Back to Journals » Cancer Management and Research » Volume 12
Radiotherapy Combined with Chemotherapy for Regional Lymph Node Recurrence in Gastric Cancer
Authors Cai L, Ouyang G , Wang X, Li Z, Shen Y
Received 8 September 2020
Accepted for publication 23 November 2020
Published 24 December 2020 Volume 2020:12 Pages 13339—13346
DOI https://doi.org/10.2147/CMAR.S280225
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Liang Cai,1,* Ganlu Ouyang,2,* Xin Wang,2 Zhiping Li,3 Yali Shen2
1Lung Cancer Center, West China School of Medicine, West China Hospital of Sichuan University, Chengdu, People’s Republic of China; 2Department of Abdominal Oncology, Cancer Center, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 3Department of Radiation Oncology, Cancer Center, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yali Shen
Department of Abdominal Oncology, Cancer Center, West China Hospital, Sichuan University, No. 37 of Wainan Guoxue Lane, Wuhou District, Chengdu 610041, People’s Republic of China
Tel/Fax +86 2885422589
Email [email protected]
Purpose: Regional lymph node recurrence (RLNR) in gastric cancer is uncommon. We investigated the effects of radiotherapy combined with chemotherapy against limited RLNR and analyzed the regularity of regional lymph node recurrence and metastasis.
Patients and Methods: This retrospective study included 34 gastric cancer patients with limited RLNR after D2 lymphadenectomy between January 2012 and May 2018. All patients received systemic chemotherapy and local radiotherapy with median dose of 52.5 Gy (30– 66 Gy in fractions of 1.8– 3.0 Gy daily, five times weekly). All sites of recurrent and metastatic lymph nodes were collected and analyzed.
Results: The median follow-up was 19 months (range 7– 60 months). After treatment, complete response and partial response were observed in 32.4% and 55.9% of patients, respectively. The median overall survival (OS) and progression-free survival (PFS) were 18 months and 13 months. On multivariate analysis, age (≤ 60 vs > 60) was associated with a significantly better OS (p = 0.025) and radiation dose (< 54 Gy vs ≥ 54 Gy) was considered as an independent prognostic factor for PFS (p = 0.000). During radiotherapy, three patients developed grade 3 gastrointestinal toxicity, and no deaths were related to the treatments. The most commonly metastatic lymph nodes were the No. 4, No. 3, No. 6, No. 5, No. 7, No. 9, and No. 8 nodes; the recurrent lymph nodes were mainly located in the No. 16b, No. 16a, No. 9, No. 14, No. 7, No. 13, and No. 8 nodes.
Conclusion: The selected gastric cancer patients with limited RLNR may benefit from radiotherapy combined with chemotherapy. High-dose radiotherapy (≥ 54 Gy) lead to better PFS and tend to extend OS. The major lymph node recurrence sites were in the gastric vascular region (especially No. 16a/b nodes).
Keywords: gastric cancer, radiotherapy, regional lymph node, D2 lymphadenectomy, recurrence
Introduction
It revealed gastric cancer is the sixth-most common cancer and the third leading cause of cancer-related mortality worldwide from 2018 global cancer statistics.1 The prognosis of advanced and metastatic gastric cancer is generally poor and lack of effective treatment.2 Growing evidence has suggested that a subset of carefully selected gastric cancer with limited metastatic exists that might achieve a significantly prolonged survival from a multimodality treatment strategy. In a recently published article, the patients with limited metastasis showed a more prolonged survival (median OS 16.7 months) after preoperative chemotherapy followed by surgical resection of metastases than without surgery.3
In the patterns of recurrence after curative resection, locoregional lymph node recurrence accounts for a small proportion.4,5 There is no standard treatment for regional lymph node recurrence (RLNR). Systemic chemotherapy is often recommended for patients. However, chemotherapy alone is not regarded as sufficient for locoregional control. And surgical resection is not preferred for RLNR because of the low resectability.6 With radiation development, radiotherapy may play an essential role as a safe and effective local treatment modality. Moreover, few studies investigated the role of RT for RLNR after radical surgery in advanced gastric cancer. Therefore, we screened out gastric cancer patients with RLNR after D2 lymphadenectomy to explore whether radiotherapy combined with chemotherapy could improve clinical outcome and analyze the regularity of regional lymph node recurrence and metastasis.
