Back to Journals » Drug Design, Development and Therapy » Volume 13
Radioprotective Potential of Sulindac Sulfide to Prevent DNA Damage Due to Ionizing Radiation
Authors Torabizadeh SA , Rezaeifar M , Jomehzadeh A, Nabizadeh Haghighi F, Ansari M
Received 1 June 2019
Accepted for publication 15 November 2019
Published 6 December 2019 Volume 2019:13 Pages 4127—4134
DOI https://doi.org/10.2147/DDDT.S218022
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Seyedeh Atekeh Torabizadeh,1 Mehdi Rezaeifar,1 Ali Jomehzadeh,2 Farzaneh Nabizadeh Haghighi,1 Mehdi Ansari3
1Pharmaceutics Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran; 2Department of Medical Physics, Faculty of Medicine, Medical Physics Department, Radiotherapy & Oncology Unit, Shafa Kerman Hospital, Kerman University of Medical Sciences, Kerman, Iran; 3Drug and Food Control Department, Kerman University of Medical Sciences, Kerman, Iran
Correspondence: Seyedeh Atekeh Torabizadeh
Pharmaceutics Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran
Tel +989132914643
Fax +983431325215
Email [email protected]
Introduction: The ionizing radiation exposure of the normal cell causes damage to DNA, which leads to cell dysfunction or even cell death. However, it is necessary to identify new radio protectives in order to protect normal cells. Sulindac sulfide (SS) is a metabolite of sulindac (a non-steroidal anti-inflammatory drug) known as a cyclooxygenase inhibitor. Free radicals and reactive oxygen species are generated in the IR-exposed cells. Also, the induced inflammation process causes damage in DNA.
Purpose: In this research, the radioprotective effect of SS was investigated against genotoxicity and lipid peroxidation induced by ionizing radiation in the human blood lymphocytes.
Methods: In this study, the human blood samples were pretreated with SS at different concentrations (10, 25, 50, 100 and 250 μM) and then were exposed to IR at a dose of 1.5 Gy. The micronucleus (MN) assay was used to indicate the radioprotective effects of SS on exposed cells. Total antioxidant activity of the SS was measured by using FRAP and DPPH assay. Also, the malondialdehyde (MDA) levels and the activity of superoxide dismutase (SOD) on the exposed cells were evaluated.
Results: It was found that SS decreased the percentage of MN induced by IR in exposed cells. Maximum reduction in the frequency of MN was observed at 250 μM of SS (87%) that provides the highest degree of protection against IR. On the other hand, pretreatment at 250 μM of SS inhibited IR-induced oxidative stress, which led to a decrease in the MN frequencies and MDA levels, while SOD activity showed an increase in the exposed cells.
Conclusion: It could be concluded that SS as a good radioprotective agent protects the human normal cells against the oxidative stress and genetic damage induced by IR.
Keywords: sulindac sulfide, DNA damage, MN, radioprotective, genotoxicity, lipid peroxidation
Introduction
Radiotherapy is commonly used in the treatment of a wide variety of malignancies. Radiation is the most important non-surgical method to cure the tumor. Nearly half of all cancer patients are given radiation during the course of their disease. Ionizing radiation, IR of normal tissues, may result in both acute and chronic toxicities that can further cause a range of symptoms and a decrease in quality of life. Radioprotectors are known as antioxidants that decrease the damage to the normal tissues by radiation.1
