Back to Journals » Cancer Management and Research » Volume 10
Radiofrequency ablation with systemic chemotherapy in the treatment of colorectal cancer liver metastasis: a 10-year single-center study
Authors Ou S, Xu R, Li K, Chen Y, Kong Y, Liu H, Li J, Ouyang Y, Yu X
Received 4 April 2018
Accepted for publication 8 August 2018
Published 31 October 2018 Volume 2018:10 Pages 5227—5237
DOI https://doi.org/10.2147/CMAR.S170160
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Shuangyan Ou,1 Ruocai Xu,1 Ke Li,1 Yong Chen,1 Yi Kong,1 Hanchun Liu,1 Jianliang Li,1 Yongzhong Ouyang,2 Xiaoping Yu3
1Department of Hepatobiliary and Pancreatic Medicine, Hunan Cancer Hospital, Changsha City, Hunan Province, China; 2Department of Gastroduodenal Surgery, Hunan Provincial Cancer Hospital, Changsha City, Hunan Province, China; 3Department of Radiology, Hunan Cancer Hospital, Changsha City, Hunan Province, China
Objective: To retrospectively evaluate the long-term efficacy and safety of radiofrequency ablation (RFA) with systemic chemotherapy (CT) in treatment of solitary liver metastasis after surgery for colorectal cancer (CRC).
Methods: This single-center study was conducted at the Hunan Provincial Cancer Hospital from June 2006 to December 2015 with median follow-up time of 26 months. Percutaneous ultrasound-guided RFA was carried out on eligible patients with solitary liver metastasis after surgery for CRC. After a week, ablation status was confirmed by MRI. Post MRI, all patients received systemic CT with or without molecular-targeted therapy. Survival rate was evaluated and survival curve was constructed with Kaplan–Meier analysis. Log-rank test and Cox regression model were used for univariate and multivariate analysis, respectively, to determine the independent prognostic factors for survival rate.
Results: A total of 109 eligible patients (mean age, 53.84±11.71; mean tumor mass diameter, 3.4+2.01 cm) were enrolled in this 10-year study. After RFA, 95 patients achieved complete ablation, and 14 patients achieved partial ablation, with median ablation time of 26 minutes (range: 12–120 minutes). The median survival time required for achieving complete and partial ablation was 56.0 and 19.0 months, respectively (P<.01). After RFA and adjuvant systemic CT, the 1-, 3-, and 5-year survival rates were 92.3%, 50.7%, and 41.6%, respectively, with the median (mean) survival time of 39.0 (56.5) months. Age was the only significant independent prognostic factor with better survival rate observed in patients aged ≥50 years than those aged <50 years (P<0.05). The incidence of complications was minimal (1.8%) with only two cases: one biliary fistula and one liver hemorrhage.
Conclusion: RFA combination with systemic CT was safe; it showed long-term efficacy in patients with solitary liver metastasis after surgery for CRC and can be a preferred treatment.
Keywords: radiofrequency assay, systemic chemotherapy, liver metastasis, colorectal cancer, ultrasound, survival
Introduction
Colorectal cancer (CRC) is one of the most commonly diagnosed cancers worldwide, and is ranked second and third in female and male patients, respectively.1 Though the incidence is the highest in North America, Europe, and New Zealand/Australia, there has been an evolving trend in Asia and eastern Europe due to change in lifestyle.1,2 According to the 2015 statistics in China, CRC is the fifth most commonly diagnosed cancer in male patients and fourth most commonly diagnosed cancer in female patients, causing one of the highest mortalities (191,000 deaths in 2015).3 A study by Dennis DL et al revealed that majority of patients with CRC were not as physically active as subjects without cancer, on a regular basis.4 Also, western lifestyle changes, such as alcohol consumption, sleep deprivation, obesity, etc correlate with higher incidences of CRC.5
Liver is one of the most common sites for metastasis in CRC and in about 50% of the metastasis of liver cases observed.6 The gold standard of treatment for colorectal liver metastasis (CRLM) is surgical resection.7 The 5-year survival after resection ranged between 35% and 58% in patients with CRLM.8–11 Due to the anatomic, functional, and medical complexities, resection is possible only in about 10%–20% of patients with liver metastasis.12 Moreover, after resection, the recurrence is about 60% in patients with CRLM.13,14 Chemotherapy (CT) is also widely used in the treatment of CRLM. The addition of irinotecan and oxaliplatin to the traditional 5-fluorouracil and leucovorin combination improved the response from 20% to