Patients and Methods
Patient Characteristics
We retrospectively studied gastric cancer patients with regional lymph node recurrence treated at our institution between January 2012 and May 2018. The patients selected for this study received radical gastrectomy with D2 lymphadenectomy and had regional lymph node recurrence lesions without any other metastasis. Regional lymph node recurrence is mainly diagnosed by lymph node biopsy or imaging studies, including contrast-enhanced computed tomography (CT) and positron emission tomography (PET). In terms of imaging, the diagnosis of recurrent lymph nodes is defined as a short diameter of lymph nodes larger than 1.0 cm or uptake of fluorine–18–2–fluoro–2–deoxy–d-glucose (FDG). We collected all available clinical data of these patients, including history, treatment, imaging, and follow-up.
Treatment
Chemotherapy was recommended after regional lymph node recurrence was performed for patients in this study. Because the optimal regimen remains to be defined, heterogeneous chemotherapy regimens were performed depending on the patient’s status and the medical oncologist’s preference. We divided the patients into two groups according to the chemotherapy regimen: group 1: dual agents chemotherapy (XELOX/SOX/TP) and group 2: single agent chemotherapy (Docetaxel/Irinotecan/Paclitaxel).
The timing of radiotherapy was fixed according to the patient’s status and the physician’s judgment. Immediate radiotherapy was defined as starting radiotherapy within eight weeks after the diagnosis of regional lymph node recurrence. The CT scan was performed for radiotherapy planning with immobilization devices. A scan thickness of 3mm was used. All patients received radiotherapy with 6 MV linear accelerator using intensity-modulated radiotherapy (IMRT). The gross tumor volume (GTV) of recurrent lymph nodes was determined by the CT scan that showed the position and size of involved lymph nodes. Adjacent regional nodal area was described as clinical target volume (CTV). Planned target volume (PTV) denoted the CTV and 0.6cm-1cm margins for geometric uncertainties.
Assessment of Response, Survival and Toxicity
We positioned distribution of recurrent lymph nodes on the CT image and measured their maximum degree change during the follow-up. The regression of the tumor was considered as a response to the treatment. And the response was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1. Overall survival (OS) and progression-free survival (PFS) were calculated from the time of diagnosis of lymph node recurrence.
Adverse events caused by chemotherapy were evaluated by the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Acute and chronic toxicities associated with radiotherapy were assessed according to the Radiation Therapy Oncology Group (RTOG) criteria (version 2.0).
Statistical Analysis
Ordinal data are presented as numbers and percentages. Kaplan–Meier method was used to draw the OS and PFS curve and conduct univariate analysis. Statistically significant variables in univariate analysis were used to conduct multivariate analysis with COX proportional risk model. Statistically significant was defined as P < 0.05. All statistical analyses were performed using SPSS, version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Patient Characteristics
During the period, a total of gastric cancer 34 patients with regional lymph node recurrence were retrieved. The median age of the patients was 57.8 years old (range 41–78 years). Most patients (67.6%) were in ECOG 0–1, and the others (32.4%) patients were in ECOG 2. There were 14 (41.1%) patients with clinical symptoms. The most common symptom was abdominal or back pain, which was rated moderate to severe. There were 19 patients with high lymph node positive rate that was greater than 0.25. “Lymph node positive rate” was defined as the ratio of positive lymph nodes to the total number of removed lymph nodes in D2 lymphadenectomy. Patients’ characteristics are presented in Table 1.
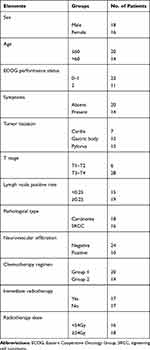 |
Table 1 Patient and Tumor Characteristics |
A total of 34 patients received both radiotherapy and chemotherapy after regional lymph node recurrence. The median total dose to GTV was 52.5 Gy (range, 30 to 66 Gy), with fractions of 1.8 to 3.0 Gy once, five times per week. The dose received by 18 patients was ≥54 Gy. Twenty patients received dual agents chemotherapy and the remaining 14 patients received single agent chemotherapy. Seventeen patients received immediate radiotherapy.
Patterns of Recurrence
At the time of recurrence, lymph node mainly occurred in No. 16b nodes (76%) and No. 16a nodes (56%). Meanwhile, No. 9 nodes (32%), No. 14 nodes (29%), No.7 nodes (26%), No. 13 nodes (24%), and No.8 nodes (21%) are secondly mainly metastatic position.
