Back to Journals » Journal of Asthma and Allergy » Volume 15
Rapid and Continuing Improvements in Nasal Symptoms with Dupilumab in Patients with Severe CRSwNP
Authors Bachert C, Khan AH , Hopkins C, Blaiss MS , Soler ZM, Nash S, Siddiqui S , Praestgaard A, Deniz Y, Rowe PJ, Jacob-Nara JA
Received 23 December 2021
Accepted for publication 15 April 2022
Published 4 May 2022 Volume 2022:15 Pages 557—563
DOI https://doi.org/10.2147/JAA.S355391
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Luis Garcia-Marcos
Claus Bachert,1– 3 Asif H Khan,4 Claire Hopkins,5 Michael S Blaiss,6 Zachary M Soler,7 Scott Nash,8 Shahid Siddiqui,8 Amy Praestgaard,9 Yamo Deniz,8 Paul J Rowe,10 Juby A Jacob-Nara10
1Upper Airways Research Laboratory and Department of Otorhinolaryngology, Ghent University, Ghent, Belgium; 2Division of ENT Diseases, CLINTEC, Karolinska Institutet, Stockholm, Sweden; 3International Airway Research Center, First Affiliated Hospital, Sun Yat-sen University, Guangzhou, People’s Republic of China; 4Global Medical Affairs, Sanofi, Chilly-Mazarin, France; 5Department of Otorhinolaryngology – Head and Neck Surgery, Guy’s and St Thomas’ Hospitals, London, UK; 6Department of Pediatrics, Medical College of Georgia at Augusta University, Augusta, GA, USA; 7Department of Otolaryngology – Head and Neck Surgery, Medical University of South Carolina, Charleston, SC, USA; 8Medical Affairs, Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA; 9Department of Biostatistics, Sanofi, Cambridge, MA, USA; 10Global Medical Affairs, Sanofi, Bridgewater, NJ, USA
Correspondence: Claus Bachert, Upper Airways Research Laboratory and Department of Otorhinolaryngology, Ghent University, Ghent, B-9000, Belgium, Tel +32 9332 5513, Email [email protected]
Purpose: In the phase 3 SINUS-24 (NCT02912468) and SINUS-52 (NCT02898454) studies in adults with severe chronic rhinosinusitis with nasal polyps (CRSwNP), dupilumab significantly improved the co-primary endpoints of change from baseline to Week 24 in nasal polyp score (NPS) and nasal congestion/obstruction (NC) vs placebo on background intranasal corticosteroids (standard of care [SOC]). This post hoc analysis of SINUS-24/-52 investigated the direction and magnitude of within-patient change in these endpoints over time.
Methods: NPS (scale 0– 8) was assessed at Weeks 4, 8, 16, 24, 40, and 52 in SINUS-52 and Weeks 8, 16, and 24 in SINUS-24. Daily patient-reported NC scores (0 [no symptoms]– 3 [severe symptoms]) were averaged over 28 days. Within-patient changes from baseline were assessed through Week 24 in pooled SINUS-24/-52 (n = 438/286 dupilumab/SOC) and through Week 52 in SINUS-52 (n = 150/153).
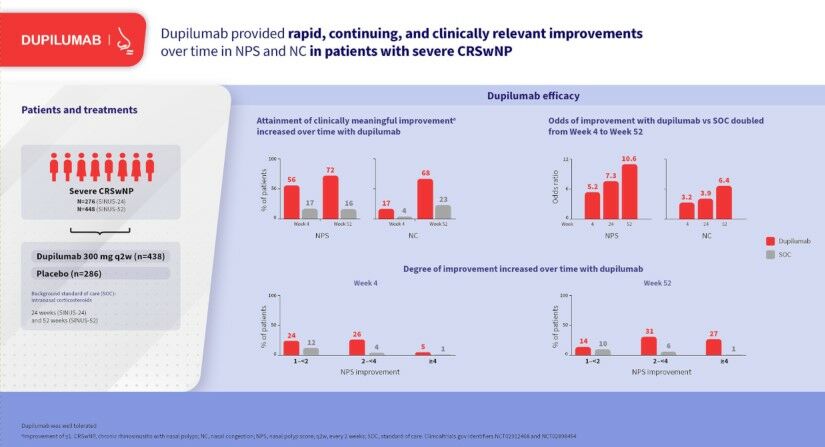
Results: In SINUS-52, NPS improved in 70.0% of dupilumab-treated patients at Week 4 vs 31.8% with SOC (odds ratio [OR] 5.2 [95% confidence interval 3.1– 8.8]) and 78.7% vs 28.2% at Week 52 (OR 10.6 [6.0– 18.7]) (all p < 0.0001). NC improved in 73.3% of dupilumab-treated patients at Week 4 vs 46.7% with SOC (OR 3.2 [2.0– 5.3]) and 86.9% vs 50.7% at Week 52 (OR 6.4 [3.5– 11.5]) (all p < 0.0001). Clinically meaningful (≥ 1 point) improvements in NPS occurred in 55.7% and 72.3% of dupilumab-treated patients at Weeks 4 and 52, respectively, vs 16.9% and 16.2% with SOC. Clinically meaningful (≥ 1 point) improvements in NC occurred in 16.7% and 67.6% of dupilumab-treated patients at Weeks 4 and 52, respectively, vs 3.9% and 20.8% with SOC. At Week 52, NPS worsening from baseline was observed in 5.7% of dupilumab-treated patients vs 40.1% with SOC and NC worsening in 2.1% vs 20.8%, respectively.
