the examination. ROP screening was performed according to the screening guidelines using RetCam (Clarity Medical Systems, Pleasanton, CA, USA). All examinations were performed by experienced ophthalmologists. The first screening examination was performed in the neonatal intensive care unit (NICU) at 30–31 weeks PMA. The examinations were repeated at specific intervals, depending on the severity of ROP, until vascularization had reached Zone III or ROP had completely regressed after treatment. The stages of ROP were classified according to the International Classification of ROP. Based on the Early Treatment of Retinopathy of Prematurity (ETROP) standards,10 the infants were divided into five groups: AP-ROP; type 1 ROP; type 2 ROP; not type 1 or 2 ROP; and no ROP. AP-ROP and type 1 ROP were designated severe ROP. All infants with AP-ROP and Zone I type 1 ROP were treated with anti-vascular endothelial growth factor (VEGF) therapy. 0.25 mg (0.025 mL) ranibizumab was injected intravitreally via the pars plana. The other type 1 ROP infants were treated with argon laser photocoagulation using indirect ophthalmoscopy with the parameters: laser power, 150 mW; laser duration, 200 ms; laser pulse interval, 200 ms.
Statistical Analysis
The statistical analysis was performed with the SPSS 24.0 software (IBM Corporation, Armonk, NY, USA). The results are presented as numbers and frequencies for categorical variables and as means ± standard deviations for continuous variables. The χ2 test was used to compare categorical variables. Student’s unpaired t-test was used to compare continuous data. Multivariate logistic regression analyses were used to evaluate the risk factors for severe ROP. P ≤ 0.05 was considered statistically significant. The adjusted odds ratio (OR) and 95% confidence interval (CI) were calculated.
Results
Study Population
During the 10-year period, a total of 104 infants met the inclusion criteria of EP and ELBW. The mean GA was 26.63 ± 0.88 weeks (range: 23.10–28.00 weeks) and the mean BW was 892.39 ± 108.06 g (range: 300–1000 g). Of the 104 infants, 52.5% were male and 47.5% were female, which did not differ significantly. The demographic data for the infants enrolled in the study are presented in Table 1.
 |
Table 1 Distribution of the Infants in the Study |
The median PMA at the onset of ROP was 34.51 ± 2.40 weeks (range: 30–40 weeks). Fewer than half the eyes (41.8%) were vascularized to zone III by 37 weeks PMA.
Distribution of Severity of ROP
Of the 104 infants, only 21/104 (20.2%) showed no ROP, whereas 83/104 (79.8%) developed ROP. The incidence rates of type 1 ROP and type 2 ROP were 13.5% (14/104) and 9.6% (10/104), respectively. Three (2.9%) infants had AP-ROP, but only one ultimately developed retinal detachment (stage 4A). None of the infants developed stage 5 ROP. All the infants with type 1 ROP received laser treatment and those with AP-ROP received anti-VEGF therapy. Of the cases of severe ROP, 47.1% occurred in infants with a GA of 26 weeks.
Distribution of Stages and Incidence of ROP by GA
The incidence of ROP among infants with GA of ≤25 weeks was 100%, but neither infant had type 1 ROP or AP-ROP. The incidence rates of ROP and type 1 ROP among infants with GA of 26–27 weeks were 82.4% (28/34) and 17.6% (6/34), respectively, and these were the highest rates observed in this research (Table 2).
 |
Table 2 Distribution of ROP by GA |
Distribution of Stages and Incidence of ROP by BW
Among infants with BW of ≤750 g, only two infants had type 1 ROP and two had type 2 ROP. ROP was detected in 71.1% (27/38) of infants with BW of 751–900 g; 10.5% (4/38) of these infants had type 1 ROP and 7.9% (3/38) had AP-ROP. The rate of ROP was higher among the infants with BW of 900–1000 g than in infants with BW of 751–900 g. However, the rate of AP-ROP was less than that of infants with BW of 751–900 g (Table 3).
 |
Table 3 Distribution of ROP by BW |
The mean BW of infants who developed severe ROP was 860.00 ± 163.48 g and their mean GA was 26.56 ± 0.68 weeks, whereas those of the infants with not type 1 or 2 ROP were 913.73 ± 85.53 g and 26.59 ± 1.00 weeks, respectively. The differences in the mean GA and BW of the two groups were not statistically significant (both P > 0.05). However, PMA at the onset of ROP was statistically significantly different between the two groups (P < 0.05). PMA at the onset of ROP was younger in infants with severe ROP than in infants with not type 1 or 2 ROP (Table 4).