IR can increase DNA damage and produce stress response and inflammation; it can even directly or indirectly cause cell death and carcinogenesis. As a direct effect, DNA molecules are hit directly by the radiation2,3 that leads to SSB (single-stranded-binding proteins) and DSB (double-stranded-binding proteins) formation.4 In indirect effect, the ionization of the water molecules produces reactive oxygen species (ROS) such as OHO, H2O2, OH, O2 that increase free radicals and other reactive species.5 Increase in the lipid peroxidation indicates the ROS-dependent cellular damage.6 ROS cause the release of arachidonic acid from membrane phospholipids and may increase the formation of prostaglandins and leukotrienes.7 Therefore, ROS are known as mediators of inflammation in vivo. Also, there is a direct relation between ROS-mediated inflammation and DNA damage.8
As previously mentioned, ionizing radiation with several mechanisms can cause damage to cells including the production of free radicals, the reduction of antioxidant stores inside the cell and the development of inflammatory processes inside the cell. Ionizing radiation increases inflammatory markers such as cytokines and interleukins, and the increased inflammatory processes activate some of the intracellular pathways and signals, which ultimately induce or exacerbate radiation damage to the cell’s DNA. There are complex and reasoned connections between the production of free radicals, the increase in inflammatory processes and DNA damage in the exposed cells, in such a way that each of these free radicals and inflammatory processes reinforces the other and increases the damage.9,10 Therefore, radioprotectors are often drugs or compounds with free radical scavenging (antioxidant) or anti-inflammatory activity that showed the role of radiation protection inside the cell.11–14
Sulindac is an inactive non-steroidal anti-inflammatory drug (NSAID) that is quickly metabolized following oral administration (Figure 1). Sulindac has two metabolites, sulindac sulphone as an inactive metabolite that does not inhibit COX, and sulindac sulfide as the pharmacologically active metabolite that has NSAID properties and inhibits COX. Sulindac sulfide can directly inhibit 5-lipoxygenase and can also penetrate into the lipid bilayer. Furthermore, it can ionize the carboxyl group of the membrane, which shows better antioxidant capacity at the membrane level and potentially protects normal cells from ROS implicated in the pathophysiology of inflammation.15,16 Thus, sulindac sulfide protects the membrane against oxy-radicals and reduces the expression of pro-inflammatory factors TNFα, iNOS, IL-1β and IL-6.17,18
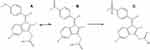 |
Figure 1 Chemical structure: (A) sulindac sulfide (B), sulindac and (C) sulindac sulfone. |
It has been reported that anti-inflammatory agents are able to mitigate pro-inflammatory biomarkers involved in IR-induced cellular toxicity.19–21 NSAIDs could protect normal tissue by arresting the cell cycle in the G1 state.22,23 Furthermore, NSAIDs not only provide radioprotection to normal tissues, but they also offer additive antitumor effects.18,24 Therefore, NSAIDs are antioxidants with the potential to minimize cellular damage that their mechanism of action is still unknown.
NSAIDs could elevate the level of superoxide dismutase as an antioxidant enzyme in cells.23,25 Therefore, these compounds are able to trap free radicals to avoid DNA damage.26 In the present study, the radioprotective effects of the SS were evaluated on the exposed human lymphocytes by IR.
Materials and Methods
Sulindac sulfide and Cytochalasin-B were purchased from Sigma Chemicals Co. (St. Louis, USA). Phytohemagglutinin (PHA), Roswell Park Memorial Institute (RPMI-1640) medium, fetal bovine serum (FBS), penicillin and streptomycin were purchased from Biosera (USA). Moreover, malondialdehyde (MDA) assay kit (zellbio, Germany) and Superoxide Dismutase (SOD) assay kit (Randox, UK) were purchased. Giemsa stain, methanol and acetic acid were obtained from Merck (Germany).
