Back to Journals » OncoTargets and Therapy » Volume 13
Radiotherapy of Pulmonary Hepatoid Adenocarcinoma with Intrahepatic Hemangioma: A Case Report
Received 11 August 2020
Accepted for publication 23 October 2020
Published 19 November 2020 Volume 2020:13 Pages 11947—11955
DOI https://doi.org/10.2147/OTT.S275340
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Wenting Wang, Guang Li
Department of Radiation Oncology, The First Hospital of China Medical University, Shenyang, People’s Republic of China
Correspondence: Guang Li
Department of Radiation Oncology, The First Hospital of China Medical University, 155 Nanjing North Street, Shenyang, People’s Republic of China
Tel +86 1380408616
Email [email protected]
Abstract: Hepatoid adenocarcinoma is defined as an extrahepatic adenocarcinoma with hepatocyte differentiation, characterized by high malignancy and poor prognosis. Herein, we report the diagnosis, treatment and survival of a patient with pulmonary hepatoid adenocarcinoma who had received radiotherapy only. The patient was a 41-year-old man diagnosed with local advanced lung cancer (T3N3M0, stage IIIC). He had an intrahepatic hemangioma and abnormal serum liver enzymes. The patient developed intermittent fever with increased white blood cells and granulocytes during radiotherapy. After 38Gy/19 fractions of radiotherapy, the blood routine results returned to normal levels. After 50Gy/25 fractions of radiotherapy, the patient’s tumor was significantly shrank in imaging. Although the patient refused to receive any treatment after radiotherapy and died 12 months after diagnosis, the data presented here represent a valuable resource for understanding the survival benefits of pulmonary hepatoid adenocarcinoma patients treated with radiotherapy alone.
Keywords: hepatoid adenocarcinoma, lung, radiotherapy, prognosis
Introduction
Hepatoid adenocarcinoma (HAC) is a special type of adenocarcinoma with hepatocyte differentiation occurring in extrahepatic organs or tissues. The tumor composition can be simple HAC or HAC with typical acinar or papillary adenocarcinoma, signet ring cell or neuroendocrine carcinoma.1 Although most HACs are associated with elevated α-fetoprotein (AFP) levels, the current pathological diagnosis is based on morphological features, regardless of serum AFP levels or immunohistochemical AFP staining.2 Usually the patient’s liver has no lesions and functions normally. The most common HAC locations are the stomach (63%), ovaries (10%), lung (5%), gallbladder (4%), pancreas (4%), and uterus (4%), and the current treatment methods depend on the primary site, as are other common types of adenocarcinoma.3 With the development of targeted therapy and immunotherapy, it is possible to achieve a longer prognosis for paired patients. The current researches on pulmonary HAC are mostly case reports. We report a unique case of primary pulmonary HAC with intrahepatic hemangioma and abnormal serum liver enzymes, so as to provide an accurate prognostic basis for clinical diagnosis and treatment.
Case Report
A 41-year-old man was admitted to our department because of fever and right chest pain for 1 month. The chest CT revealed a 5.2×5.8cm mass in the upper lobe of the right lung. Plain CT value was about 30~42HU, while enhanced CT showed obvious uneven enhancement, with a CT value of about 47~72HU. Meanwhile, the right hilar lymph nodes were enlarged and enhanced (Figure 1A,C,E,G). Cervical ultrasound showed several grade III~IV lymph node echoes in the double supraclavicular fossa, with cortical thickening and rich blood flow. Head CT and whole-body bone imaging showed no obvious abnormality.
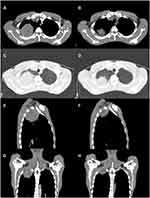 |
Figure 1 (A,C,E,G) Chest CT showed a 5.2x5.8cm mass in the upper lobe of the right lung. (B,D,F,H) After 50Gy/25 fractions of radiotherapy, the tumor shrank significantly on the radiograph. |
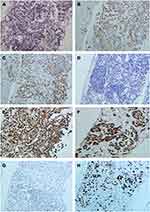
After disinfection and anesthesia, the right lung lesion was punctured with an 18G *15cm cutting needle in the prone position under CT guidance. Microscopically, heteromorphic cells were seen to be nest-like or lamellar with dense arrangement and hyperchromatic nuclei (Figure 2A). Immunohistochemistry showed CK(+), CK7(+), PAS-AB(+), Vimentin(+), Hepatocyte(+), Napsin-A(-), TTF-1(-), Ki-67 (60%) (Figure 2B–H). CD56, CD31, Desmin, ERG, HMB-45, Melan A, MiTF, S-100, SMA and STAT6 are negative. Hepatoid adenocarcinoma or metastatic hepatocellular carcinoma are not excluded. EGFR and KRAS gene mutations were not detected by fluorescence PCR.