about 56%.15–18 Target therapies, such as bevacizumab or cetuximab in combination with CT with FOLFIRI showed a response rate of about 70% in patients with CRC.19 The overall survival (OS) rate for bevacizumab and FOLFOXIRI combination was reported to be 24.9%,20 whereas that for cetuximab and FOLFOXIRI combination was 46.2%.21 However, CT has serious complications such as steatohepatitis and sinusoidal obstruction syndrome, causing hepatic injury in patients undergoing liver resection for CRLMs.22 Hence, less-invasive ablative procedures like radiofrequency ablation (RFA), microwave coagulation therapy, and percutaneous ethanol injection have emerged as treatment of choice in nonsurgical CRLM cases.23–26 RFA has proved advantageous compared with other local ablative techniques in hepatocellular carcinoma due to several factors, including its minimal invasiveness, tumor control with good long-term survival with acceptable morbidity.27–29 CRLM patients treated with RFA have shown a 5-year survival of about 26%–33%.30,31 Though RFA has shown a local recurrence of 12%–18%,32,33 few studies have shown a recurrence as high as 47%.34 This has led to the need for adjuvant therapy. However, few studies used a combination of RFA and CT in the treatment of CRLM patients and found a recurrence of about 15% (26 out of 168 subjects) with no recurrence at the site of RFA.35 Similar study with combination of RFA and CT in 202 subjects reported an OS rate of 48% though 32% showed recurrence at RFA site; however, retreatment with ablation increased their OS compared with those who received no retreatment (45.5 vs 31.1 months, P<.001),36 suggesting that combination therapy improves long-term survival in patients with CRLM. In a country like China, where the mortality rate is high, there is an unmet need of large-scale studies to improve the OS in this challenging area of cancer. Therefore, the present study was designed in China to evaluate the efficacy and safety of RFA therapy combined with systemic CT in patients with solitary CRLM.
Materials and methods
Study design and patient selection
This single-center study was conducted at the Hunan Provincial Cancer Hospital from June 2006 to December 2015. The eligibility criteria included male or female patients aged between 18 and 85 years, who had a histologically confirmed adenocarcinoma after surgery for CRC, an Eastern Cooperative Oncology Group score between 0 and 1,37 single intrahepatic metastatic lesion confirmed by MRI or CT, etc. All subjects included in this study had clinically diagnosed liver metastasis after CRC surgery.
The study was approved by the Hunan Provincial Cancer Hospital review board. Written informed consent was taken from patients before enrolling into the study. There was no bias in selecting the patient population. Based on patient’s affordability and tissue biopsy and gene detection test for KRAS and NRAS genes were conducted prior to RFA.
RFA procedure and CT
An ultrasound-guided RFA through percutaneous approach under general anesthesia was performed in all patients. The devices used for the procedure were as follows: (i) RFA treatment system using internally cooled tip electrodes (Valleylab, Boulder, CO, USA) for generating radiofrequency; and (ii) ultrasound equipment (GE LOGIQ P5 Color and Philips CX50 portable color Doppler ultrasound, convex array probe, the frequency of 3.5–4.0 MHz) that acts as a guide for the needle electrode to reach the tumor location. Examination of blood, urine, liver, and renal function, and electrolytes was examined prior to RFA. For pathologic analysis, biopsy tissue sample was obtained by tumor puncture from those who agreed for biopsy test and centesis. Due to age and economic reasons, liver tissue was taken only from some patients to examine KRAS and NRAS genes.
The RFA technique and design depended on the morphology of the lesion: (i) for the long lesion, single-needle multipoint ablation for 12 minutes per hour; ii) for flat oval lesions, multi-needle multipoint ablation for 12 minutes per point; iii) for cylindrical lesions, multi-needle point up and down for 12 minutes per hour; and iv) for the liver surface, RF needle was placed 5 mm from the organ with caution for high-risk areas like gallbladder, bile duct, etc. MRI was performed 1 week after RFA to check on the ablation status. Blood, liver, and renal function were repeated about 1 week after RFA, then to prolong the effects of RFA and prevent recurrence, systemic CT or systemic CT along with molecular-targeted therapy (cetuximab or bevacizumab) was initiated.