Determination of Antioxidant Activity of Sulindac Sulfide Using DPPH (1,1-Diphenyl-2-Picrihyrazyl)
The antioxidant activity of the SS was determined using a stable free radical α,α-diphenyl-β-picrylhydrazyl (DPPH). Various concentrations of SS (10, 25, 50,100, 250 μM) were mixed thoroughly with an equal volume of ethanol solution of 0.1 M, DPPH. The mixture was allowed to stand for 15 mins in the dark. The absorbance was measured at 517 nm. The experiment was performed in triplicate. Ascorbic acid was used as an antioxidant standard. The scavenging activity was calculated using the following formula:
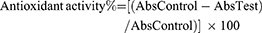
Total Antioxidant Capacity Assay
The total antioxidant potential of the SS was determined using ferric reducing the antioxidant power (FRAP) assay of Benzie and Strain.27 At low pH, the sample is able to reduce ferric tripyridyltriazine (Fe III–TPTZ) complex to an intense blue-colored ferrous (Fe II) form. This complex has an absorbance maximum at 593 nm and the blue color intensity is proportional to the antioxidant capacity of the sample as described elsewhere. First, 10–250 µM (5 µL) of SS and 70 μL of FRAP reagent were mixed. Distilled water was used as a blank, the mixture was incubated at 37°C for 5 mins and absorbance was read at 593 nm. The FRAP values were expressed as micromoles per liter (µM) and a standard curve that showed millimole Fe2+ to absorbance was used to read these values.28
Blood Treatment
This study obtained permission from research and ethical committees of the Kerman University of Medical Sciences (IR.KMU.REC.1396.2488) and all volunteers provided written informed consent, in accordance with the Declaration of Helsinki. This study enrolled three healthy and non-smoking male volunteers, aged from 21 to 25 years. Twelve milliliters of whole blood were collected in the heparinized tubes and allocated in microtubes each containing 0.9 mL. Blood samples were incubated with different concentrations of sulindac sulfide including 10, 25, 50, 100 and 250 μmol/l. These samples were incubated for 2 hrs at 37°C. Control samples were treated with diluted DMSO in RPMI with the same concentration as Sulindac sulfide. DMSO concentration was the same in control and SS solutions (0.1%).
Ionizing Radiation and Micronucleus Test
At each concentration and for each volunteer, peripheral blood samples were irradiated in micro tubes at 37°C with 6 MV photon beam. The photon beam was produced by a medical linear accelerator (Elekta Compact™ Linear accelerator, Crawley, UK) with a total dose of 1.5 Gy delivered in mid-line of microtubes and at a dose rate of 200 cGy/min. Dose calculation was performed using Treatment Planning System (ISOgray TPS, version 5.2, Dosisoft, Cachan, France). Tree microtubes were distributed among three volunteers in the control group (non-irradiated samples). Moreover, microtubes containing blood samples were placed on the plastic box filled with water as a phantom, which were exposed to irradiation. Finally, after irradiation, 0.5 mL of each sample (control and irradiated samples in duplicate) was added to 4.4 mL of RPMI 1640 culture medium which contained a blend of 10% FBS and 100 µL PHA. All cultures were incubated at 37°C in a humidified atmosphere of 5% CO2. After 44 hrs, cytochalasin B (final concentration, 6 µg/mL) was added to the culture. In the subsequent 72 hrs of incubation, the cells were harvested by centrifugation for 10 mins at 3000 rpm and suspended in cold potassium chloride. Then, cells were immediately stabilized in a fixative solution made of methanol:acetic acid (6:1) three times. The fixed cells were spotted onto clean microscopic slides (the triple slide for each concentration), air-dried and stained with 20% Giemsa solution. The slides were coded and evaluated at 100x magnification in order to determine the frequency of MN in the cytokinesis blocked binucleated cells with a well-preserved cytoplasm.29 The MN frequency was determined as 1000 binucleate cells for each volunteer in the treated group. Totally, 3000 binucleated lymphocytes were counted for three volunteers in each treated group, and finally 36,000 binucleated lymphocytes were counted for 12 treated groups in this examination.
Isolation of Lymphocytes
The peripheral blood used in the experiment was obtained from three healthy, non-smoking young male volunteers. Then, blood samples were collected in heparinized sterile tubes and lymphocytes were isolated by Ficoll-Hypaque using the protocol reported in the previous experiments. Briefly, blood was diluted with an equal volume of a serum-free RPMI medium, layered carefully over Ficoll-Hypaque solution (without intermixing) and centrifuged at 400 g for 30 mins. Then, the lymphocyte layer was aspirated and diluted with serum-free medium and centrifuged at 300 g for 5 mins. The lymphocyte was washed again with serum-free medium and was re-suspended in the RPMI-1640 media. The number of viable cells was determined by the Trypan Blue dye exclusion test. The viable cells were more than 99%.