Ultrasound revealed a strong echogenic mass in the patient’s liver with clear boundaries. And the patient’s liver function was abnormal: ALT 55U/L, ALP 646U/L, GGT 369U/L, ALB 29.1g/L, PA 5.70mg/dl. To further confirm the nature of hepatic lesion, hepatic enhancement MR showed a 14mm sized nodule in the upper segment of the right lobe of the liver near the inferior vena cava. The edge was strengthened in the arterial phase after enhancement, indicating that it was an intrahepatic hemangioma, rather than hepatocellular carcinoma or liver metastasis. Because of the rich blood supply of this lesion, the risk of bleeding after puncture is high, we did not obtain the pathology of liver lesion. Of the tumor markers, neuron specific enolase (NSE) was increased to 18.34ng/mL, and squamous cell carcinoma antigen (SCC) was increased to 7.20ng/mL. Levels of other tumor markers such as AFP, CEA, and Cyfra21-1 were all within normal limits.
The patient was 182-cm tall and weighed 50 kg. His Karnofsky performance status (KPS) score was 90. The patient had a 180 pack‑year smoking history of 20 years. He has a cephalospres allergy history and tumor family history (His grandmother was died of gingival carcinoma).
The patient was diagnosed with local advanced lung cancer (T3N3M0, stage IIIC) and began IMRT on November 1, 2018. During the treatment, patients had intermittent fever, up to 38.5°C. The white blood cells, PCT and CRP were all increased. The blood routine examination after the 19th radiotherapy showed that the white blood cells returned to normal level and the patient’s temperature returned to normal. The changes in complete blood count during radiotherapy are shown in Figure 3. After 50Gy/25 fractions of radiotherapy, the tumor shrank significantly on the CT scan (Figure 1B,D,F,H). After the radiotherapy of 60Gy/30f, the patient refused to accept further treatment, and regular review was recommended. On June 5, 2019, the patient, with a right chest pain NRS score of 7, began to take 5mg of compound capsule of oxycodone and acetaminophen (Tylox) every 6 hours and 10mg of morphine sulfate controlled-release tablets (MS Contin) every 12 hours. The patient developed convulsions and suspected brain metastasis in August 2019. However, the patient refused further examination and treatment and was controlled by oral gabapentin. The patient succumbed due to disease progression on December 8, 2019. The ultimate survival benefit from radiotherapy was 12 months.
 |
Figure 3 The changes in complete blood count (WBC, lymphocyte and granulocyte) depended on the date (days 0–30). |
Discussion
Hepatoid adenocarcinoma is defined as an extrahepatic adenocarcinoma with hepatocyte differentiation. HAC of the lung is rare. Patients often come to see a doctor because of chest pain and hemoptysis. According to the reported cases, the clinical features, treatments and prognosis of the pulmonary HAC patients are shown in Table 1 (palliative radiotherapy to bone or other metastatic sites is not included in the table).1,4–21
 |
Table 1 Clinical Features, Treatments and Prognosis of the Pulmonary Hepatoid Adenocarcinoma |
The incidence of pulmonary HAC is higher in men, which is similar to the incidence of other pathological types of lung cancer. The age of the patients ranges from 47 to 71, with an average age of 58.91 years. The patient in this case is 41 years old, the youngest ever reported. The upper lobe of the lung is the most common site of HAC. HAC grows faster and has a more malignant nature. It is usually found at a large size and at a later clinical stage. In most cases, AFP increased to varying degrees, but there are still patients with normal levels of AFP.5 In our case, serum AFP was not increased in the patient, but NSE and SCC were increased. Therefore, the ability of serum tumor markers to diagnose HAC is limited, and pathology is the gold standard of diagnosis. Microscopically, heteromorphic cells are arranged in nests or clumps with hepatoid differentiation. Immunohistochemically, AFP, CK7, HepPar-1 and hepatocyte are commonly positive markers.
Patients with HAC usually have no lesions in the liver with normal liver function. Our case had a hemangioma in the liver needing to be identified clinically. His liver enzyme and albumin levels are abnormal, which may be associated with a decrease in liver functioning mass. A mild increase in ALT may be caused by a virus (mainly hepatitis C) or alcohol abuse, while an increase in ALP may be caused by long-term medication, metastatic liver cancer, lymphoma, or invasive diseases such as sarcoidosis. Many diseases can cause an increase in GGT, including liver disease and non-liver disease, and taking drugs and alcohol can also cause an increase in GGT.22 This patient had no long-term medication history, and all hepatitis virus test results were negative. Combined with his long-term alcoholism history, it could be considered that the cause of his liver function decline was alcoholism.