Data collection, outcomes measured, and follow-up
All cases were followed up till June 30, 2016 via telephone, letters, personal visit, and household registration survey from police department, for determining survival and cause of death. Survival time was calculated from the time of hospitalization for liver metastasis. No patient was lost to follow-up. All patients who underwent RFA and systemic CT were evaluated. The patients were followed up at least once a year during the study. The outcomes studied included as follows: (i) 1-, 3-, and 5-year survival rate; and ii) survival difference with respect to gender (male and female), age (<50 and ≥50 years old), tumor site (left and right liver), tumor size (≤5 - and >5 cm), and CT regimen after RFA (systemic CT and systemic CT combined with molecular-targeted therapy).
Statistical analysis
The statistical analysis was performed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics was used to summarize baseline characteristics. Continuous variables (age, tumor mass size, tumor mass location, survival time, number of subjects receiving CT, and number of subjects receiving CT along with molecular-targeted therapy) were expressed as mean, median, and SD. Kaplan–Meier method was used to predict long-term survival. To compare the survival difference with respect to gender, age, tumor mass site, tumor mass size, and CT regimen after RFA, univariate analysis was performed by log-rank test. P value <0.05 was considered statistically significant. For those variables which were significant in the univariate analysis, Cox regression model was used for multivariate analysis to predict the independent prognostic factors for survival rate.
Results
Patient demographics and clinicopathologic features
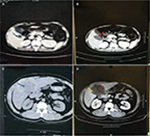
A total of 109 patients with CRC and single liver metastasis were enrolled over the 10-year study period. The median follow-up time was 26.0 months, with the longest survival time of 113 months. Most of the patients visited hospital 2–4 times in 1 year to be reviewed. Although no cases dropped out of the study, 54 out of 109 patients died and the cause of all deaths were tumor-related and not RFA-related. The mean age of the study population was 53.84+11.71 years. The mean tumor mass size among all subjects was 3.4+2.01 cm (maximum: 12.4 cm), with 80.7% of subjects having the right liver lobe as the tumor location. There were 92 patients with tumor measuring ≤5 cm and 17 with tumor measuring >5 cm. Ablation was complete (Figure 1A, B, blue arrow marks lesion and red arrow points toward ablated area) in 95 (87.2%) patients and partial (Figure 1C, D, yellow arrow points toward remanant lesion) in 14 (12.8%) patients with median RFA time of 26 minutes (range: 12–120 minutes). Post RFA, the number of patients who underwent standardized systemic CT and standardized systemic CT combined with molecular-targeted therapy was 83.4% and 16.5%, respectively. The demographic and clinicopathologic data are summarized in Table 1.
  | Table 1 Patient demographics and clinicopathological features Abbreviations: CT, chemotherapy; RFA, radiofrequency ablation. |
Biopsy of liver lesions is important; however, some patients disagreed for biopsy, and some were not suitable for percutaneous needle biopsy because of special locations of the lesions (n=39).
Survival outcome
The 1-, 3-, and 5-year cumulative survival rates were 92.3%, 50.7%, and 41.6%, respectively. The overall mean survival time was 56.5 months and the overall median survival time was 39.0 months. The 1-, 3-, and 5-year survival rates for standardized systemic CT were 94.4%, 46.2%, and 37.2%, respectively, with mean survival time of 52.8 months and median survival time of 31.0 months (Figure 2). The 1-, 3-, and 5-year survival rates for standardized systemic CT+ molecular targeting were 93.8%, 74.0%, and 63.5%, respectively, with mean survival time of 50.3 months.
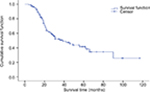  | Figure 2 Overall survival rate. |
Factors affecting survival outcome
Log-rank method was used to compare survival rate difference between different groups, including age, gender, tumor size, tumor location, and CT regimen after RFA (Table 2). No significant difference in survival rates were observed between male and female patients (P=0.985; Figure 3). A significant difference in the survival rate was observed among the different age groups (P=0.025; Figure 4) with the survival rate being better for patients aged ≥50 years. For ≥50 years group, the 1-, 3-, and 5-year survival rates were 91.0%, 58.0%, and 49.7%, respectively, with median (mean) survival time of 56.0 (67.3) months. For <50 years group, the 1-, 3-, and 5-year survival rates were 94.7%, 39.2%, and 29.8%, respectively, with median (mean) survival time of 24.0 (40.8) months. There was no significant difference in survival between different tumor sites and tumor mass size groups (P=0.331 and P=0.123; Figures 5 and 6, respectively). Also, no statistically significant difference in survival was noticed between the different CT regimen after RFA (P=0.091; Figure 7). Age was included as an independent prognostic factor for the final equation by Cox regression analysis. Those patients aged ≥50 years were 0.550 times at risk of death than patients aged <50 years (Table 3). Other factors were excluded in the equation.
