Back to Journals » OncoTargets and Therapy » Volume 12
Radiologic Complete Response In Lung Adenocarcinoma With Symptomatic Brain Metastasis After Systematic Therapy: A Case Study
Authors Chen J, Wang J, Zheng Q, Weng M, Wu X
Received 10 August 2019
Accepted for publication 23 October 2019
Published 11 November 2019 Volume 2019:12 Pages 9551—9557
DOI https://doi.org/10.2147/OTT.S226735
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Jianxin Chen,1 Junhui Wang,2 Qinhong Zheng,1 Meiling Weng,1 Xilin Wu1
1Department of Medical Oncology, Quzhou People’s Hospital, Quzhou, Zhejiang 324000, People’s Republic of China; 2Department of Radiation Oncology, Quzhou People’s Hospital, Quzhou, Zhejiang 324000, People’s Republic of China
Correspondence: Xilin Wu
Department of Medical Oncology, Quzhou People’s Hospital, Quzhou, Zhejiang 324000, People’s Republic of China
Email [email protected]
Abstract: Patients diagnosed as lung adenocarcinoma with brain metastasis usually result in poor prognosis with limited survival time. Palliative systematic therapy has emerged as the primary choice for non-small cell lung cancer patients with brain metastasis harboring wild-type drive genes. However, the objective response rate and long-term survival for patients treated with this therapy remained unsatisfied. Herein, we present a case with lung adenocarcinoma accompanied with symptomatic brain metastasis who achieved radiologic complete response after receiving combined therapy including stereotactic body radiation therapy, anti-angiogenesis, and chemotherapy. He has achieved a duration of disease-free survival of thirty-six months, and is still in extension.
Keywords: complete response, lung adenocarcinoma, brain metastasis, systematic therapy
Introduction
Lung adenocarcinoma has emerged as the most common subtype of non-small cell lung cancer (NSCLC), which has been the leading cause of cancer-related mortalities worldwide. There were only approximately 2% of patients diagnosed with metastatic lung adenocarcinoma surviving over five years without the administration of immunotherapy.1 Brain metastasis was one of the most common events at the time of diagnosis in patients with NSCLC, which usually resulted in poorer prognosis and extremely significant mortalities compared to patients without brain metastasis. The median overall survival time for NSCLC patients with brain metastasis, those absent from any treatment, or those who received glucocorticoid treatment or whole brain radiation therapy (WBRT) was merely one month, two months, or five months, respectively.2,3 In addition, the efficacy of cytotoxic agents was significantly limited because of poor penetration of the blood–brain barrier. Hence, it still remains challenging to prolong the survival time in patients with lung adenocarcinoma accompanied with brain metastasis.
Systematic therapy is recommended as a primary choice for NSCLC patients with brain metastasis according to National Comprehensive Cancer Network (NCCN) guidelines. As one of the effective strategies to control brain disease and release symptoms, brain radiation therapy including WBRT, stereotactic radiosurgery (SRS), and stereotactic ablative radiotherapy (SABR) played a significant role in the management of brain metastasis. However, a high recurrence rate of brain disease and the radiation dose constraint of brain tissue made it difficult to receive a satisfactory outcome. What is worse, brain metastasis was deemed to be unresponsive to chemotherapeutic agents or macromolecular ones because of obstruction of the blood–brain barrier. In recent decades, combined treatment of platinum-based double-agent regimens and antiangiogenesis with bevacizumab was recommended as the primary choice for metastatic non-squamous NSCLC patients without sensitive gene mutations. It was reported that the addition of bevacizumab significantly contributed to the extension of progression-free survival (PFS) and overall survival (OS) time according to the results of clinical trials ECOG 4599 and BEYOND.4,5 However, neither of the studies recruited NSCLC patients with brain metastasis, let alone symptomatic brain disease. In recent years, several prospective research studies with small sample size and retrospective ones were conducted to evaluate the safety and efficacy profile of the addition of bevacizumab in NSCLC patients with brain metastasis,6–9 the results of which revealed that chemotherapy plus bevacizumab might be more effective for NSCLC patients with brain metastasis, along with similar risk of developing cerebral hemorrhage, independent of bevacizumab. However, because of the limited size of samples, single-armed design, and retrospective investigation of the existing proof, convincing evidence from a randomized, controlled, prospective trial might still be necessary to sustain the application value of bevacizumab in such patients.