Moreover, we analyzed the location of pathologically confirmed metastatic lymph nodes in all patients after surgery. It shows that metastatic lymph nodes occur mainly in the perigastric lymph nodes, including No. 4 nodes (50%), No. 3 nodes (38%), No. 6 nodes (35%), No. 5 nodes (26%), No. 7 nodes (41%), No. 9 nodes (29%), and No. 8 nodes (24%). Details of lymph node distribution are shown in Figure 1.
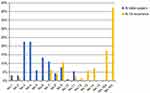 |
Figure 1 Distribution of lymph nodes in patients after surgery and distribution of metastatic lymph nodes. Abbreviation: LN, lymph nodes. |
Response to Treatment
All 34 patients with abdominal LN metastasis who received radiation, 11 patients (32.4%) showed a complete response and 19 patients (55.9%) showed partial response, resulting in an objective regression rate of 88.3%. Other 4 patients (11.8%) had stable disease control. Among the symptomatic patients, 11 patients (78.6%) experienced pain relief after the radiotherapy.
Survival and Prognostic Factors
The median follow-up was 19 months (range 7–60 months). The median OS and PFS were 18 months and 13 months (Figure 2), with 91.2% 1-yr OS (%) and 70.6% 1-yr PFS (%). On univariate analysis, five factors appeared to be associated with OS: age, lymph node-positive rate, chemotherapy regimens, immediate radiotherapy and radiation dose. On multivariate analysis, younger age (≤60 vs >60) was associated with a significantly better OS (HR 0.425; 95% CI 0.200–0.900; p=0.025). Additionally, higher radiation dose (<54 Gy vs ≥54 Gy) has a tendency to get better survival (OS HR 2.864; 95% CI 1.000–8.202; p = 0.050). Univariate analyses, age, lymph node-positive rate, chemotherapy regimens, immediate radiotherapy, and radiation dose are related to PFS. And radiation dose (<54Gy vs ≥54Gy) was proved to be an independent prognostic factor for PFS (HR 15.985; 95% CI 3.546–72.049; p = 0.000). The full details of univariate and multivariate analyses of OS and PFS are provided in Tables 2 and 3.
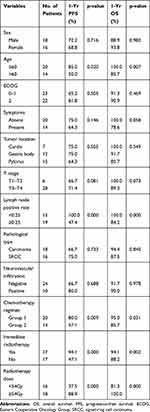 |
Table 2 Univariate Analysis for Prognostic Factors of Survival |
 |
Table 3 Multivariate Analysis for Overall Survival and Progression-Free Survival |
Treatment Toxicities
The toxicity of treatment was tolerable. In the course of radiotherapy, acute gastrointestinal toxicity was observed in all patients. The most common symptoms were nausea and vomiting, with Grade 1 in 19 (55.9%), Grade 2 in 12 (35.3%), and Grade 3 in 3 (8.8%). For neutropenia, 27 patients experienced 1–2 grade toxicities and grade≥3 neutropenia was observed in remaining seven patients. No deaths were related to chemoradiotherapy.
Discussion
Lymph node recurrence after gastrectomy is uncommon in clinical practice.7 Due to the different biological behavior of gastric cancer and the complexity of RLNR, there is a lack of high-level clinical evidence. The aims of this study are to evaluate the safety and efficacy of RT with chemotherapy for selected patients with abdominal LN involvement alone from gastric cancer after D2 lymphadenectomy.
Perioperative treatment is recommended for advanced gastric cancer patients following the evidence from MAGIC and MRC trials.8,9 Prospective study studies have shown that surgery combined with perioperative chemotherapy can effectively prolong the survival of oligometastatic gastric cancer patients.10 No standard treatment has been established yet for patients with RLNR after radical surgery for gastric cancer. In most cases, recurrences after gastrectomy are considered unsuitable for reoperation due to the low resectability and postoperative complications.11,12 With the development of systemic therapy and radiation technique, successful abdominal recurrence management using radiotherapy combined with chemotherapy has been reported. Sun et al reported the median OS in the chemoradiotherapy group was 11.4 months, while in the chemotherapy group was 4.8 months (P = 0.002) for gastric cancer patients with lymph node recurrence.13 Kim et al reported that the radiotherapy group’s survival time was much longer than that of the chemotherapy group (36 months vs 16 months; P = 0.007).14 Lee et al demonstrated that PFS of the radiotherapy group was superior to that of the non-radiotherapy group (25 vs 8 months; P = 0.021), and OS showed an increasing trend in the radiotherapy group (29 vs 20 months; P = 0.095).15 In this study, our data also demonstrate that OS and PFS were significantly associated with chemoradiotherapy: 13 months PFS and 18 months OS.