Conclusion: Dupilumab provided rapid, continuing, and clinically relevant improvements over time in NPS and NC in most patients with severe CRSwNP in the SINUS studies.
Keywords: chronic rhinosinusitis with nasal polyps, dupilumab, nasal polyp score, nasal congestion
Graphical Abstract:
Introduction
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a predominantly type 2-mediated inflammatory disease of the nasal cavity and paranasal sinuses that is associated with a high symptom burden, poor health-related quality of life, and high economic burden.1–3 Dupilumab is a fully human monoclonal antibody that binds to interleukin (IL)‐4Rα, the shared receptor component for IL-4 and IL-13, which are key and central drivers of type 2 inflammation in multiple diseases including CRSwNP.4,5 In the phase 3 SINUS-24 and SINUS-52 trials in adults with uncontrolled CRSwNP, dupilumab on a background of intranasal corticosteroids reduced polyp size, sinus opacification, and severity of symptoms, and was well tolerated.6 Both studies achieved their co-primary efficacy endpoints of reductions in bilateral nasal polyp score (NPS) and nasal congestion/obstruction (NC) at Week 24.
Clinical trial data on the efficacy of treatments for CRSwNP, including responder rates, are commonly reported at study protocol predefined timepoints, such as after 24 weeks or 52 weeks. Data on the evolution of within-patient responses over time are less commonly reported. Such data may provide additional granular evidence on the direction and magnitude of within-patient changes over time observed with new treatments and also with comparator regimens of optimized standard of care (SOC).
The aim of the current post hoc analysis was to investigate the proportion of patients with improved, stable, or worsened NPS and NC over time following treatment with dupilumab or placebo (optimized SOC) in the SINUS trials. We also report the proportion of patients who achieved different categories of improvement in NPS and NC over time. These analyses provide additional evidence to support decision making by patients, physicians, and policy makers on the magnitude of change observed in patients treated with dupilumab and in patients treated with optimized SOC.
Materials and Methods
This was a post hoc analysis of the SINUS-24 (NCT02912468) and SINUS-52 (NCT02898454) trials, details of which have been published previously.6 In brief, eligible patients had severe CRSwNP on a background of mometasone furoate nasal spray (MFNS; 2 × 50 µg actuations in each nostril twice daily [400 µg daily dose] or once daily [200 µg daily dose] if twice daily was not tolerated) and prior history of treatment with systemic corticosteroids and/or surgery for nasal polyposis. Patients were randomized 1:1 to dupilumab 300 mg subcutaneously (SC) or matching placebo every 2 weeks (q2w) for 24 weeks in SINUS-24 and 1:1:1 to dupilumab 300 mg SC q2w for 52 weeks, placebo q2w for 52 weeks, or dupilumab 300 mg SC q2w for 24 weeks, followed by dupilumab 300 mg SC every 4 weeks for 28 weeks in SINUS-52. All patients received SOC throughout the study with MFNS as described above.
