Back to Journals » Journal of Blood Medicine » Volume 6
Rate and predictors of low serum ferritin levels among healthy parturient women in Enugu, Nigeria
Authors Emegoakor FCJ, Iyoke CA , Ezegwui HU, Ezugwu FO, Umeora OU, Ibeagha IO
Received 7 February 2015
Accepted for publication 10 July 2015
Published 18 September 2015 Volume 2015:6 Pages 261—267
DOI https://doi.org/10.2147/JBM.S82411
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Fausta Chioma J Emegoakor,1 Chukwuemeka Anthony Iyoke,1 Hyginus Uzo Ezegwui,1 Frank Okechukwu Ezugwu,2 Odidika Ugochukwu Umeora,3 Izuchukwu Obumneme Ibeagha4
1Department of Obstetrics and Gynaecology, University of Nigeria Teaching Hospital, Ituku-Ozalla, 2Department of Obstetrics and Gynaecology, Enugu State University Teaching Hospital, Park Lane, Enugu, 3Department of Obstetrics and Gynaecology, Federal Teaching Hospital, Abakaliki, 4Department of Haematology, University of Nigeria Teaching Hospital, Ituku-Ozalla, Enugu, Nigeria
Background: Low serum ferritin levels signify low iron stores and this could predispose to iron deficiency anemia.
Objective: To determine the rate and predictors of low serum ferritin levels during the puerperium in Enugu, Southeast Nigeria.
Study design: A hospital-based prospective longitudinal study involving parturient women who delivered singleton fetuses at term. Venous blood samples were collected to determine the serum ferritin concentration at 48 hours and 6 weeks postpartum. Data analysis involved descriptive and inferential statistics at 95% confidence interval (CI) using Statistical Package for Social Sciences (SPSS) computer software version 20.0.
Results: Two-hundred and two women who carried singleton pregnancies to term were studied. The mean serum ferritin levels at 48 hours and 6 weeks were 27.82±18.41 µg/L and 36.12±21.53 µg/L, respectively. Forty-eight hours postdelivery, 29.2% had low ferritin levels and this decreased to 12.4% at 6 weeks postpartum. There was a significant positive correlation between the serum ferritin level at 48 hours postdelivery and the serum ferritin level at 6 weeks postpartum (r=0.89, P<0.001). Predictors of the low ferritin level at 6 weeks included age <20 years (odds ratio [OR] =0.70, 95% CI =0.53, 0.93), multiparity (OR =63.7, 95% CI =3.18, 127.5), anemia at 48 hours postpartum (OR =61.7, 95% CI =13.27, 116.6), a low ferritin level at 48 hours (OR =78.1, 95% CI =8.8, 108.3), and intake of antenatal hematinics for <3 months (OR =0.04, 95% CI =0.01, 0.20).
Conclusion: There was a significant occurrence of low ferritin levels during the puerperium in the study centers, and this was associated mainly with pregnancy and delivery factors. Efforts to improve the iron stores in parturient women could benefit from early booking and compliance with antenatal hematinics and optimizing hemoglobin and iron levels before delivery.
Keywords: iron deficiency, iron stores, puerperium, predictors, postpartum
Introduction
Iron deficiency, the common cause of anemia, underlies 115,000 maternal deaths worldwide per year.1 Iron is a major constituent of hemoglobin, the oxygen-carrying pigment of the red blood cells. Its level varies with different trimesters of pregnancy, and this, in turn, is a function of the net effect of absorption, utilization, and body losses. There is an increased demand for iron during pregnancy as a result of fetal and placental growth as well as expansion of blood volume. It has been shown that iron stores at conception strongly predict the maternal iron status and the risk of anemia later in pregnancy.2,3
Several biomarkers have been used to assess the iron status in individuals. These include hemoglobin, serum ferritin, zinc protoporphyrrin, total iron-binding capacity, and transferrin saturation.4 Serum ferritin is an iron-storage glycoprotein whose concentration is a true reflection of body iron stores in the absence of inflammatory/chronic infectious changes.4,5 The serum ferritin level is considered the best in assessing iron deficiency in pregnancy5 and together with hemoglobin level is regarded as the most efficient combination of indicators for monitoring the change in the iron status of a population.6
It is known that hemoglobin and iron concentrations vary during pregnancy, immediate postpartum, throughout the puerperium, and sometimes up to 12 weeks postpartum. When these parameters are below the normal threshold, complications such as iron-deficiency anemia may arise. Iron-deficiency anemia during pregnancy has been associated with increased risks of low birth weight, prematurity, and maternal morbidity.7 Studies carried out in Africa and other regions of the world have identified iron deficiency as a common cause of anemia.8 It was reported in a study performed in pregnant women from Zimbabwe, People’s Republic of China, India, and Mexico (between 1996 and 2008) that 43%–73% of the subjects were iron deficient, of which 7%–33% had iron-deficiency anemia.8
Puerperal hematological status is important for optimal maternal health for breastfeeding and restoration of the maternal iron and hemoglobin status before subsequent conception. The prevalence of iron deficiency during the puerperium has not been documented in this area before this study. The aim of this study was to determine the rate and predictors of low serum ferritin levels during the puerperium among apparently healthy women in Enugu, Southeast Nigeria.