Herein, based on the integrated medical record, superior compliance, and preferable efficacy, we report a patient with lung adenocarcinoma accompanied with symptomatic brain metastasis achieved radiologic complete response after receiving combined therapy including stereotactic body radiation therapy (SBRT), anti-angiogenesis, and chemotherapy. He has achieved a duration of disease-free survival time of thirty-six months, and is still in extension.
Case Presentation
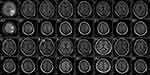
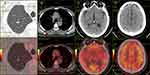
A 54-year-old man was admitted to our institution on June 25th, 2016 with a four-day history of weakness in the right upper limb. The patient had a smoking history of thirty-six years with fifteen to twenty cigarettes a day. Otherwise, he denied any other medical or family history. According to the findings of cranial magnetic resonance imaging (MRI), two lesions in the brain with surrounding edema were detected in the parietal lobe and occipital lobe, separately (Figure 1, A1/A2). Subsequent chest computed tomography (CT) showed a thoracic mass in the right upper lung (Figure 2, A1), with enlarged mediastinal lymph nodes suggesting metastases (Figure 2, A2). Based on the biopsy findings from transthoracic needle pneumocentesis (Figure 3), the patient was finally diagnosed as lung adenocarcinoma with metastases on the mediastinal lymph nodes and brain, suggesting metastatic disease, according to the American Joint Committee on Cancer staging system, seventh edition.10 Drive genes tested with the NGS panel were detected as wild types for potential mutations of anaplastic lymphoma kinase (ALK), ROS proto-oncogene 1 (ROS1), V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS), neuroblastoma RAS viral oncogene homolog (NRAS), RET proto-oncogene (RET), V-raf murine sarcoma viral oncogene homolog B1 (BRAF), receptor tyrosine-protein kinase erbB-2 (ERBB2), RAC-alpha serine/threonine-protein kinase (AKT1), discoidin domain receptor tyrosine kinase 2 (DDR2), fibroblast growth factor receptor 1 (FGFR1), MNNG HOS transforming gene (MET), phosphatase and tensin homolog (PTEN), phosphatidylinositol-4,5-bisphosphate 3-kinase (PIK3CA), and mitogen-activated protein kinase 1 (MAP2K1).
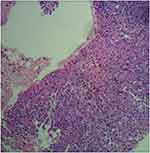 |
Figure 3 Histological finding with hematoxylin and eosin–stained biopsy specimen from percutaneous lung biopsy on June 30th, 2016. |
With the definite diagnosis made, the patient received SBRT for occupations in the brain because of the related symptoms on July 8th, 2016. After that, he received four cycles of first-line combined therapy (bevacizumab 7.5 mg/kg plus pemetrexed 500 mg/m2 day 1 plus cisplatin 75 mg/m2 day 1, every twenty-one days) in our hospital from July 11th, 2016 to November 10th, 2016. During the whole administration of the first-line combined treatment, the patient suffered adverse events including neutropenia at grade 1, leukocytopenia at grade 2, and fatigue at grade 1 (National Cancer Institute Common Toxicity Criteria, version 4.03). However, any discontinuation or interruption of the treatment was not executed because of the tolerable toxicities. The efficacy assessment with chest CT and cranial MRI was evaluated as partial response (PR) (Figure 1, B1/B2; Figure 2, B1/B2). From then on, the patient received another 31 months of maintenance therapy (bevacizumab 7.5 mg/kg plus pemetrexed 500 mg/m2 day 1, every twenty-one days) until June 28th, 2019. During the whole period of maintenance therapy, he received imaging review regularly every one to two months (cranial MRI in Figure 1, from C1/C2 to P1/P2; chest CT in Figure 2, from C1/C2 to X1/X2). During the first follow-up on September 9th, 2016, the primary tumor in the right upper lobe was found empty (Figure 2, B1), the status of which sustained to the seventeenth follow-up on August 20th, 2018 (Figure 2, Q1). On October 28th, 2018, the chest CT scan revealed an emerging streak, high-density mass in the original location in the right upper lung (Figure 2, R1). After further general imageological examination, there was no other emerging occupation discovered. In addition, the shrunken lesions in the brain and mediastinum still remained stable (Figure 1, N1/N2; Figure 2, R2). Based on that, positron emission tomography–computed tomography (PET-CT) was conducted on June 28th, 2019 to detect the activity of residual lesions in the brain and mediastinum. As a result, there was no obvious β-2-[18F]-fluoro-2-deoxy-D-glucose (FDG) uptake detected for the suspicious lesions in the lung, brain, or mediastinum, suggesting residual scars (Figure 4). The last maintenance treatment before the submission of the present report was administered on June 26th, 2019. After that, we suggested the patient should suspend the maintenance treatment because of the absence of active tumors detected by PET-CT, and the potential toxicities in the liver, kidney, or marrow. However, he insisted on receiving