Determination of MDA
The lipid peroxidation (MDA) was determined using MDA assay kit according to the protocol of the manufacturer (zellbio, Germany).30 Briefly, butylated hydroxytoluene BHT was added to 106 lymphocytes (from every group) and used directly in the assay. Samples (100 μL) were mixed with sodium dodecyl sulfate and chromogenic solution containing thiobarbituric acid, alkali and acetic acid. All the microtubes were placed on vigorously boiling water for 60 mins. Then, the tubes were shifted to an ice-bath and centrifuged at ×3500 g for 15 mins. The amount of MDA formed in each sample was assessed by measuring the absorbance of the supernatant at 535 nm with an ELISA reader (BioTek Inc., Winooski, USA). Tetramethoxypropane was used as a standard and MDA content was expressed as nmol/mg protein.31
The Determination of SOD Activity
The total SOD activity was determined according to the protocol of Randox kit (UK). Superoxide dismutase (SOD) functioned as a catalyst in the dismutation of the superoxide radical (O2-) into hydrogen peroxide (H2O2) and elemental oxygen (O2). Lymphocytes cells were harvested and cell lysates were prepared according to kit specifications. The results were read absorbance at 560 nm. Superoxide dismutase enzyme activity level was calculated using the following formula:
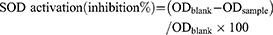
Statistical Analysis
For each concentration of SS, the amount of IR-induced micronuclei was recorded for each volunteer. Statistical analysis was performed using one-way analysis of variance (ANOVA) and post hoc Tukey multiple comparison tests. P value <0.05 was considered significant and highly significant (SPSS software 16 for windows, 2007, USA).
Results
Free Radical Scavenging
The results of free radical scavenging at different concentrations of SS are shown in Figure 2. The antioxidant radical scavenging activity of SS results was comparable with that of ascorbic acid. The SS and ascorbic acid showed a dose-dependent manner in the scavenging of DPPH free radicals. The higher the concentration of the SS is, the better the percentage of its antioxidant activity will be. At a maximum scavenging activity was recorded at a concentration of 250 μM of SS and the crude SS antioxidant activity was 62.26 ± 0.20 compared to 71.16 ± 0.40 of ascorbic acid, as an antioxidant standard.
 |
Figure 2 Sulindac sulfide radical scavenging effects at different concentrations at 517 nm. |
Total Antioxidant Capacity Assay
The results of total antioxidant capacity of SS using FRAP are shown in Figure 3. The antioxidant capacity of SS is increased at a gentle slope. It also revealed a concentration-dependent rise up to 250 μM, the highest concentration assessed.
 |
Figure 3 Ferric reducing antioxidant power of the sulindac sulfide. |
Micronucleus Test
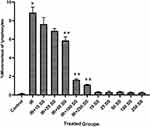
A model for binucleated lymphocyte with micronucleus is shown in Figure 4. The mean percentage of micronuclei in three volunteers treated with 1.5 Gy X-ray was 8.86 ± 0.66, while it was 0.14± 0.05 in non-irradiated control samples.
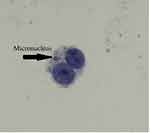 |
Figure 4 A typical binucleated lymphocyte with micronucleus in our experiment. The arrow shows a micronucleus. |
Exposure of blood samples to IR significantly increased the frequency of micronuclei (40-fold rise) in irradiated lymphocytes (P < 0.0001) (Table 1). The frequency of micronuclei after pretreatment with SS at doses of 10, 25, 50, 100, or 250 μM was 7.63 ± 0.76, 6.9 ± 0.26, 5.86± 0.41, 1.63 ± 0.11, and 1.10± 0.04, respectively (Figure 5 and Table 1). The data proved that human blood incubated with SS and then exposed in vitro to X-ray radiation, shows significant reduction in micronuclei frequency compared to blood samples incubated with X-ray alone (without SS, P < 0.001). Total micronuclei frequencies in irradiated samples pre-treated with SS were reduced to 13%, 22%, 33%, 81% and 87% at concentrations of 10, 25, 50, 100 or 250 μM, respectively, compared to irradiated samples (Table 1). Amongst irradiated samples with SS, there was no statistically significant difference in micronuclei between doses 100 and 250 μM of SS. The maximum protection of lymphocytes was observed at a concentration of 250 μM through SS treatment. The frequency of MN was significantly reduced in the irradiated sample treated by SS at a concentration of 250 μM as compared to irradiated samples with 10, 25 and 50 μM SS concentration (P < 0.001). There was no increased genotoxicity in non-irradiated samples with SS treatments at all concentrations as compared to the control group.