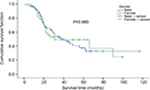  | Figure 3 Survival comparison in male and female patients. |
  | Figure 4 Comparison of survival rates in different age groups. |
  | Figure 5 Comparison of survival rates of different tumor sites. |
  | Figure 6 Comparison of survival rates of different tumor sizes. |
  | Figure 7 Comparison of survival rates of different treatment regimens. Abbreviations: CT, chemotherapy; RFA, radiofrequency ablation. |
  | Table 3 Multivariate analysis of independent prognostic factors for survival rates Notes: P value <0.05 was considered statistically significant.
|
The 1-, 3-, and 5-year survival rates for patients who underwent complete ablation were 93.5%, 59.5%, and 49.1%, respectively. The 1- and 2-year survival rates for patients who underwent partial ablation were 84.6% and 23.1%, respectively, while the 3- and 5-year survival rate cannot be calculated. The median survival time for patients who underwent complete ablation and partial ablation on liver tumor were 56.0 and 19.0 months, respectively, with a statistically significant difference observed (P<0.01) (Figure 8).
  | Figure 8 Comparison of survival rates of different ablations. |
Treatment complications
The incidence of serious complications was 1.8% (2/109). There was a case of biliary fistula, which was treated by B-guided puncture followed by anti-inflammatory agents. One case of liver hemorrhage was treated through hepatic artery catheterization embolization. No other serious complications like intestinal fistula, intestinal or gallbladder perforation were reported.
Discussion
In this study, we studied the efficacy of RFA with systemic CT in the treatment of solitary liver metastasis after surgery for CRC. In our study, post RFA and systemic CT, the survival rate of patients was found to be satisfactory with age emerging as the independent prognostic factor. On follow-up, minimal complications were observed in the 10-year study period.
Surgical resection is the standard therapy for CRLM associated with high survival rate and low mortality and morbidity.9,11,38 The mortality rate reported in our study was comparable with other published reports. There was no case of mortality related to RFA. There was no case of mortality related to RFA. All deaths reported were due to tumor. Similarly, Solbiati L et al did not observe procedure-related death,39 whereas in study by Boame N et al,40 postoperative death was reported in 1.2% of subjects. CT and RFA are the proposed alternative methods in unresectable hepatic neoplasms.41,42 RFA is a minimal invasive procedure based on the principle of tumor cell death by coagulative necrosis at a temperature >60°C. The RF waves produce thermal energy, causing intracellular protein denaturation, destruction of the cell membrane, and thrombosis of the microvasculature.28,29,43 Though RFA has advantages of easy applicability and safety, it is not suggested for larger tumors (>5–6 cm) as it is incapable of complete necrosis in these cases.44 The biggest drawback of RFA in tumors of liver tissue is its high recurrence rate.45,46
In case of solitary metastasis of liver, RFA is a better choice.31,47,48 Multiple studies have compared surgical resection and RFA in patients with CRLM. A 10-year retrospective study on patients with CRLM reported overall recurrence to be common after RFA (84%) compared with RFA + resection (64%) and resection alone (52%, P<0.001).11 The OS at 5 years was the highest (58%) after resection. Both liver-only recurrence and true local recurrence were significantly more common after RFA compared with resection.11 The 4-year survival was significantly lower in patients with RFA compared with resection and combination therapy of resection and RFA (22%, 65%, 36%; P<0.0001).11 However, in cases where resection was not possible, the survival in RFA + resection or RFA monotherapy was higher than CT alone (P<0.0017).11 Another retrospective study analyzing 12-year data showed significantly lower recurrence (5% vs 37%, P<0.001); a significantly higher 5-year OS was observed in patients with CRLM, who were treated with hepatic resection, compared with RFA (71% vs 27%; P<0.001).49 The local recurrence was higher in RFA compared with resection and the 5-year recurrence-free survival was significantly lower in RFA treated patients compared with patients who underwent resection (P<0.001).49 On the contrary, Leblanc et al reported that the local recurrence and survival rates were comparable and not statistically different between RFA, surgical resection, and combination therapy when size and topographic characteristics of liver metastases were considered for RFA.50 Similar results were observed in other studies on recurrence rates and OS between RFA and hepatic resection in small tumors (<3 cm) in solitary CRLM.51,52 Thus, these studies support that RFA could be an alternative in case of unresectable tumors of small size. In our study, we kept 5 cm as the tumor diameter because threshold has been used in previously reported studies on colon cancer53,54 and safety and efficacy of RFA in smaller tumor (average range of tumor diameter: 2.8 and 2.1–3.4 cm)40,55 are already reported.