Improvements in local control and survival were associated with the increase of the radiation dose. In this study, IMRT is applied for all patients and IGRT is also used when necessary. The median dose to GTV was 52.5 Gy and 18 patients received radiation dose above 54 Gy. The objective regression rate reached 88.3% (including 11 CR and 19 PR). Meanwhile, the toxicity related with radiation was tolerated, resulting from IMRT which decreased volume and radiation dose of gastrointestinal tissue around GTV. For prognostic factors, higher radiation dose (≥54 Gy) is a positive prognostic factor for PFS and had a tendency to obtain better OS.
Not all metastatic gastric cancer may achieve long-term control if using aggressive multimodality strategies.16 So the proper selection of advanced gastric cancer patients who is more likely to benefit from a combined approach is the key to optimize treatment outcome. In this study, the physical state of patients is good with ECOG ≤ 2, and the median number of lymph node recurrence was 3 (from 1 to 8), which was regarded as oligometastatic lesions. Based on the above results, the efficacy of radiotherapy for RLNR can be preliminarily confirmed, but it still needs to be clarified by prospective randomized study.
D2 lymphadenectomy has a wide range of lymphatic dissection. However, 21.8–49.5% of gastric cancer patients who received surgery also experienced recurrence.17–19 The recurrence pattern of D2 lymphadenectomy should be further clarified. Only one study from South Korea has explored the lymph node recurrence pattern for gastric cancer after D2 lymphadenectomy.20 Our study analyzed both the lymph node metastasis patterns at the time of initial surgery and recurrence pattern in all patients. The results showed the difference between the lymph node metastasis pattern and recurrence pattern: No.1 nodes to No.6 nodes have high metastasis rate and low recurrence rate, No.7 nodes to No.9 nodes have high metastasis rate and high recurrence rate, and No.10 nodes to No.16 nodes have the trend of low metastasis rate and high recurrence rate. In all, the recurrent lymph nodes were mainly located in the gastric vascular region, like No.9 nodes (32%), No.14 nodes (29%), No.7 nodes (26%), No.13 nodes (24%), and No. 8 nodes (21%). Meanwhile, some lymph nodes areas had a relatively rare recurrence, like splenic hilum (No.10) and below the superior mesenteric artery (No.14a). Notably, No.16a/b nodes were the most common recurrence (56% and 76%) region regardless of location of primary tumor. The results of Yoon et al were consistent with our study, No. 16a/b nodes were most likely to relapse after D2 lymphadenectomy.20 What is more, a Phase III study conducted by Sasako et al showed no significant difference in survival rate between gastric cancer patients received with addition of para-aortic nodal dissection to D2 lymphadenectomy compared with patients received with D2 lymphadenectomy alone.21 In addition, compared with D2 lymphadenectomy alone, the addition of para-aortic nodal dissection to D2 lymphadenectomy does not significantly increase the rate of surgical complications. Therefore, it is valuable to discuss the benefited patients from paraaortic nodal dissections. Moreover, in our study, there was higher recurrence occurred in No.16a/b nodes in N2/N3 patients compared to N0/N1 patients. So it is necessary to further explore the factors related to No16a/b nodes recurrence after D2 lymphadenectomy.
Limited by the number of gastric cancer patients with regional lymph node recurrence after D2 lymphadenectomy, sample size is small and no control group was set in this retrospective study. For rare disease, like gastric cancer patients with limited RLNR, it is better to carry out multi-center clinical research to obtain more clinical experience.
Conclusion
In conclusion, these patients with limited regional lymph node recurrence benefit from multimodality treatment strategies, including radiotherapy combined with chemotherapy. Besides, high-dose radiotherapy (≥54 Gy) lead to better PFS and tend to extend OS. The major lymph node recurrence sites were in the gastric vascular region (especially No. 16a/b nodes), which is valuable to discuss the benefited patients from paraaortic nodal dissections.
Ethical Approval
Ethical approval was obtained from Ethics committee on Biomedical Research, West China Hospital of Sichuan University (2020-908). Patient consent to review their medical records was not required. The reasons for the waiver were as follows. Firstly, this is a retrospective study. There was no additional risk to patients. In the process of ethical approval, we have submitted to the ethics committee the application for exemption from informed consent of patients. Additionally, we abided by the Declaration of Helsinki. We collected de-identified data of patients. And the final results of the study would be anonymity.