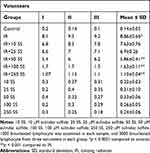 |
Table 1 The Frequency of Micronuclei Induced in vitro by 1.5 Gy X-Ray Radiation (IR) in Cultured Blood Lymphocytes at Different Doses of Sulindac Sulfide |
Lipid Peroxidation
MDA, as an oxidative stress marker, was assayed in the lymphocyte cells. The effect of IR on lipid peroxidation is shown in Figure 6. IR led to a significant increase in the level of MDA compared to control group whereas pretreatment with SS (250 μM), inhibited LP (lipid peroxidation) in lymphocytes as the concentration-dependent manner. A considerable difference was observed in the MDA level between SS (250 μM)+IR versus IR alone.
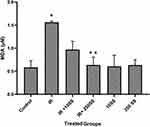 |
Figure 6 MDA assays on the lymphocytes, *p < 0.001 compared to control, **p < 0.001 compared to IR. |
Superoxide Dismutase SOD
Reduced activities of SOD in irradiated lymphocytes were observed in the results of this study (Figure 7). The activity of this antioxidant enzyme was significantly increased after pretreatment with SS (250 μM) + IR. Hence, the results of this study showed that SS increases SOD activity in lymphocytes in a dose-dependent manner.
 |
Figure 7 SOD assays on the lymphocytes, *p < 0.001 compared to control, **p < 0.001 compared to IR. |
Discussion
In this study, it was exhibited that priming of human lymphocytes with SS remarkably reduced genotoxicity and stress oxidative induced by IR. The frequency of micronuclei was reduced with pretreatment of SS. Sulindac, as a non-steroidal anti-inflammatory drug (NSAID), is widely used in clinical anti-infection medicine that, in vivo, is reversibly changed to its anti-inflammatory active compound, sulindac sulfide (SS), with the biological effects of inhibition on both cyclooxygenase-1 (COX-1) and COX-2 activities and the decrease in prostaglandin (PG) synthesis.32–34 SS was reported as the most active O2 scavenger which gives reliability to a possible contribution of O2 scavenging activity for the final therapeutic activity of sulindac.35,36 The results of the previous studies demonstrated that SS scavenged HOCl, O2•, HO•, •NO, ONOO−, and SS are a much more potent O2• scavenger than sulindac as parent compound. The SS showed that reactive nitrogen species RNS (•NO and ONOO-) and reactive oxygen species ROS (O2•, HO•) scavenging activity may contribute strongly to the radioprotective efficacy.37 Therefore, these activity species were decreased by SS.37,38 Previous studies investigated that the radical scavengers can be used to protect DNA from free radicals that were generated by radiation.39 In the normal cells, exposure to IR initiates released inflammatory cytokines, which resulted in DNA damage.40 Also, NSAIDs could reduce radiation-induced chromosomal instability in vivo.41 In this study, it was demonstrated that SS relieved IR-induced genotoxicity in human lymphocytes. Clearly, free radicals and inflammation are the main factors for IR-induced DNA damage. A normal cell is going to dysfunction in the inflammatory process. It is documented that the anti-inflammatory effect of SS with inhibition of COX and the decrease in the secretion of cytokines are the main proposed mechanisms for the radioprotective effect of SS. In the present study, IR caused inhibition of SOD activities in irradiated lymphocytes. Since antioxidant enzymes like SOD have the effect of protection against IR, the balance of these enzymes in the cell is important for maximal radioprotection. Here, the SS caused an increase in the activity of SOD in lymphocytes and avoided the accumulation of superoxide radicals and H2O2. ROS and the free radicals influence the membrane lipids and cause extensive membrane lipid peroxidation. Therefore, increased levels of lipid peroxidation induced by IR are accompanied by a decrease in the activity of SOD.38
With its main protective mechanisms including the anti-inflammatory activity, COX inhibition, antioxidant properties, reduction of oxidative stress markers such as LP, and increase of SOD enzyme content, SS can be effective as a radioprotective agent. In fact, there is a crosstalk between rise of oxidative stress level and frequency of MN after the exposure. On the other hand, several researches revealed that the natural agent and anti-inflammatory drugs against oxidant challenge might decrease the rate of mutation and genotoxicity; hence, they helped prevent genotoxic induced by IR.19,42,43 This result shows a new indication of SS for the protection of normal cells during radiation therapy in the treatment of cancer patients.