CT used after resection has proven to lower recurrence compared with resection alone.56 Based on these data, studies using RFA and CT were conducted in patients with CRLM. In our study, the 1-, 3-, and 5-year cumulative survival rates were 92.3%, 50.7%, and 41.6%, respectively, after RFA and CT. The mean survival time was 56.5 months and the median survival time was 39.0 months. Our results are comparable with several other studies that reported survival benefits of RFA and systemic CT in patients with solitary CRLM. A study by Gillams et al reported a median survival of 59 months with 1-, 3-, and 5-year survival rates being 97%, 84%, and 40%, respectively in 40 patients with solitary, unresectable CLRM, with an average diameter of 2.3 cm (range: 0.8–4.0 cm).57
Another study reported similar survival outcomes in 100 patients with CRLM, who underwent RFA, as the first- and second-line treatment after CT. The 1-, 3-, and 5-year survival rates were 90%, 42%, and 30.5%, respectively.58 A study, comparing systemic therapy and RFA+ systemic therapy, reported a survival rate of 61.7% for 30 months in combination arm with median OS of 45.3 months. There was also a significant improvement in the 3-year progression-free survival rate in case of combination therapy.59 A retrospective study conducted by Knudsen et al evaluated patients with CRLM treated with RFA after systemic CT. The results showed a 5-year survival of 34% with a median survival time of 39 months.60 Gillams et al conducted a study of 69 patients with CRLM who were not eligible for resection. The patients underwent RFA with CT (either before, after, or along with RFA). The median survival time was 27 months with 1-, 3-, and 4-year survival of 90%, 34%, and 22%, respectively.61
Complete ablation was defined as no abnormal enhancement in and around lesions by enhanced CT or MRI 1 week after RFA on liver tumor. Partial ablation was defined as abnormal enhancement in and around lesions by enhanced CT or MRI 1 week after RFA on liver tumor. The previous studies reported safety and efficacy of this treatment regimen in relatively smaller tumors (average tumor size: 2.8 cm55; tumor diameter range: 2.1–3.4 cm40), whereas in our study, we have compared safety and efficacy of RFA in tumors with a diameter of ≤5 and >5 cm. Out of 109 patients in this study, there were 92 patients with a tumor diameter of ≤5 cm, 17 with that of >5 cm, which averages the diameter to 3.4+2.01 cm (maximum: 12.4 cm). The size of tumor substantially affects the efficacy and thereby, the recurrence rate of RFA.55,62 The 1-, 3-, and 5-year survival rates were 92.3%, 50.7%, and 41.6%, respectively, with mean survival at 56.5 months and median at 39.0 months. Our results were lower compared with Gillams’ results in terms of median survival (39 vs 59 months) and 3-year survival rate (50.7% vs 84%), while the results were comparable in terms of 5-year survival rate (41.6% vs 40%). Our results were better than those reported by Machi48 (5-year survival rate: 30.8%, median survival: 28 months) and Treska52 regarding long-term survival rate. Some of these studies included multi-liver metastatic lesions and some only included ≤3 cm solitary lesion, and most of the studies did not include cases with lesions >3 or even >5 cm.