Within-patient changes from baseline in NPS and NC were assessed for dupilumab 300 mg q2w and placebo through Week 24 in the pooled SINUS-24/-52 intention-to-treat population (n=286/438 [placebo/dupilumab]) and through Week 52 in SINUS-52 (n=153/150). NPS (range 0–8) was assessed at visits including at Weeks 4, 8, 16, 24, and 52 in SINUS-52, and Weeks 8, 16, and 24 in SINUS-24. NPS was scored as the average from 2 independent masked central readers of the nasoendoscopy video. NC score was recorded daily by patients using an eDiary (0, no symptoms; 1, mild symptoms; 2, moderate symptoms; 3, severe symptoms). NC score was calculated as the average of the prior 7 days for the baseline score, and prior 28 days for monthly post-baseline scores.
Data are presented as the percentage of patients with improvement (score decreased), stable (change = 0), or worsened (score increased) from baseline in NPS or NC. Estimated odds ratios were calculated for improvement vs [stable + worsened] with 95% confidence intervals using a logistic regression model adjusted for treatment group, asthma/non-steroidal anti-inflammatory drug-exacerbated respiratory disease status, prior surgery history, regions, baseline NPS or NC score, and (for pooled analyses) study. Data are also presented as percentage of patients by categories of improvement from baseline by >0–<1, ≥1–<2, ≥2–<3, ≥3–<4, ≥4–<5, and ≥5 points (NPS) or >0–<1, ≥1–<2, ≥2–<3, and = 3 points (NC); stable; or worsened. The proportions of patients achieving the minimum clinically important difference (≥1-point improvement)7 for both outcomes are also presented.
Results
NPS
At the earliest post-baseline assessment (Week 4 in SINUS-52), 70.0% of dupilumab-treated patients showed an improvement in NPS compared with 31.8% with optimized SOC (placebo) (Figure 1). At Week 24, the proportions of patients with improvement were 76.2% with dupilumab and 32.4% with SOC, and at Week 52, the proportions were 78.7% and 28.2%, respectively (Figure 1). A similar pattern of results was observed from Week 8 through Week 24 in the SINUS-24/-52 pooled analysis (Supplementary Figure 1).
The most frequent improvement in NPS at Week 4 (SINUS-52) was improvement of ≥1–<2 points with dupilumab (24.3% of patients) and >0–<1 point with SOC (14.9% of patients) (Figure 2). By Week 52 the most frequent improvement was ≥3–<4 with dupilumab (17.7% of patients) and >0–<1 with SOC (12.0% of patients). A similar trend was observed from Week 8 through Week 24 in the SINUS-24/-52 pooled analysis (Supplementary Figure 2).
Clinically relevant improvements in NPS (≥1 point)7 were seen by Week 4 (SINUS-52) in 55.7% of dupilumab-treated patients compared with 16.9% with SOC (Figure 2). By Week 52, clinically relevant improvements were seen in 72.3% of dupilumab-treated patients compared with 16.2% with SOC. A similar trend was observed from Week 8 through Week 24 in the SINUS-24/-52 pooled analysis (Supplementary Figure 2).
Worsening of NPS relative to baseline was seen in 10.7% and 33.8% of patients with dupilumab and SOC, respectively, at Week 4, and in 5.7% and 40.1% of patients, respectively, at Week 52 in SINUS-52 (Figures 1 and 2). A similar trend was observed from Week 8 through Week 24 in the SINUS-24/-52 pooled analysis (Supplementary Figures 1 and 2).
NC
At Week 4 in SINUS-52, 73.3% of dupilumab-treated patients showed an improvement in NC score compared with 46.7% with optimized SOC (placebo) (Figure 1). At Week 12 the proportions of patients with improvement were 80.9% with dupilumab and 57.3% with SOC, at Week 24 the proportions were 81.5% and 52.4%, and at Week 52 the proportions were 86.9% and 50.7%, respectively (Figure 1). A similar pattern of results was observed from Week 4 through Week 24 in the SINUS-24/-52 pooled analysis (Supplementary Figure 1).
The most frequent improvement in NC at Week 4 in SINUS-52 was improvement of >0–<1 point with dupilumab (56.7% of patients) and >0–<1 point with SOC (42.8% of patients) (Figure 2). By Week 52 the most frequent improvement was ≥1–<2 with dupilumab (31.0% of patients) and >0–<1 with SOC (27.8% of patients). A similar trend was observed from Week 4 through Week 24 in the SINUS-24/-52 pooled analysis (Supplementary Figure 2).