Methods
Study area
Enugu State is a mainland state in southeastern Nigeria, with a land area of ~8,727.1 km2 (3,369.6 mile2) located between the latitude 60 301°N and longitude 70 301°E. The state shares borders with Abia and Imo States to the south, Ebonyi State to the east, Benue State to the northeast, Kogi State to the northwest, and Anambra State to the west. Enugu State lies partly within the semitropical rain forest belt of the south, and its vegetation changes gradually from the tropical rain forest to open woodlands and then to Savannah. The 2006 population census estimated the Enugu State population to be 3,257,298.9 The females constitute 50.1% of the population, and the population of women of reproductive age (15–49 years) was estimated to be 716,600.9
Study centers
The study took place in the University of Nigeria Teaching Hospital, Ituku-Ozalla, and Enugu State University Teaching Hospital, Park Lane, both of which are tertiary health care centers that offer multidisciplinary services at primary and tertiary levels. In addition to serving as referral centers to other southeastern states, these institutions are also training and research centers. The annual delivery rate ranges between 1,500 and 1,800. The obstetric units conduct daily antenatal and postnatal clinics.
Study population
The study population included women with uncomplicated pregnancies who delivered singleton babies at term in the two study centers. Exclusion criteria included women with hematological disorders like bleeding disorders, sickle cell anemia, or such conditions requiring blood transfusion or iron infusion; women with other comorbidities like renal diseases, pre-eclampsia/eclampsia, diabetes mellitus, HIV infections; and women with fever during the peripartum period. Fever, here, is defined as a temperature ≥38°C.
Study design/study period
This was a prospective longitudinal study carried out between December 2013 and March 2014.
Sampling
Pregnant women who booked for antenatal care in the two centers were told about the study by the researcher/assistants during the antenatal clinic visits. The importance of serial blood sample collection and the need for compliance to follow-up were explained to the antenatal attendees. Only those women whose pregnancies had reached term were given consent forms to sign and bring to the hospital while coming for delivery, if they consented. Term was defined as 37 completed weeks to 41+6 weeks.
On admission for delivery at the study centers, the counseling on the proposed study was repeated. Then, review of their antenatal records, preliminary history taking, and physical examination were done to exclude confounders. The actual enrollment took place 48 hours postdelivery after exclusion of those with postpartum hemorrhage requiring blood transfusion or iron infusion. Forty-eight hours after delivery, a venous blood sample was collected in plain bottles to assay the serum ferritin levels. Also, a blood sample was collected for hemoglobin estimation at 48 hours after delivery.
Women received their postpartum care according to the hospitals’ protocols. This included the continuation of the routine hematinics given to them during pregnancy for at least 2 weeks postpartum, which consists tablets of ferrous sulfate (200 mg) twice daily and folic acid once daily for 6 weeks. Women with normal vaginal delivery were discharged 24 hours after delivery but stayed for 48 hours’ sample collection at no extra cost during the study period.
Follow-up was facilitated by obtaining the phone numbers from the patients and their partners or spouses in order to remind them of their scheduled appointment 2–3 days before time. Participants were told to report any morbidity as they arose during the puerperium before the scheduled postnatal clinic visits at 6 weeks. At the 6-week postpartum visit, clinical evaluation of the subjects was carried out to assess the general well-being and if there was any form of morbidity necessitating medical intervention since discharge from the hospital. Information regarding the commencement of menses or persistence of lochia discharge was also elicited. Finally, venous blood samples were taken for hemoglobin and serum ferritin assay.