 |
Table 4 Characteristics in Patients with Severe ROP, Those with Not Type 1 or 2 ROP and No ROP |
Multiple Logistic Regression Analysis
In previous studies of older premature infants, GA and BW were significant predictors of the development of severe ROP.11–13 However, in our study, a multivariate logistic regression showed that neither BW (OR: 0.996; 95% CI: 0.990–1.001; P = 0.110) nor GA (OR: 1.151; 95% CI: 0.601–2.205; P = 0.671) was a risk factor for the development of severe ROP in this EP and ELBW infant population. Our conclusion does not contradict those of other major articles because the inclusion criteria for BW and GA were quite different.
Characteristics of Infants with Severe ROP
The mean PMA at first diagnosis of AP-ROP and type 1 ROP was 33.33 ± 3.06 weeks and 33.14 ± 1.96 weeks, respectively. The distribution of severe ROP in different GA and BW groups were showed in Tables 2 and 3.
Most (10/14, 71.4%) of the infants with type 1 ROP developed stage 3 ROP in zone II with plus disease at a mean PMA of 33.20 ± 1.75 weeks, whereas the other three infants with type 1 ROP developed stage 2 ROP in zone II with plus disease at a mean PMA of 31.67 ± 0.58 weeks, and only one developed stage 3 ROP in zone I without plus disease. Ten infants with type 2 ROP all showed stage 3 ROP in zone II without plus disease (Table 5).
 |
Table 5 Characteristics of Infants with Severe ROP and Type 2 ROP |
The mean GA and BW of infants with severe ROP were 26.55 ± 0.62 weeks and 863.15 ± 142.64 g, respectively. The incidence of ROP in infants with BW ≤ 750 g and BW 750–1000 g was 77.8% (7/9) and 80.0% (76/95), respectively, and the incidence rates of severe ROP were 22.2% (2/9) and 15.8% (15/95), respectively. The percentage of severe ROP was greater in infants with BW < 750 g than in infants with BW of 751–1000 g. However, a statistical analysis showed no significant differences in the characteristics of the two BW groups, except in the mean GA (Table 6).
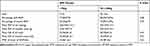 |
Table 6 Characteristics of Different Birth Weight Groups |
Discussion
ROP is an important cause of low visual acuity or blindness especially in developing countries with low infant mortality rates. With advances in neonatology, the survival rates among ELBW and very low birth weight (VLBW) premature infants have increased. As a result, the number of preterm babies with ROP has increased.14 However, few studies of the ROP epidemic in EP infants born with ELBW in developing countries have been published, and these infants are considered to be at significantly higher risk of severe ROP. This retrospective study investigated the incidence and clinical characteristics of ROP in EP and ELBW infants at a single center in eastern China.
The incidence of ROP in ELBW infants varies across different populations and races, ranging from 90% in Brazil to 32.8% in India. In this study, our results showed an incidence of 79.8% ROP in EP and ELBW Chinese infants who met the established criteria for ROP screening. Our findings are consistent with some of the other studies. In Brazil, Graziano et al reported that 78.5% of infants with BW <1000 g and 72.7% of infants with GA <30 weeks had ROP.15 Another multicenter study from Brazil by Zin et al found that the rates of ROP in infants with BW <1000 g were 61.9%.16 In a study in Brunei Darussalam, the incidence of ROP among ELBW infants (86.7%) was much higher than that among VLBW infants (27.8%).17 Moraes et al reported that 90% of preterm infants with BW < 1000 g developed ROP.18 A report from India showed that 32.8% of preterm babies with BW < 1000 g developed ROP which was lower than the proportions in other reports.19 According to the CRYO-ROP study, 81.6% of ELBW infants developed ROP,20 and the ETROP study showed that 82.5% of infants with BW < 1000 g developed ROP.21 A multi-country research project by Brian A. Darlow showed that the rate of any ROP in infants born between 24 and 28 weeks, with BW < 1500 g, ranged from 25.2% to 91.0% in Switzerland and Japan, respectively, and that the rate of treatment varied from 4.3% to 30.4%.22
The age of ROP onset may differ in EP and ELBW infants from that in older premature infants. An unnecessarily early screening examination would be an unjustifiable economic burden, but the later detection of ROP may cause the optimal window for its treatment to be missed. Therefore, it is important to perform the initial ROP examination of EP and ELBW infants at the optimal time. According to the latest joint statement of the American Academy of Pediatrics, the American Academy of Ophthalmology, the American Association for Pediatric Ophthalmology and Strabismus, and the American association of certified orthoptists published in 2018, 31 weeks PMA is the recommended time to perform the initial ROP screening examination for infants with a GA ≤ 28 weeks.23 In this study, the median PMA at the onset of ROP was 34.51 ± 2.40 weeks (range: 30–40 weeks), which was earlier than in the studies of infants with BW > 1000 g. These data are similar to those of other ELBW studies and indicate that the more premature the infant, the earlier that ROP might occur, increasing the risk of developing severe ROP.24