Conclusion
In this study, sulindac sulfide as active metabolite of sulindac with anti-inflammatory and antioxidant properties can decrease genotoxicity and reduce levels of MDA. Moreover, sulindac sulfide can increase the antioxidant activity of SOD enzyme that induced by ionizing irradiation in human lymphocytes. Furthermore, sulindac sulfide showed the low toxicity as non-steroidal anti-inflammatory drugs and free radical scavenging properties. It can help the protection of the body against side effects’ ionizing irradiation in human.
Acknowledgments
This study was supported by a grant from Kerman University of Medical Sciences, Kerman, Iran. The ethic approval code is IR.KMU.REC.1396.2488. This research was the subject of a PharmD thesis by Farzaneh Nabizadeh Haghighi as a PharmD student of Kerman University of Medical Sciences.
Disclosure
The authors report no potential conflicts of interest relevant to this article.
References
1. Bai H, Chen X, Zhang L, Dou X. The effect of sulindac, a non-steroidal anti-inflammatory drug, attenuates inflammation and fibrosis in a mouse model of chronic pancreatitis. BMC Gastroenterol. 2012;12:115. doi:10.1186/1471-230X-12-115
2. Desouky O, Ding N, Zhou G. Targeted and non-targeted effects of ionizing radiation. J Radiat Res Appl Sci. 2015;8(2):247–254. doi:10.1016/j.jrras.2015.03.003
3. Kawanishi S, Ohnishi S, Ma N, Hiraku Y, Murata M. Crosstalk between DNA damage and inflammation in the multiple steps of carcinogenesis. Int J Mol Sci. 2017;18(8):1808. doi:10.3390/ijms18081808
4. Milligan JR, Aguilera JA, Ward JF. Variation of single-strand break yield with scavenger concentration for plasmid DNA irradiated in aqueous solution. Radiat Res. 1993;133(2):151–157. doi:10.2307/3578350
5. Yamamori T, Yasui H, Yamazumi M, et al. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free Radic Biol Med. 2012;53(2):260–270. doi:10.1016/j.freeradbiomed.2012.04.033
6. Kwiecien S, Jasnos K, Magierowski M, et al. Lipid peroxidation, reactive oxygen species and antioxidative factors in the pathogenesis of gastric mucosal lesions and mechanism of protection against oxidative stress-induced gastric injury. J Physiol Pharmacol. 2014;65(5):613–622.
7. Baselet B, Sonveaux P, Baatout S, Aerts A. Pathological effects of ionizing radiation: endothelial activation and dysfunction. Cell Mol Life Sci. 2019;76(4):699–728.