Due to the rarity of pulmonary HAC, there is no effective treatment currently. Patients in the early stages had a relatively longer survival after surgery and postoperative chemoradiotherapy.4,8,10,11,14,17 The therapy for local advanced (stage III) patients are still inconsistent. Survival of patients who only underwent surgery were 14 and 18 months.1,19 After surgery, radiotherapy and chemotherapy, and immunotherapy, the reported survival was 14 months.21 And the survival period after radiotherapy and chemotherapy was 19 months.7 Our case only received thoracic radiation therapy, and the survival period reached 12 months. Overall, the average survival for III stage patients was 15 months. Chemotherapy may prolong the survival of local advanced patients.
Targeted drugs may offer hope to patients with specific genetic mutations. EGFR mutations occur in 30~60% of lung cancer patients in Asia.23 Chen HF reported a case of EGFR mutation with significant therapeutic effect of icotinib after surgery and adjuvant chemotherapy. Subsequently, EGFR T790M mutation related to resistance was discovered and osimertinib and anlotinib were administered. The patient is still alive after 29 months of follow-up.14 Gavrancic T reported an EGFR wild-type case treated with palliative chemotherapy and sorafenib. After 4 cycles of carboplatin/paclitaxel and sorafenib, chest CT showed stable disease. Three months later, the lung lesion was enlarged with the liver metastasis disappeared. The second line of vinorelbine and third line of gemcitabine with sorafenib both failed, and the patient eventually died 11 months after diagnosis.9 The progression-free survival of a patient with ALK gene rearrangement using crizotinib is 6 months.5 A patient with TP53 mutation who received erlotinib was reported to have a progression-free survival of 3 months.18 From the above, patients with EGFR mutations have a relatively good prognosis, and multiline targeted therapy may effectively delay disease progression. Targeted drugs can be used as adjuvant therapy to control tumor development, but may not be used alone to completely control tumor progress.
Immunotherapy is an emerging tumor treatment method in recent years, and it is mostly used as a rescue treatment method after failure of multiline tumor therapy. Chen L reported a case of pulmonary HAC with positive PD-L1 staining. After receiving multi-line chemotherapy, the PD-1 inhibitor sintilimab was used, and the overall survival benefit of anti-PD-1 treatment was 6 months.20 Tonyali O reported a case of pulmonary HAC with disease progression after surgery, radiotherapy and chemotherapy. His PD-L1 status was not reported, and there was no remission after nivolumab treatment. The survival benefit of immunotherapy was 3 months.21 Basse et al reported a HAC case used durvalumab anti-PD-L1 therapy. Although PD-L1 status was negative, the patient still had partial response to immunotherapy.24 This may be related to the patient’s mismatch-repair status.25 Mismatch-repair status patients without effective treatment may benefit from PD-1 blockade. In general, for patients with pulmonary HAC after multi-line therapy, immunotherapy is worth trying to prolong survival.
Conclusion
In summary, our report first presented an unresectable pulmonary HAC patient treated with only thoracic radiotherapy. This finding would provide valuable information for the treatment decisions of similar patients with pulmonary HAC.
Ethical Approval and Consent to Participate
Written informed consent for publication of their details was obtained from the parent. The First Hospital of China Medical University approved to publish the case details.
Acknowledgments
We would like to thank the researchers and study participants for their contributions. The authors did not receive funding for this research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Haninger DM, Kloecker GH, Bousamra IM, Nowacki MR, Slone SP. Hepatoid adenocarcinoma of the lung: report of five cases and review of the literature. Mod Pathol. 2014;27(4):535–542. doi:10.1038/modpathol.2013.170
2. Xie Y, Zhao Z, Li P, et al. Hepatoid adenocarcinoma of the stomach is a special and easily misdiagnosed or missed diagnosed subtype of gastric cancer with poor prognosis but curative for patients of pN0/1: the experience of a single center. Int J Clin Exp Med. 2015;8(5):6762–6772.