Clinically relevant improvements in NC (≥1 point)7 were seen by Week 4 (SINUS-52) in 16.7% of dupilumab-treated patients compared with 3.9% with SOC (Figure 2). By Week 52 clinically relevant improvements were seen in 67.6% of dupilumab-treated patients compared with 22.9% with SOC. A similar trend was observed from Week 4 through Week 24 in the SINUS-24/-52 pooled analysis (Supplementary Figure 2).
Worsening of NC relative to baseline was seen in 5.3% and 17.8% of patients with dupilumab and SOC, respectively, at Week 4, and in 2.1% and 20.8% of patients, respectively, at Week 52 in SINUS-52 (Figures 1 and 2). A similar trend was observed from Week 4 through Week 24 in the SINUS-24/-52 pooled analysis (Supplementary Figures 1 and 2).
Odds of Improvement Over Time
The odds ratios for improvement with dupilumab vs SOC increased from Week 4 to Week 24 and from Week 24 to Week 52 in SINUS-52. The odds of improvement in NPS with dupilumab vs SOC were 5.2 at Week 4 and increased to 7.3 and 10.6 at Weeks 24 and 52, respectively (Figure 1). The odds of improvement in NC with dupilumab vs SOC were 3.2 at Week 4 and increased to 3.9 and 6.4 at Weeks 24 and 52, respectively.
Discussion
Within-patient changes over time (worsened, stable, improved), and the magnitude of these changes, provide granular evidence on the efficacy of treatments assessed in clinical trials. The present analysis provides evidence on efficacy of dupilumab and also on optimized SOC in patients with severe CRSwNP in the SINUS trials. For each of the co-primary endpoints, NPS and NC, a significantly greater proportion of patients showed improvement from baseline with dupilumab than with optimized SOC from the first post-baseline visit to the last visit. Furthermore, in the dupilumab group, the proportion of patients with improvement increased from the first post-baseline visit to Week 24 and increased further from Week 24 to Week 52 for both NPS and NC. These findings indicate that while many patients show early response to dupilumab, in some patients the response can be manifested later. In contrast, with SOC the proportion of patients with improvement remained generally unchanged between Weeks 4, 24, and 52, indicating no change over time. The difference between dupilumab and SOC in prevalence of response over time is demonstrated by the odds of improvement with dupilumab vs SOC, which doubled between Week 4 and Week 52 for both NPS and NC. Even from Week 24 to Week 52 the odds of improvement with dupilumab vs SOC increased substantially, demonstrating the benefit of continuous treatment with dupilumab and that chronic treatment increases the odds of improvement.
The magnitude of within-patient improvements observed with dupilumab was greater than the magnitude of improvements observed with SOC at all analyzed timepoints. Moreover, the magnitude of within-patient improvements with dupilumab increased from the first post-baseline visit to the last for both NPS and NC. More than 10% of patients treated with dupilumab in SINUS-52 had a reduction in NPS of at least 5 points at Week 52, while >8% reported the maximum improvement of 3 points in NC at the same time point, representing complete resolution of symptoms from “severe” to “no symptom.” In contrast, with SOC at Week 52 no patients achieved 3-point improvement in NC or 5-point improvement in NPS.
Thresholds for clinically meaningful change in objective and patient-reported outcomes in patients with CRSwNP have recently been estimated using data from SINUS-24 and SINUS-52, with improvement of ≥1 point established as clinically meaningful for both NPS and NC.7 More than half of patients treated with dupilumab achieved clinically meaningful improvement in NPS by Week 4, rising to almost three-quarters by Week 52. In contrast, fewer than one-fifth of patients treated with SOC achieved clinically meaningful improvement in NPS by Week 4, and this proportion remained unchanged at Week 52. Clinically meaningful improvements in NC took longer to manifest than those for NPS: at Week 4, approximately 1 in 6 dupilumab-treated patients had achieved a ≥1-point improvement in NC and this increased to approximately 4 in 6 patients by Week 52. In contrast, fewer than 1 in 25 patients treated with optimized SOC achieved clinically meaningful improvement in NC by Week 4, and fewer than 1 in 4 by Week 52.