Procedure for serum ferritin assay
This was performed by ELISA technique using ELISA machine Stat Fax-2600 (Awareness Tech Inc., Palm City, FL, USA). The blood samples were first centrifuged to separate the serum from the cellular components. The sera now in well-labeled cryovials were stored at −80° in a freezer till a complete batch for one kit of reagent was obtained. Prior to the biochemical analysis of each batch, the samples were thawed and kept at room temperature. The kit used was Assay Max Human Ferritin ELISA kit, and it ran between 90 and 96 samples per batch. The assay employed the quantitative sandwich enzyme immuno assay technique that measured the ferritin levels in <4 hours at room temperature. The samples of sera were made to undergo a series of incubations and washed at different intervals using the reagent and buffer solutions till optimal color density developed. The absorbance was then read immediately on a microplate reader at a wavelength of 450 nm.
Data collection and analysis
The data collection tool used was a structured proforma. The proforma contained the patients’ sociodemographic variables, breastfeeding information, gestational age at delivery, estimated blood loss at delivery, weight of the baby, compliance with and duration of intake of routine hematinics, levels of serum ferritin and hemoglobin 48 hours and 6 weeks after delivery, and information regarding the return of menses. The data collection commenced at enrollment and was completed by 6 weeks. The principal investigator (Emegoakor FCJ) together with the trained research assistants collected samples for the 48-hour postdelivery hemoglobin and serum ferritin estimation for the two centers. Two registrars from each of the study centers were trained on the study protocol, as research assistants, to help in the recruitment of patients and sample collection. All the samples were taken to University of Nigeria Teaching Hospital for the biochemical analysis.
The data collected were analyzed using the Statistical Package for Social Sciences (SPSS) computer software version 20.0 for Windows. Anemia was defined as hemoglobin concentration <11.0 g/dL. The normal range of serum ferritin for the study laboratory was 15–200 ng/mL. Simple descriptive statistics was used to calculate the mean serum ferritin 48 hours after delivery and at 6 weeks. Categorical variables were compared using chi-square and Fisher’s exact tests where appropriate. A comparison of means was done with student’s t-test. Pearson’s correlation coefficient was used to ascertain the association of continuous variables. Logistic regression was done to determine the predictors of low serum ferritin levels at 6 weeks postpartum. The dependent variable was low serum ferritin. The presence of low serum ferritin (ie, <15 ng/mL) was coded as 1 while normal ferritin was coded as 0; independent predictor variables included in the model were parity, age, use of antenatal hematinics, educational status, anemia at 48 hours postpartum, low serum ferritin at 48 hours postpartum, anemia at term, birth interval, and employment status. The results of logistic regression were reported as adjusted odds ratios (aOR) and 95% confidence intervals (CI) for odds ratios. The P-value for significance was set at ≤0.05. Ethical consent was obtained from the Research Ethics Committee of the University of Nigeria Teaching Hospital.
Results
Of the 220 women who were recruited during the period of the study, 202 completed the study and were used for analysis. This gave a completion rate of 91.8%. Figure 1 shows a flowchart of the study.
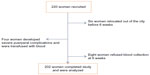  | Figure 1 Flowchart of the study. |
The average age of the participants was 30.3±4.6 years. The mean gestational age at delivery was 38.3 weeks. The mean blood loss at delivery was 276.09 mls. The mean birth weight of the babies was 3.3±0.4 kg. Ninety-five percentage of the women had at least secondary education and at least 64% were employed. Table 1 shows the sociodemographic and obstetric characteristics of the study participants.
  | Table 1 Sociodemographic and obstetric characteristics of the study subjects (n=202) |
Forty-eight hours postdelivery, 29.2% women had low ferritin levels and this decreased to 12.4% at 6 weeks postpartum. The mean serum ferritin levels at 48 hours and 6 weeks were 27.82±18.41 μg/L (range: 5–85 μg/L) and 36.12±21.53 μg/L (range: 10–97 μg/L), respectively. There was a statistically significant difference between the mean serum ferritin levels at 48 hours and 6 weeks postpartum (t =−11.73; P-value <0.001).
There was a significant positive correlation between hemoglobin level at 48 hours postpartum and serum ferritin level at 6 weeks postpartum (r=0.39, P<0.001). There was, also, a significant positive correlation between serum ferritin level at 48 hours postdelivery and at 6 weeks postpartum (r=0.89, P<0.001).