further maintenance therapy regularly because of the free medicine policy of bevacizumab and pemetrexed (costs of the two drugs were gratuitous after established cycles of treatment). The variations of tumor markers including CEA, SCC, CA72-4, and CA19-9 during the whole treatment are presented in Figure 5. During the administration of pemetrexed and bevacizumab as maintenance therapy, a slight skin rash on the chest at grade 1 was observed. With the symptomatic treatment of glucocorticoid, the symptom of rash was relieved, without any reduction of drugs or discontinuation of treatment. The latest follow-up was conducted on June 26th, 2019 for the present report, without any symptoms or adverse events discovered then. The patient diagnosed as metastatic NSCLC with symptomatic brain metastases has achieved radiologic complete response, with disease-free survival time of thirty-six months, and is still in extension.
Discussion
The present case report presented the long-term survival (thirty-six months and still in extension) achieved by a NSCLC patient with symptomatic brain metastases after the combined treatment of SBRT, anti-angiogenesis, and platinum-based chemotherapy. Because of the integrated medical record, superior compliance, and preferable efficacy, we reported the representative case to provide some inspiration for clinicians, as well as confidence for selected patients.
Patients with symptomatic brain metastasis were always accompanied with poor prognosis, especially in those harboring wild-type sensitive drive genes.11,12 Definitive therapy for brain disease played a significant role during the whole administration according to NCCN guidelines. In selected NSCLC patients with limited brain metastasis, local treatment strategies such as SBRT and surgical resection were recommended as the standard choice before systematic treatment. Even so, the median OS for NSCLC patients with metastatic brain disease, who had benefited from systematic therapy including local treatment, was only 19.7 months.13 The patient in the present case was eligible for local treatment of brain metastases because of the tumor-related weakness in the right upper limb and limited disease in the brain. He received SBRT with our suggestion. Although the results of the drive genes test did not provide a targeted approach for him, the efficacy of combined therapy including bevacizumab, pemetrexed, and cisplatin seemed significantly effective. With the systematic treatment, he achieved a PR in the first imageological evaluation on September 9th, 2016, and then a duration of disease-free survival of thirty-six months, which was much longer than that in the ECOG 4599 (median OS for bevacizumab/paclitaxel/carboplatin, 12.3 months) and BEYOND (median OS for bevacizumab/paclitaxel/carboplatin, 24.3 months) trials.4,5 Meanwhile, maintenance therapy in the present case may also play a significant role during the whole administration. Data from the AVAPERL study, which used bevacizumab/pemetrexed as maintenance therapy, showed a 3.7-month increase (7.4 months versus 3.7 months) on PFS compared to bevacizumab alone.14,15 However, the priority of the maintenance therapy on the benefit of PFS seems limited, whether with single-agent regimens or combined regimens.16–18 Therefore, it might not be appropriate to ascribe the significantly advantageous survival to the maintenance therapy in the present case. In recent years, there were several research studies conducted to evaluate the predicted value of the neutrophil to lymphocyte ratio (NLR) on clinical efficacy of chemotherapy in patients with lung cancer. Accordingly, the NLR was supposed to be well connected with outcomes and response to chemotherapy in patients with lung cancer.19–21 Specifically, a lower NLR seemed to be associated with better prognosis in patients with NSCLC, as well as a higher response rate to treatment.21 Although the cut-off level of the NLR remained discrepant, NLR of 5 was deemed a consensual value in the several reported research studies.20,21 In the present report, the NLR was 2.42 at the diagnosis of the disease, which varied from 2.12 to 2.80 stably during the whole treatment. It was revealed that the NLR might be available as a predictive factor of clinical efficacy from the combined treatment as well in the present case. In addition, tumor markers including CEA, CA19-9, CA72-4, and SCC varied in the normal range most of the time during the whole therapy for the present patient, which came to be another feature. It has been reported that high levels of tumor markers including CEA and CA125 at baseline were correlated with worse survival in stage III–IV NSCLC patients.22 It was revealed that tumor cells which were unable to secrete tumor-related protein, or stimulated the secretion function of normal cells, might be more indolent compared to active ones.22–24 That might be stood as another potential reason to explain the advantageous prognosis of the present case.