3. Metzgeroth G, Ströbel P, Baumbusch T, Reiter A, Hastka J. Hepatoid adenocarcinoma - review of the literature illustrated by a rare case originating in the peritoneal cavity. Onkologie. 2010;33(5):263–269. doi:10.1159/000305717
4. Hayashi Y, Takanashi Y, Ohsawa H, Ishii H, Nakatani Y. Hepatoid adenocarcinoma in the lung. Lung Cancer. 2002;38(2):211–214. doi:10.1016/S0169-5002(02)00214-3
5. Khozin S, Roth MJ, Rajan A, et al. Hepatoid carcinoma of the lung with anaplastic lymphoma kinase gene rearrangement. J Thorc Oncol. 2012;7(11):e29–31. doi:10.1097/JTO.0b013e3182697a23
6. Mokrim M, Belbaraka R, Allaoui M, et al. Hepatoid adenocarcinoma of the lung: a case report and literature review. J Gastrointest Cancer. 2012;43(Suppl S1):S125–127. doi:10.1007/s12029-011-9318-5
7. Che YQ, Wang S, Luo Y, Wang JB, Wang LH. Hepatoid adenocarcinoma of the lung: presenting mediastinal metastasis without transfer to the liver. Oncol Lett. 2014;8(1):105–110. doi:10.3892/ol.2014.2064
8. Shaib W, Sharma R, Mosunjac M, Farris AB, El Rayes B. Hepatoid adenocarcinoma of the lung: a case report and review of the literature. J Gastrointest Cancer. 2014;45(Suppl S1):99–102. doi:10.1007/s12029-013-9558-7
9. Gavrancic T, Park YH. A novel approach using sorafenib in alpha fetoprotein-producing hepatoid adenocarcinoma of the lung. J Natl Compr Canc Netw. 2015;13(4):
10. Motooka Y, Yoshimoto K, Semba T, et al. Pulmonary hepatoid adenocarcinoma: report of a case. Surg Case Rep. 2016;2(1):1. doi:10.1186/s40792-016-0129-6
11. Sun JN, Zhang BL, Li LK, Yu HY, Wang B. Hepatoid adenocarcinoma of the lung without production of alpha-fetoprotein: a case report and review of the literature. Oncol Lett. 2016;12(1):189–194. doi:10.3892/ol.2016.4559
12. Valle L, Thomas J, Kim C, et al. Hepatoid adenocarcinoma of the lung metastasizing to the tonsil. Mol Clin Oncol. 2017;6(5):705–707. doi:10.3892/mco.2017.1215
13. Ayub A, Nunez Lopez O, Booth A, Okereke I. Pulmonary hepatoid adenocarcinoma. J Thorac Cardiovasc Surg. 2019;158(4):e139–e140. doi:10.1016/j.jtcvs.2019.06.023
14. Chen HF, Wang WX, Li XL, et al. Hepatoid adenocarcinoma of the lung with EGFR mutation and the response to tyrosine kinase inhibitors. J Thorc Oncol. 2019;14(10):e217–e219. doi:10.1016/j.jtho.2019.04.032
15. Kuan K, Khader SN, El Hussein S. Hepatoid adenocarcinoma of the lung. Diagn Cytopathol. 2019;47(8):831–833.
16. Li J, Qi H, Xu B, et al. Genomic profiles of a patient of pulmonary hepatoid adenocarcinoma with high AFP level: a case report. Front Oncol. 2019;9:1360. doi:10.3389/fonc.2019.01360
17. Shi YF, Lu JG, Yang QM, et al. Primary hepatoid adenocarcinoma of the lung in Yungui Plateau, China: a case report. World J Clin Cases. 2019;7(13):1711–1716. doi:10.12998/wjcc.v7.i13.1711
18. Wang C, Xu G, Wu G, et al. Hepatoid adenocarcinoma of the lung metastasizing to the gingiva. Onco Targets Ther. 2019;12:8765–8768. doi:10.2147/OTT.S222974
19. Yang K, Jiang H, Li Q. Primary pulmonary hepatoid adenocarcinoma: a case report and review of the literature. Medicine. 2019;98(14):e15053. doi:10.1097/MD.0000000000015053
20. Chen L, Han X, Gao Y, et al. Anti-PD-1 therapy achieved disease control after multiline chemotherapy in unresectable KRAS-positive hepatoid lung adenocarcinoma: a case report and literature review. Onco Targets Ther. 2020;13:4359–4364. doi:10.2147/OTT.S248226
21. Tonyali O, Gonullu O, Ozturk MA, Kosif A, Civi OG. Hepatoid adenocarcinoma of the lung and the review of the literature. J Oncol Pharm Pract. 2020;1078155220903360.
22. Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. Can Med Assoc J. 2005;172(3):367–379. doi:10.1503/cmaj.1040752
23. Zhao D, Chen X, Qin N, et al. The prognostic role of EGFR-TKIs for patients with advanced non-small cell lung cancer. Sci Rep. 2017;7:40374. doi:10.1038/srep40374
24. Basse V, Schick U, Gueguen P, et al. A mismatch repair-deficient hepatoid adenocarcinoma of the lung responding to anti-PD-L1 durvalumab therapy despite no PD-L1 expression. J Thorc Oncol. 2018;13(7):e120–e122. doi:10.1016/j.jtho.2018.03.004
25. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi:10.1056/NEJMoa1500596
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.