Table 2 summarizes the logistic regression to determine the predictors of serum ferritin levels at 6 weeks postpartum. It showed that low ferritin levels at 48 hours postpartum increased the likelihood of a low ferritin level at 6 weeks postpartum (OR =78.1, 95% CI =8.8, 108.3). Multiparity increased the likelihood of low ferritin levels at 6 weeks, (OR =63.7, 95% CI =3.18, 127.5). The intake of antenatal hematinics for <12 weeks increased the likelihood of low ferritin levels at 6 weeks postpartum, (aOR =2.80, 95% CI =1.74, 5.42). Maternal age <20 years increased the likelihood of low ferritin levels at 6 weeks postpartum, (aOR =0.7, 95% CI =0.53, 0.93). Anemia at 48 hours postpartum increased the likelihood of a low ferritin level at 6 weeks postpartum, (aOR =61.7, 95% CI =3.3, 116.6).
  | Table 2 Logistic regression model showing predictors of low serum ferritin at 6 weeks postpartum |
Discussion
This work is the second part of a study on the hematological status of healthy parturient women in Enugu. Available local studies on iron stores and other hematological parameters were performed during pregnancy and none aimed at the iron stores with regard to the postpartum period. Therefore, most of the comparisons made here relied more on data published outside our immediate environment.
Values of serum ferritin levels obtained at 6 weeks in our study were in agreement with the values that were obtained by Mara et al among women who received no iron supplementation.10 However, our results show lower ferritin levels than what were obtained among women who received iron supplementation in the studies by Mara et al10 and Milman et al.11 However, both studies differed slightly from our study in that they assayed serum ferritin at 8 weeks postpartum. Since the return to the pre-pregnancy state is a continuous process and does not abruptly end at 6 weeks postpartum, the 2-week difference in the timing of the measurements could account for the higher values obtained in the quoted studies. Unlike in our study, these researchers selected women who were of a relatively better nutritional status, which impacts positively on the iron stores even prior to conception. It is known that iron stores at conception are a strong predictor of the maternal iron level status and the risk of anemia later during pregnancy.2 Also, the compromised prenatal iron status limits the amount of iron that would return to maternal iron stores following delivery.12 The women in the abovementioned studies were not just supplemented early but monitoring for adherence was instituted. This was not the case in our study which was not an interventional study.
Our results suggest that the women with uncomplicated pregnancies on an average do not have a high prevalence of iron depletion in the study centers. This is based on the previously documented fact that a ferritin concentration <15 μg/L indicates iron depletion and levels ≥15 μg/L implies the presence of iron stores.5,13 Also, in women of reproductive age, a serum ferritin level <15 μg/L has shown a specificity of 98% and sensitivity of 75% for iron deficiency, as defined by no stainable bone marrow iron.14 Our results, therefore, agree with previous studies on the ferritin level of pregnant and non-pregnant subjects, which concluded that iron deficiency did not have a high prevalence in Nigeria.15,16 Of importance, in this study, however, is the observation of different degrees of iron deficiency and sideropenia in which some women who had no anemia were found with iron deficiency. This may stem from the fact that iron deficiency occurs in stages, and the women observed with sideropenia may be in the early stage where serum ferritin is significantly reduced with no obvious change in the hemoglobin level.5,17 Other stages of iron deficiency are the stage of iron-deficient erythropoiesis and finally iron-deficiency anemia.5,18 Similar observations were also noted by previous researchers despite the use of iron/folate supplements.10,19 Our results also show that antenatal and delivery factors were strong predictors of low serum ferritin levels in the puerperium. The fact that increasing duration of the use of antenatal hematinics reduced the likelihood of sideropenia at 6 weeks postpartum is not surprising. This is because antenatal hematinics help to replenish iron stores apart from replacing the iron lost during delivery.
On its part, blood loss at delivery significantly correlated negatively with serum ferritin levels at 24 hours and 6 weeks. This is in keeping with previous studies that observed blood loss at delivery to be a strong predictor/risk factor for postpartum anemia and iron deficiency.20–23 It is known that each mL of blood lost from the body results in a loss of 0.5 mg of iron and that each gram of hemoglobin contains 3.47 g of iron.24 This implies that the more the blood loss at delivery, the higher the incidence of iron deficiency and anemia at 6 weeks.