Acknowledgments
This research was supported by project of the Science and Technology Department in Sichuan province (2019YJ0142).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064.
3. Carmona-Bayonas A, Jimenez-Fonseca P, Echavarria I, et al. Surgery for metastases for esophageal-gastric cancer in the real world: data from the AGAMENON national registry. Eur J Surg Oncol. 2018;44(8):1191–1198. doi:10.1016/j.ejso.2018.03.019
4. Liu D, Lu M, Li J, et al. The patterns and timing of recurrence after curative resection for gastric cancer in China. World J Surg Oncol. 2016;14(1):305. doi:10.1186/s12957-016-1042-y
5. Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87(2):236–242. doi:10.1046/j.1365-2168.2000.01360.x
6. Song KY, Park SM, Kim SN, Park CH. The role of surgery in the treatment of recurrent gastric cancer. Am J Surg. 2008;196(1):19–22. doi:10.1016/j.amjsurg.2007.05.056
7. Chang JS, Lim JS, Noh SH, et al. Patterns of regional recurrence after curative D2 resection for stage III (N3) gastric cancer: implications for postoperative radiotherapy. Radiother Oncol. 2012;104(3):367–373. doi:10.1016/j.radonc.2012.08.017
8. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi:10.1056/NEJMoa055531
9. Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79(9–10):1522–1530. doi:10.1038/sj.bjc.6690243
10. Al-Batran SE, Homann N, Pauligk C, et al. Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer: the AIO-FLOT3 trial. JAMA Oncol. 2017;3(9):1237–1244. doi:10.1001/jamaoncol.2017.0515
11. Lehnert T, Rudek B, Buhl K, Golling M. Surgical therapy for loco-regional recurrence and distant metastasis of gastric cancer. Eur J Surg Oncol. 2002;28(4):455–461. doi:10.1053/ejso.2002.1260
12. Badgwell B, Cormier JN, Xing Y, et al. Attempted salvage resection for recurrent gastric or gastroesophageal cancer. Ann Surg Oncol. 2009;16(1):42–50. doi:10.1245/s10434-008-0210-x
13. Sun J, Sun YH, Zeng ZC, et al. Consideration of the role of radiotherapy for abdominal lymph node metastases in patients with recurrent gastric cancer. Int J Radiat Oncol Biol Phys. 2010;77(2):384–391. doi:10.1016/j.ijrobp.2009.05.019
14. Kim BH, Eom KY, Kim JS, Kim HH, Park DJ. Role of salvage radiotherapy for regional lymph node recurrence after radical surgery in advanced gastric cancer. Radiat Oncol J. 2013;31(3):147–154. doi:10.3857/roj.2013.31.3.147
15. Lee J, Yoon HI, Rha SY, et al. Integration of radiotherapy and chemotherapy for abdominal lymph node recurrence in gastric cancer. Clin Transl Oncol. 2017;19(10):1268–1275. doi:10.1007/s12094-017-1665-7
16. Salati M, Valeri N, Spallanzani A, Braconi C, Cascinu S. Oligometastatic gastric cancer: an emerging clinical entity with distinct therapeutic implications. Eur J Surg Oncol. 2019;45(8):1479–1482. doi:10.1016/j.ejso.2018.11.006
17. Lee SE, Ryu KW, Nam BH, et al. Prognostic significance of intraoperatively estimated surgical stage in curatively resected gastric cancer patients. J Am Coll Surg. 2009;209(4):461–467. doi:10.1016/j.jamcollsurg.2009.06.001
18. Nurwidya F, Takahashi F, Takahashi K. Meeting report: current cancer perspectives from the 9(th) Annual Meeting of the Japanese Society of Medical Oncology. Thorac Cancer. 2012;3(1):94–97. doi:10.1111/j.1759-7714.2011.00076.x
19. Wu CW, Lo SS, Shen KH, et al. Incidence and factors associated with recurrence patterns after intended curative surgery for gastric cancer. World J Surg. 2003;27(2):153–158. doi:10.1007/s00268-002-6279-7
20. Yoon HI, Chang JS, Lim JS, et al. Defining the target volume for post-operative radiotherapy after D2 dissection in gastric cancer by CT-based vessel-guided delineation. Radiother Oncol. 2013;108(1):72–77. doi:10.1016/j.radonc.2013.05.025
21. Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359(5):453–462. doi:10.1056/NEJMoa0707035
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