There are also several limitations in the present case. First of all, the histological features at the time of radiologic complete response were not obtained. Although imageological results of chest CT, cranial MRI, and PET-CT suggested complete remission of the previous lesions without any evidence of active tumors, pathologic findings still remained as the standard of the definite evaluation. We tried to suggest to the patient conducting complete resection of the imageological mass in the lung and mediastinum. However, he refused to receive the active operation. Furthermore, the drive gene panel used in the present case only included fourteen common genes, which may result in controversy of potential rare mutations of genes. In addition, the CT and MRI images presented in the present report were not completely consistently among each visit. Because of the bustling arrangement of CT scans every day, we could not guarantee that CT scans on each visit be conducted in the same machine, except for clinical trials. The different choices of the machines might lead to a potential, minor diversity in each examination. In addition, the deviation of position, including the head and body in each visit, might lead to another discrepancy for a patient. We understand that the consistency of images is very important. We have tried our best to fix it but failed. Finally, the leading limitation comes from the nature of a case report, the results of which need further identification in clinical practice and basic experiments.
Even so, based on the integrated medical record and preferable efficacy of the present case, we suggest that systematic administration including actively local treatment might be necessary for selected patients with metastatic disease, especially in patients with potential benign prognostic factors, such as wild-type drive gene, negative tumor markers, and lower NLR status. With active management, select patients with metastatic disease might be able to obtain a result of remission of cancer.
Consent For Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Acknowledgments
The authors thank the patient for his participation and his agreement to publication of the report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stinchcombe TE, Socinski MA. Current treatments for advanced stage non-small cell lung cancer. Proc Am Thorac Soc. 2009;6:233–241. doi:10.1513/pats.200809-110LC
2. Schuler M, Wu YL, Hirsh V, et al. First-line afatinib versus chemotherapy in patients with non-small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol. 2016;11:380–390. doi:10.1016/j.jtho.2015.11.014
3. Fan Y, Huang Z, Fang L, et al. Chemotherapy and egfr tyrosine kinase inhibitors for treatment of brain metastases from non-small-cell lung cancer: survival analysis in 210 patients. Onco Targets Ther. 2013;6:1789–1803. doi:10.2147/OTT.S52172
4. Sandler A, Yi J, Dahlberg S, et al. Treatment outcomes by tumor histology in eastern cooperative group study e4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:1416–1423. doi:10.1097/JTO.0b013e3181da36f4
5. Zhou C, Wu YL, Chen G, et al. Beyond: A randomized, double-blind, placebo-controlled, multicenter, phase iii study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33:2197–2204. doi:10.1200/JCO.2014.59.4424
6. Zustovich F, Ferro A, Lombardi G, Farina P, Zagonel V. Bevacizumab-based therapy for patients with brain metastases from non-small-cell lung cancer: preliminary results. Chemotherapy. 2014;60:294–299. doi:10.1159/000376605
7. Besse B, Lasserre SF, Compton P, Huang J, Augustus S, Rohr UP. Bevacizumab safety in patients with central nervous system metastases. Clin Cancer Res. 2010;16:269–278. doi:10.1158/1078-0432.CCR-09-2439