Other factors like anemia at term and 48 hours after delivery were predictive iron deficiencies at 6 weeks like was observed in previous studies12,20,25 This is expected considering that uncorrected prepartum iron deficiency anemia is already a risk factor for postpartum iron deficiency and anemia.26–28 Again, prepartum iron insufficiency and anemia make women vulnerable to the effects of blood loss at delivery thereby limiting the amount of iron that will return to the iron stores at 6 weeks.12,22,29 Some sociodemographic characteristics such as age and parity were observed to predict low ferritin levels at 6 weeks. An increase in parity increased the likelihood of low serum ferritin levels and an increase in age reduced the likelihood of low ferritin levels at 6 weeks. The effect of high parity on serum ferritin may be due to an inadequate replacement of iron utilized in pregnancy (and pregnancy has a high demand for iron as previously documented). Therefore, the more the number of pregnancies carried to term, the more the iron status is depleted, worse if the birth interval is short. On its part, the effect of age on serum ferritin level seen in our study may possibly be due to the less demand of iron on growth and development seen in older than younger people as also observed in a previous study.30 A similar effect of parity and age on serum ferritin levels was also noted in a previous study.31
Limitations
The limitations of this study include the fact that it was hospital based, which limits the external validity of our findings. Factors such as nutritional status/dietary habits as well as possible parasitic (hookworm) infestations which may affect iron stores were not sought or controlled.
Nigeria comprises an ethnically diverse population while this study was conducted on Igbo women only. A multicenter study of puerperal women belonging to the different ethnic groups will throw more light on the possible differences, if any, on the patterns of hematological changes after childbirth.
Conclusion/recommendation
Changes in ferritin levels during puerperium in this cohort of women followed the same trend as observed in other parts of the world. However, there was a significant occurrence of low ferritin levels during the puerperium in the study centers, and this was associated mainly with pregnancy and delivery factors. Efforts to improve the iron stores in parturient women could benefit from improving the compliance with antenatal hematinics and optimizing hemoglobin and iron levels before delivery.
Disclosure
The authors report no conflicts of interest in this work.
References
Sanghvi TG, Harvey PW, Wainwright E. Maternal iron folic acid supplementation programs: evidence of impact and supplementation. Food Nutr Bull. 2010;31(2 Suppl):S100–S107. | |
Casanueva E, Pfeffer F, Drijanski A, Fernández-Gaxiola AC, Gutiérrez- Valenzuela V, Rothenberg SJ. Iron and folate status before pregnancy and anemia during pregnancy. Ann Nutr Metab. 2003;47:60–63. | |
Viteri FE. The consequences of iron deficiency and anemia in pregnancy on maternal health, the foetus and infant. 1994. Available from: http://www.unsystem.org/SCN/archives/scnnews11/ch07.htm. Accessed August 05, 2012. | |
Cameron BM, Neufeld LM. Estimating the prevalence of iron deficiency in the first 2 years of life: technical and measurement issues. Nutr Rev. 2011;69(Suppl 1):S49–S56. | |
Pavord S, Myers B, Robinson S, Allard S, Strong J, Oppenheimer C. UK Guideline on the management of iron deficiency in pregnancy. British Journal of Haematology. 2012;156(5):588–600. | |
Mei Z, Cogswell ME, Parvanta I, et al. Hemoglobin and Ferritin are currently the most efficient indicators of population response to iron interventions: an analysis of nine randomized controlled trials. J Nutr. 2005;135:1974–1980. | |
Pasricha SRS, Flecknoe-Brown SC, Allen KJ, et al. Diagnosis and management of iron deficiency anemia: a clinical update. Med J Aust. 2010;193(9):525–532. | |
Mc Mahon LP. Iron deficiency in pregnancy. Obstet Med. 2010;3:17–24. | |
Enugu State. National Population Commission; 2006. Available from: http://www.population.gov.ng. Accessed December 13, 2011. | |