This study included 104 infants with BW ≤ 1000 g and GA ≤ 28 weeks. Of the 83 (79.8%) infants who developed ROP, three (2.9%) had AP-ROP, 14 (13.5%) had type 1 ROP, 10 (9.6%) had type 2 ROP, and only one developed retinal detachment. Nearly half the cases of AP-ROP or type 1 ROP occurred in infants with a GA of 26–27 weeks. The incidence of ROP was also highest in infants born with a GA of 26–27 weeks. Therefore, the incidence and severity of ROP were not directly inversely related to GA and BW in the EP and ELBW infants. Although the other major articles suggested that low GA and low BW were associated with severe ROP. In fact, the two approaches are not conflicting but complementary. Our research mainly focuses on the EP and ELBW infants whose GA and BW were low enough. This means that we continue to refine the analysis in their low GA and BW subgroup. In general, there is no doubt that low GA and low BW are perinatal risk factors for ROP. Moreover, our research provides a refinement and supplement to the above conclusions.
We also compared the characteristics of ROP between infants with BW ≤ 750 g and BW 750–1000 g. The rate of severe ROP was greater in infants with BW ≤ 750 g than in infants with BW of 751–1000 g. However, a statistical analysis showed that the two groups did not differ significantly in many characteristics, except their mean GA.
There were several limitations to this study. First, the retrospective design of the study meant that the data were inconsistent and that the follow-up period was not standardized and varied among patients. Second, it is well recognized that in large series, lower GA and lower BW indicate a greater the risk of ROP. However, these risk factors may lose much significance when other risk factors are included. In this study, many other systemic postnatal factors were not included when the risk of ROP was calculated because the data were incomplete. Therefore, there may have been some bias in our results. Third, our sample size was insufficient to generalize our findings to all EP and ELBW infants in the Chinese population.
However, our study still provides useful information on the incidence and clinical characteristics of ROP in EP and ELBW infants in eastern China. With economic growth, improvements in neonatal care, and the development of the two-child policy throughout the Chinese populations of mainland China, it is predicted that the survival rate of EP and ELBW infants will continue to increase. Therefore, data on the clinical characteristics of and risk factors for severe ROP are important for its timely screening and treatment in China.
Data Sharing Statement
The data and materials used in this study are available from the corresponding author.
Ethics Approval and Consent to Participate
The study was approved by the Institutional Review Board of Eye and ENT Hospital of Fudan University, and all the patients’ guardians provided written informed consent.
Consent for Publication
All the patients’ guardians provided written informed consent so that the results could be analyzed for scientific publication.
Acknowledgments
We thank the Retina and Vitreous Department of the Eye and ENT Hospital of Fudan University for their collaboration with the diagnosis and treatment of ROP, as well as their suggestions and comments of our research.
Author Contributions
All authors read and approved the final manuscript. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. The manuscript is approved by all authors for publication.
Funding
This work was supported by National Natural Science Foundation of China (81570855, 81770944) and National Natural Science Foundation for Young Scholar of China (81400410).
Disclosure
The authors report no conflicts of interest for this work.
References
1. O’Connor AR, Stephenson T, Johnson A, et al. Long-term ophthalmic outcome of low birth weight children with and without retinopathy of prematurity. Pediatrics. 2002;109(1):12–18. doi:10.1542/peds.109.1.12
2. Beck K, Young R, Read S, Harper H, Desireddi J, Harper CA. The severity and associated comorbidities of retinopathy of prematurity among micro-premature infants with birth weight less than 750 grams. J Neonatal Perinatal Med. 2019;12(1):41–45.
3. Shah PK, Prabhu V, Karandikar SS, Ranjan R, Narendran V, Kalpana N. Retinopathy of prematurity: past, present and future. World J Clin Pediatr. 2016;5(1):35–46.