8. De Braganca KC, Janjigian YY, Azzoli CG, et al. Efficacy and safety of bevacizumab in active brain metastases from non-small cell lung cancer. J Neurooncol. 2010;100:443–447. doi:10.1007/s11060-010-0200-2
9. Tang N, Guo J, Zhang Q, Wang Y, Wang Z. Greater efficacy of chemotherapy plus bevacizumab compared to chemo- and targeted therapy alone on non-small cell lung cancer patients with brain metastasis. Oncotarget. 2016;7:3635–3644. doi:10.18632/oncotarget.6184
10. Edge SB, Compton CC. The american joint committee on cancer: the 7th edition of the ajcc cancer staging manual and the future of tnm. Ann Surg Oncol. 2010;17:1471–1474. doi:10.1245/s10434-010-0985-4
11. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi:10.1200/JCO.2011.38.0527
12. Lukas RV, Kumthekar P, Rizvi S, Salgia R. Systemic therapies in the treatment of non-small-cell lung cancer brain metastases. Future Oncol. 2016;12:1045–1058. doi:10.2217/fon.16.17
13. Ashworth A, Rodrigues G, Boldt G, Palma D. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer. 2013;82:197–203. doi:10.1016/j.lungcan.2013.07.026
14. Barlesi F, Scherpereel A, Rittmeyer A, et al. Randomized phase iii trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: avaperl (mo22089). J Clin Oncol. 2013;31:3004–3011. doi:10.1200/JCO.2012.42.3749
15. Barlesi F, Scherpereel A, Gorbunova V, et al. Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: updated survival analysis of the avaperl (mo22089) randomized phase iii trial. Ann Oncol. 2014;25:1044–1052. doi:10.1093/annonc/mdu098
16. Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (paramount): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13:247–255. doi:10.1016/S1470-2045(12)70063-3
17. Paz-Ares LG, de Marinis F, Dediu M, et al. Paramount: final overall survival results of the phase iii study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:2895–2902. doi:10.1200/JCO.2012.47.1102
18. Patel JD, Socinski MA, Garon EB, et al. Pointbreak: a randomized phase iii study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage iiib or iv nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:4349–4357. doi:10.1200/JCO.2012.47.9626
19. Liu D, Jin J, Zhang L, Li L, Song J, Li W. The neutrophil to lymphocyte ratio may predict benefit from chemotherapy in lung cancer. Cell Physiol Biochem. 2018;46:1595–1605. doi:10.1159/000489207
20. Cedres S, Torrejon D, Martinez A, et al. Neutrophil to lymphocyte ratio (nlr) as an indicator of poor prognosis in stage iv non-small cell lung cancer. Clin Transl Oncol. 2012;14:864–869. doi:10.1007/s12094-012-0872-5
21. Peng B, Wang YH, Liu YM, Ma LX. Prognostic significance of the neutrophil to lymphocyte ratio in patients with non-small cell lung cancer: a systemic review and meta-analysis. Int J Clin Exp Med. 2015;8:3098–3106.
22. Cedres S, Nunez I, Longo M, et al. Serum tumor markers cea, cyfra21-1, and ca-125 are associated with worse prognosis in advanced non-small-cell lung cancer (nsclc). Clin Lung Cancer. 2011;12:172–179. doi:10.1016/j.cllc.2011.03.019
23. Duan X, Cui Y, Gong M, et al. Variations in serum cea and cyfra21-1 levels before and after surgery facilitate prognosis of non-small cell lung cancer patients. Zhongguo Fei Ai Za Zhi. 2015;18:358–364. doi:10.3779/j.issn.1009-3419.2015.06.05
24. Molina R, Filella X, Auge JM, et al. Tumor markers (cea, ca 125, cyfra 21-1, scc and nse) in patients with non-small cell lung cancer as an aid in histological diagnosis and prognosis. Comparison with the main clinical and pathological prognostic factors. Tumour Biol. 2003;24:209–218. doi:10.1159/000074432
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.