Mara M, Zivny J, Eretova V, et al. Changes in markers of anemia and iron metabolism and how they are influenced by anti anaemics in postpartum period. Acta Obstet Gynecol Scand. 2001;80(2):142–148. | |
Milman N, Bergholt T, Byg K-E, Eriksen L, Hvas A-M. Reference intervals for haematological variables during normal pregnancy and postpartum in 434 healthy Danish women. Eur J Hematol. 2007;79:39–46. | |
Bodnar LM, Cogswell ME, McDonald T. Have we forgotten the significance of postpartum iron deficiency? Am J Obstet Gynecol. 2005;193:36–44. | |
Assessing the iron status of populations: report of a joint WHO/CDC and Prevention Technical Consultation on the assessment of iron status at the population level, 2nd ed. Geneva: World Health Organisation; 2007. Available from: http://www.who.int/nutrition/publiications/micronutrients/anaemia.iron deficiency/9789241596106.pdf. Accessed October 30, 2012. | |
Hallberg L, Bengtsson C, Lapidus L, Lindstedt G, Lundberg P-A, Hultén L. Screening for iron deficiency: an analysis based on bone-marrow examinations and serum ferritin determinations in a population sample of women. Br J Haematol. 1993;85:787–798. | |
Adediran A, Gbadegesin A, Adeyemo TA, et al. Cord blood hemoglobin and ferritin concentrations in newborns of anemic and non-anemic mothers in Lagos. Niger Med J. 2013;54(1):22–26. | |
Abudu OO, Macaulay K, Oluboyede OA. Serial serum ferritin and other hematological parameters in normal Nigerian primigravidae. Int J Gynaecol Obstet. 1988;26:33–39. | |
Beutler E. Disorders of iron metabolism. In: Lichtman MA, editor. Williams Haematology. 7th ed. New York: McGraw-Hill; 2006;1221. | |
Cook JD. Diagnosis and Management of Iron deficiency anemia. Best Pract Res Clin Haematol. 2005;18:319–332. | |
Krafft A, Perewusnyk G, Hanseler E, Quack K, Huch R, Breymann C. Effect of Postpartum Iron Supplementation on red cell and iron parameters in non-anaemic iron deficient women: a randomized placebo controlled study. BJOG. 2005;112(4):445–450. | |
Chan SM, Nelson EAS, Leung SSF, Li CY. Postnatal iron status of Hong Kong Chinese women in a longitudinal study of maternal nutrition. Eur J Clin Nutr. 2001;55:538–546. | |
Petraro P, Duggan C, Urassa W, et al. Determinants of anemia in postpartum HIV negative women in Dar es Salaam, Tanzania. Eur J Clin Nutr. 2013;67(7):708–717. | |
Milman N. Oral iron prophylaxis in pregnancy: not too little and not too much! J Pregnancy. 2012;2012:514345. | |
Bergmann RL, Richter R, Bergmann KE, Dudenhausen JW. Prevalence and risk factors for early post-partum anemia. Eur J Obstet Gynecol Reprod Biol. 2010;150(2):126–131. | |
Harper JL, Conrad ME, Besa EC, Sacher RA, Schick P, Talavera F. Iron deficiency anemia. Available from: http://www.emedicine.medscape.com/article/202333. Accessed August 2, 2014. | |
Bodnar LM, Siega-Riz AM, Miller WC, Cogswell ME, McDonald T. Who should be screened for postpartum anemia? An evaluation of current recommendations. Am J Epidemiol. 2002;156:903–912. | |
Milman N. Postpartum anemia I: definition, prevalence, causes and consequences. Ann Hematol. 2011;90:1247–1253. | |
Bodnar LM, Scanlon KS, Freedman DS, Siega-Riz M, Cogswell ME. High prevalence of post-partum anemia among low-income women in the United States. Am J Obstet Gynecol. 2001;185(2):438–443. | |
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 95: anemia in pregnancy. Obstet Gynecol. 2008;112(1):201–207. | |
Kavle JA, Stoltfuz RJ, Witter F, Tielsch JM, Khalfan SS, Caulfield LE. Association between Anaemia during pregnancy and blood loss at and after delivery among women with vaginal births in Pemba Island, Zanzibar, Tanzania. J Health Popul Nutr. 2008;26(2):232–240. | |
Iannotti LL, O’Brien KO, Chang S-C, et al. Iron deficiency anemia and depleted body iron reserves are prevalent among pregnant African-American adolescent. J Nutr. 2005;135:2572–2577. | |
Vandevijvere S, Amsalkhir S, Van Oyen H, Ines E, Moreno-Reyes R. Iron status and its determinants in nationally representative sample of pregnant women. J Acad Nutr Diet. 2013;113(5):659–666. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