8. Hosseinimehr SJ. Flavonoids and genomic instability induced by ionizing radiation. Drug Discov Today. 2010;15(21–22):907–918. doi:10.1016/j.drudis.2010.09.005
9. Mukherjee D, Coates PJ, Lorimore SA, Wright EG. Responses to ionizing radiation mediated by inflammatory mechanisms. J Pathol. 2014;232(3):289–299. doi:10.1002/path.2014.232.issue-3
10. Rodrigues MA, Beaton-Green LA, Wilkins RC. Validation of the cytokinesis-block micronucleus assay using imaging flow cytometry for high throughput radiation biodosimetry. Health Phys. 2016;110(1):29–36. doi:10.1097/HP.0000000000000371
11. Soriano-Hernandez AD, Galvan-Salazar HR, Montes-Galindo DA, et al. Antitumor effect of meclofenamic acid on human androgen-independent prostate cancer: a preclinical evaluation. Int Urol Nephrol. 2012;44(2):471–477. doi:10.1007/s11255-011-0012-0
12. Alok A, Adhikari J, Chaudhury N. Radioprotective role of clinical drug diclofenac sodium. Mutat Res Genet Toxicol Environ Mutagen. 2013;755(2):156–162. doi:10.1016/j.mrgentox.2013.06.015
13. Asghari M, Shaghaghi Z, Farzipour S, Ghasemi A, Hosseinimehr SJ. Radioprotective effect of olanzapine as an anti-psychotic drug against genotoxicity and apoptosis induced by ionizing radiation on human lymphocytes. Mol Biol Rep. 2019. doi:10.1007/s11033-019-05024-x
14. Pouri M, Shaghaghi Z, Ghasemi A, Hosseinimehr SJ. Radioprotective effect of gliclazide as an anti-hyperglycemic agent against genotoxicity induced by ionizing radiation on human lymphocytes. Cardiovasc Hematol Agents Med Chem. 2019;17(1):40–46. doi:10.2174/1871525717666190524092918
15. Steinbrink SD, Pergola C, Buhring U, et al. Sulindac sulfide suppresses 5-lipoxygenase at clinically relevant concentrations. Cell Mol Life Sci. 2010;67(5):797–806. doi:10.1007/s00018-009-0206-0
16. Costa D, Gomes A, Lima JL, Fernandes E. Singlet oxygen scavenging activity of non-steroidal anti-inflammatory drugs. Redox Rep. 2008;13(4):153–160. doi:10.1179/135100008X308876
17. Santos F, Teixeira L, Lucio M, et al. Interactions of sulindac and its metabolites with phospholipid membranes: an explanation for the peroxidation protective effect of the bioactive metabolite. Free Radic Res. 2008;42(7):639–650. doi:10.1080/10715760802270326
18. Rice PL, Goldberg RJ, Ray EC, Driggers LJ, Ahnen DJ. Inhibition of extracellular signal-regulated kinase 1/2 phosphorylation and induction of apoptosis by sulindac metabolites. Cancer Res. 2001;61(4):1541–1547.
19. Hosseinimehr SJ, Nobakht R, Ghasemi A, Pourfallah TA. Radioprotective effect of mefenamic acid against radiation-induced genotoxicity in human lymphocytes. Radiat Oncol J. 2015;33(3):256–260. doi:10.3857/roj.2015.33.3.256
20. Hosseinimehr SJ, Fathi M, Ghasemi A, Shiadeh SN, Pourfallah TA. Celecoxib mitigates genotoxicity induced by ionizing radiation in human blood lymphocytes. Res Pharm Sci. 2017;12(1):82–87. doi:10.4103/1735-5362.199051
21. Salehifar E, Hosseinimehr SJ. The use of cyclooxygenase-2 inhibitors for improvement of efficacy of radiotherapy in cancers. Drug Discov Today. 2016;21(4):654–662. doi:10.1016/j.drudis.2016.02.019
22. Perugini RA, McDade TP, Vittimberga FJ
23. Lee TK, Stupans I. Radioprotection: the non-steroidal anti-inflammatory drugs (NSAIDs) and prostaglandins. J Pharm Pharmacol. 2002;54(11):1435–1445. doi:10.1211/00223570254
24. Li X, Pathi SS, Safe S. Sulindac sulfide inhibits colon cancer cell growth and downregulates specificity protein transcription factors. BMC Cancer. 2015;15:974. doi:10.1186/s12885-015-1956-8
25. Nivsarkar M. Improvement in circulating superoxide dismutase levels: role of nonsteroidal anti-inflammatory drugs in rheumatoid arthritis. Biochem Biophys Res Commun. 2000;270(3):714–716. doi:10.1006/bbrc.2000.2503
26. Hahn MB, Meyer S, Schroter MA, Kunte HJ, Solomun T, Sturm H. DNA protection by ectoine from ionizing radiation: molecular mechanisms. Phys Chem Chem Phys. 2017;19(37):25717–25722. doi:10.1039/C7CP02860A
27. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi:10.1006/abio.1996.0292
28. Ohadi M, Forootanfar H, Rahimi HR, et al. Antioxidant potential and wound healing activity of biosurfactant produced by Acinetobacter junii B6. Curr Pharm Biotechnol. 2017;18(11):900–908. doi:10.2174/1389201018666171122121350
29. Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455(1–2):81–95. doi:10.1016/S0027-5107(00)00065-8
30. Gholamine B, Houshmand G, Hosseinzadeh A, Kalantar M, Mehrzadi S, Goudarzi M. Gallic acid ameliorates sodium arsenite-induced renal and hepatic toxicity in rats. Drug Chem Toxicol. 2019;1–12. doi:10.1080/01480545.2019.1591434
31. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310.