4. Zin AA, Moreira ME, Bunce C, Darlow BA, Gilbert CE. Retinopathy of Prematurity in 7 neonatal units in Rio de Janeiro: screening criteria and workload implications. Pediatrics. 2010;126(2):e410–e417.
5. Smith LEH, Hellström A, Stahl A, Fielder A, Chambers W, Moseley J. Development of a Retinopathy of prematurity activity scale and clinical outcome measures for use in clinical trials. JAMA Ophthalmol. 2019;137(3):305–311.
6. Chen Y, Xun D, Wang YC, et al. Incidence and risk factors of retinopathy of prematurity in two neonatal intensive care units in North and South China. Chin Med J. 2015;128(7):914–918.
7. Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84(2):77–82.
8. Gilbert C, Rahi J, Eckstein M, O’Sullivan J, Foster A. Retinopathy of prematurity in middle-income countries. Lancet. 1997;350(9070):12–14.
9. Ni YQ, Huang X, Xue K, et al. Natural involution of acute retinopathy of prematurity not requiring treatment: factors associated with the time course of involution. Invest Ophthalmol Vis Sci. 2014;55(5):3165–3170.
10. Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121(12):1684–1689.
11. Lundgren P, Kistner A, Andersson EM, Hansen Pupp I, Holmström G, Ley D. Low birth weight is a risk factor for severe retinopathy of prematurity depending on gestational age. PLoS One. 2014;9(10):e109460. doi:10.1371/journal.pone.0109460
12. Hwang JH, Lee EH, Kim EA-R. Retinopathy of prematurity among very-low-birth-weight infants in Korea: incidence, treatment, and risk factors. J Korean Med Sci. 2015;Suppl 30(Suppl 1):S88. doi:10.3346/jkms.2015.30.S1.S88
13. Wu W-C, Chen Y-H, Lien R, et al. Retinopathy of Prematurity in Neonatal Patients with Birth Weight Greater than 1500 g in Taiwan. Biomedical Journal. 2013;36(2):84–89. doi:10.4103/2319-4170.110399
14. Wu T, Zhang L, Tong Y, Qu Y, Xia B, Mu D. Retinopathy of prematurity among very low-birth-weight infants in China: incidence and perinatal risk factors. Invest Ophthalmol Vis Sci. 2018;59(2):757–763. doi:10.1167/iovs.17-23158
15. Graziano RM, Leone CR, Cunha SL, Pinheiro AC. Prevalence of retinopathy of prematurity in very low birth weight infants. J Pediatr (Rio J). 1997;73(6):377–382. doi:10.2223/JPED.564
16. Zin AA, Moreira ME, Bunce C, Darlow BA, Gilbert CE. Retinopathy of prematurity in 7 neonatal units in Rio de Janeiro: screening criteria and workload implications. Pediatrics. 2010;126(2):410–417. doi:10.1542/peds.2010-0090
17. Ali NA, George J, Joshi N, Chong E. Prevalence of retinopathy of prematurity in Brunei Darussalam. Int J Ophthalmol. 2013;3:381–384.
18. Moraes NSB, Farah ME, Bonomo PP, Almeida MFB. Diode laser versus cryotherapy for the treatment of the retinopathy of prematurity: a comparative study. Arq Bras Oftalmol. 1997;60(6):635–638.
19. Kumar P, Sankar MJ, Deorari A, et al. Risk factors for severe retinopathy of prematurity in preterm low birth weight neonates. Indian J Pediatr. 2011;78(7):812–816.
20. Palmer EA, Flynn JT, Hardy RJ. Incidence and early course of retinopathy of prematurity. Ophthalmology. 1991;98:1628–1640.
21. Good WV, Hardy RJ, Dobson V, et al. The incidence and course of retinopathy of prematurity: findings from the early treatment for retinopathy of prematurity study. Pediatrics. 2005;116(1):15–23.
22. Darlow BA, Lui K, Kusuda S, et al. International variations and trends in the treatment for retinopathy of prematurity. Br J Ophthalmol. 2017;101(10):1399–1404.
23. Fierson WM. American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2018;142(6):e20183061.
24. Subhani M, Combs A, Weber P, Gerontis C, DeCristofaro JD. Screening Guidelines for Retinopathy of Prematurity: the Need for Revision in Extremely Low Birth Weight Infants. Pediatrics. 2001;107(4):656–659.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