Worsening of NPS and NC was observed in only a small minority of patients treated with dupilumab, and the proportion of patients with worsening declined over the course of the study. In contrast, in patients treated with SOC, worsening of NPS was observed in more than 3 in 10 patients at Week 4, increasing to 4 in 10 patients by Week 52, and worsening of NC was seen in approximately 1 in 6 patients at Week 4, increasing slightly to approximately 1 in 5 by Week 52. These results illustrate the extent of unmet need with current SOC of intranasal corticosteroid spray for severe CRSwNP, despite this treatment being optimized in a clinical trial setting, which is likely better than would be achieved in a real-world setting. Worsening of disease, with dupilumab or placebo/SOC, may reflect temporal variation in disease severity among patients who are not responding to treatment. Further analysis will be required to investigate this subgroup of patients.
Conclusion
In conclusion, these results add to the evidence of dupilumab efficacy in patients with CRSwNP, and offer new insights into the time course of dupilumab’s treatment effects. Dupilumab provided rapid, continuing, and clinically relevant improvements over time in NPS and NC in the majority of patients with severe CRSwNP, and longer treatment led to a greater proportion of patients becoming responders.
Abbreviations
CI, confidence interval; CRSwNP, chronic rhinosinusitis with nasal polyps; IL, interleukin; MFNS, mometasone furoate nasal spray; NC, nasal congestion/obstruction; NPS, nasal polyp score; OR, odds ratio; q2w, every 2 weeks; SC, subcutaneously; SOC, standard of care.
Ethics Approval and Informed Consent
The studies were conducted in accordance with the Declaration of Helsinki, approved by the local institutional review board or ethics committee at each study site (Supplementary Table 1), and all patients provided written informed consent.
Data Sharing Statement
Qualified researchers may request access to patient-level data and related study documents including clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org/
Acknowledgments
Research sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. ClinicalTrials.gov Identifiers: NCT02912468 (SINUS-24) and NCT02898454 (SINUS-52). The authors thank Nadia Daizadeh, PhD (formerly of Sanofi), for statistical analyses and Neil Anderson, PhD, of Adelphi Group, Macclesfield, UK, for medical writing/editorial assistance funded by Sanofi and Regeneron Pharmaceuticals, Inc. in accordance with Good Publications Practice.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all of these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc.
Disclosure
C.B. reports advisory board membership and/or speakers’ fees from ALK, AstraZeneca, GlaxoSmithKline, Mylan, Novartis, Sanofi, Stallergenes Greer. C.H. reports advisory board membership from AstraZeneca, GlaxoSmithKline, OptiNose, Sanofi, Smith & Nephew. M.S.B. reports consultancy from ALK, Amgen, AstraZeneca, Bellus, Covis, Merck, Pfizer, Regeneron, Sanofi, TerSera. Z.M.S. reports advisory board membership and/or consultancy from Lyra, Novartis, Olympus, OptiNose, and is medical director of Healthy Humming and Sinusonic. A.H.K., A.P., P.J.R., J.A.J.-N. are employees and may hold stock and/or stock options in Sanofi. S.N., Y.D., S.S. are employees and shareholders in Regeneron Pharmaceuticals, Inc. The authors report no other conflicts of interest in this work.
References
1. Bachert C, Bhattacharyya N, Desrosiers M, Khan AH. Burden of disease in chronic rhinosinusitis with nasal polyps. J Asthma Allergy. 2021;14:127–134. doi:10.2147/JAA.S290424
2. Bhattacharyya N, Villeneuve S, Joish VN, et al. Cost burden and resource utilization in patients with chronic rhinosinusitis and nasal polyps. Laryngoscope. 2019;129(9):1969–1975. doi:10.1002/lary.27852
3. Khan A, Huynh TMT, Vandeplas G, et al. The GALEN rhinosinusitis cohort: chronic rhinosinusitis with nasal polyps affects health-related quality of life. Rhinology. 2019;57(5):343–351. doi:10.4193/Rhin19.158
4. Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425–437. doi:10.1080/1744666X.2017.1298443
5. Le Floc’h A, Allinne J, Nagashima K, et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Ralpha antibody, is required to broadly inhibit type 2 inflammation. Allergy. 2020;75(5):1188–1204. doi:10.1111/all.14151
6. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–1650. doi:10.1016/S0140-6736(19)31881-1
7. Han JK, Bachert C, Lee SE, et al. Estimating clinically meaningful change of efficacy outcomes in inadequately controlled CRSwNP. The Laryngoscope. 2021. doi:10.1002/lary.29888
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