32. Gruber BM, Bubko I, Krzyszton-Russjan J, Anuszewska EL. Synergistic action of doxorubicin and sulindac in human cervix carcinoma cells – studies on possible mechanisms. Med Sci Monit. 2010;16(1):Br45–Br51.
33. Haanen C. Sulindac and its derivatives: a novel class of anticancer agents. Curr Opin Investig Drugs. 2001;2(5):677–683.
34. Frahm S, Kurtz A, Kluwe L, Farassati F, Friedrich RE, Mautner VF. Sulindac derivatives inhibit cell growth and induce apoptosis in primary cells from malignant peripheral nerve sheath tumors of NF1-patients. Cancer Cell Int. 2004;4(1):4. doi:10.1186/1475-2867-4-4
35. Laube M, Kniess T, Pietzsch J. Development of antioxidant COX-2 inhibitors as radioprotective agents for radiation therapy – a hypothesis-driven review. Antioxidants. 2016;5(2):14. doi:10.3390/antiox5020014
36. Pangburn HA, Ahnen DJ, Rice PL. Sulindac metabolites induce proteosomal and lysosomal degradation of the epidermal growth factor receptor. Cancer Prev Res (Phila). 2010;3(4):560–572. doi:10.1158/1940-6207.CAPR-09-0159
37. Fernandes E, Toste SA, Lima JL, Reis S. The metabolism of sulindac enhances its scavenging activity against reactive oxygen and nitrogen species. Free Radic Biol Med. 2003;35(9):1008–1017. doi:10.1016/S0891-5849(03)00437-4
38. Cosar M, Kaner T, Sahin O, et al. The neuroprotective effect of sulindac after ischemia-reperfusion injury in rats. Acta Cir Bras. 2014;29(4):268–273. doi:10.1590/S0102-86502014000400008
39. Runge R, Oehme L, Kotzerke J, Freudenberg R. The effect of dimethyl sulfoxide on the induction of DNA strand breaks in plasmid DNA and colony formation of PC Cl3 mammalian cells by alpha-, beta-, and auger electron emitters (223)Ra, (188)Re, and (99m)Tc. EJNMMI Res. 2016;6(1):48. doi:10.1186/s13550-016-0203-x
40. Mavragani IV, Laskaratou DA, Frey B, et al. Key mechanisms involved in ionizing radiation-induced systemic effects. A current review. Toxicol Res (Camb). 2016;5(1):12–33. doi:10.1039/C5TX00222B
41. Mukherjee D, Coates PJ, Lorimore SA, Wright EG. The in vivo expression of radiation-induced chromosomal instability has an inflammatory mechanism. Radiat Res. 2012;177(1):18–24. doi:10.1667/RR2793.1
42. Modi JP, Prentice H, Wu J-Y. Sulindac for stroke treatment: neuroprotective mechanism and therapy. Neural Regen Res. 2014;9(23):2023. doi:10.4103/1673-5374.147919
43. Hosseinimehr SJ, Ahmadi A, Mahmoudzadeh A, Mohamadifar S. Radioprotective effects of daflon against genotoxicity induced by gamma irradiation in human cultured lymphocytes. Environ Mol Mutagen. 2009;50(9):749–752. doi:10.1002/em.v50:9
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.