Although several factors were studied, including gender, age group, tumor location, tumor size, the association between survival outcomes and systemic CT, and the association between systemic CT and molecular-targeted therapy after RFA, age was the only independent prognostic predictor for survival on multivariate analysis as subjects <50 years of age had higher risk of death than those aged ≥50 years. We suspect that the comparatively higher survival rate observed in patients aged >50 years might be due to presence of more aggressive tumors in younger subjects (aged <50 years).63–65 Similar results were observed in another study that used RFA as first- or second-line treatment after CT; age (70 years) was found to be the significant parameter, affecting survival, on multivariate analysis.36,58
Although RFA is easy to operate and is less invasive, some serious complications have been reported. The complications include bleeding, needle track seeding, biliary fistula, intestinal fistula, liver abscess, portal vein thrombosis, biliary perforation, and in some cases, pulmonary problem.28 The incidence of complication in the present study was 1.8%, which was similar to another study that reported 1.3% of adverse events.36
To the best of our knowledge, this was the first long-term study conducted in China, which evaluated the efficacy and safety of ultrasound-guided RFA along with systemic CT and molecular-targeted therapy used in the treatment of solitary CRLM. The results were satisfactory with improvement in OS. We also believe that: (i) single-operation technology can significantly reduce the blindness and number of punctures, thereby improving the accuracy and efficacy; (ii) according to the morphology and size of solitary lesion, single-needle multipoint or multi-needle multipoint, 12 minutes per point design could be used, thereby expanding ablation area and reducing the residual tissue and local recurrence. In this study, the median ablation duration was 26 minutes and the complete ablation rate was 87.2%. The median survival time of patients of complete and partial ablation were 56.0 and 19.0 months, respectively, with a statistically significant difference observed (P<0.01). Although survival rate was higher in the RFA along with systemic CT and molecular-targeted therapy compared with RFA and systemic CT, it could not reach any statistical significance due to the small sample size. More large-scale randomized studies are warranted to explore the efficacy of RFA in combination with systemic CT and RFA in combination with systemic and targeted therapies in increasing the OS benefit of solitary CRLM; and (iii) solitary large liver metastatic lesion was reported in this study. A total of 17 patients had lesions of >5 cm diameter with the largest among them being of 12.4 cm diameter. This is the first study reporting RFA targeted at large tumors.
Conclusion
RFA along with systemic CT is effective and safe in patients with solitary liver metastasis after surgery for CRC. However, randomized control trials with more frequent follow-ups are warranted in future to ascertain its efficacy in treating solitary liver metastasis after surgery for CRC.
Acknowledgments
The authors would like to acknowledge Wu Shengqi, Chief Physician, Hunan Provincial Cancer Hospital, China, who helped in the treatment protocol and has extended support in carrying out the statistical analysis for the study. All the investigators involved in the study contributed in development and approval of the manuscript. The authors acknowledge Dr Priyanka Nair and Dr Anuradha Nalli’s support (Indegene Pvt Ltd) in providing medical writing assistance in the development of this manuscript, funded by Medtronic China Co Ltd. The study was funded by Medtronic China Co Ltd.
Disclosure
The authors report no conflicts of interest in this work.
References
Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012: Global Cancer Statistics, 2012. CA Cancer J Clin. 2015;65:87–108. | ||
Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59(6):366–378. | ||
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015: Cancer Statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. | ||
Dennis DL, Waring JL, Payeur N, Cosby C, Daudt HM. Making lifestyle changes after colorectal cancer: insights for program development. Curr Oncol. 2013;20(6):493–511. | ||
Durko L, Malecka-Panas E. Lifestyle Modifications and Colorectal Cancer. Curr Colorectal Cancer Rep. 2014;10:45–54. | ||
Geoghegan JG, Scheele J. Treatment of colorectal liver metastases. Br J Surg. 1999;86(2):158–169. | ||
Mcloughlin JM, Jensen EH, Malafa M. Resection of colorectal liver metastases: current perspectives. Cancer Control. 2006;13(1):32–41. | ||
Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241(5):715–724. | ||
Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235(6):759–766. | ||
Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77(11):1241–1246. | ||
Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239(6):818–827. | ||
Feliberti EC, Wagman LD. Radiofrequency ablation of liver metastases from colorectal carcinoma. Cancer Control. 2006;13(1):48–51. | ||
Nordlinger B, Quilichini MA, Parc R, et al. Hepatic resection for colorectal liver metastases. Influence on survival of preoperative factors and surgery for recurrences in 80 patients. Ann Surg. 1987;205(3):256–263. | ||
Bozzetti F, Doci R, Bignami P, Morabito A, Gennari L. Patterns of failure following surgical resection of colorectal cancer liver metastases. Rationale for a multimodal approach. Ann Surg. 1987;205(3):264–270. | ||
de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938–2947. | ||
Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22(2):229–237. | ||
Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343(13):905–914. | ||
Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–1047. | ||
Eng C, Shalan N. Biological agents versus chemotherapy in the treatment of colorectal cancer. Expert Opin Pharmacother. 2006;7(10):1251–1271. | ||
Azvolinsky A. Colorectal cancer: to stack or sequence therapy? J Natl Cancer Inst. 2015;107(5):djv138–djv138. | ||
Folprecht G, Gruenberger T, Bechstein W, et al. Survival of patients with initially unresectable colorectal liver metastases treated with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM study). Ann Oncol. 2014;25(5):1018–1025. | ||
Khan AZ, Morris-Stiff G, Makuuchi M. Patterns of chemotherapy-induced hepatic injury and their implications for patients undergoing liver resection for colorectal liver metastases. J Hepatobiliary Pancreat Surg. 2009;16(2):137–144. | ||
Shiina S, Teratani T, Obi S, et al. Nonsurgical treatment of hepatocellular carcinoma: from percutaneous ethanol injection therapy and percutaneous microwave coagulation therapy to radiofrequency ablation. Oncology. 2002;62(Suppl 1):64–68. | ||
Okada S. Local ablation therapy for hepatocellular carcinoma. Semin Liver Dis. 1999;19(3):323–328. | ||
Bartolozzi C, Lencioni R. Ethanol injection for the treatment of hepatic tumours. Eur Radiol. 1996;6(5):682–696. | ||
Lee MJ, Mueller PR, Dawson SL, et al. Percutaneous ethanol injection for the treatment of hepatic tumors: indications, mechanism of action, technique, and efficacy. AJR Am J Roentgenol. 1995;164(1):215–220. | ||
Livraghi T, Goldberg SN, Lazzaroni S, et al. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210(3):655–661. | ||
Minami Y, Kudo M. Radiofrequency ablation of hepatocellular carcinoma: Current status. World J Radiol. 2010;2(11):417. | ||
Rhim H, Lim HK. Radiofrequency ablation of hepatocellular carcinoma: pros and cons. Gut Liver. 2010;4 Suppl 1:S113. | ||
Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol. 2009;19(5):1206–1213. | ||
Gwak JH, Oh BY, Lee RA, Chung SS, Kim KH. Clinical applications of radio-frequency ablation in liver metastasis of colorectal cancer. J Korean Soc Coloproctol. 2011;27(4):202. | ||
van Tilborg AA, Meijerink MR, Sietses C, et al. Long-term results of radiofrequency ablation for unresectable colorectal liver metastases: a potentially curative intervention. Br J Radiol. 2011;84(1002):556–565. | ||
Jakobs TF, Hoffmann RT, Trumm C, Reiser MF, Helmberger TK. Radiofrequency ablation of colorectal liver metastases: mid-term results in 68 patients. Anticancer Res. 2006;26(1B):671–680. | ||
van Duijnhoven FH, Jansen MC, Junggeburt JM, et al. Factors influencing the local failure rate of radiofrequency ablation of colorectal liver metastases. Ann Surg Oncol. 2006;13(5):651–658. | ||
Boame N, Gresham G, Jonker D, et al. Use of chemotherapy and radiofrequency ablation to treat colorectal cancer metastases: a retrospective review of The Ottawa Hospital Cancer Centre over 7 years. Curr Oncol. 2014;21(4):557. | ||
Solbiati L, Ahmed M, Cova L, et al. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265(3):958–968. | ||
Watanabe A, Yang C, Cheung WY. ECOG performance status as a predictor of adjuvant chemotherapy (AC) toxicities in stage III colorectal cancer (CRC) patients. J Clin Oncol. 2017;35(4 Suppl):789–789. | ||
Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann Surg. 2004;240(3):438–450. | ||
Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265(3):958–968. | ||
Boame N, Gresham G, Jonker D, et al. Use of chemotherapy and radiofrequency ablation to treat colorectal cancer metastases: a retrospective review of The Ottawa Hospital Cancer Centre over 7 years. Curr Oncol. 2014;21(4):557. | ||
Bilchik AJ, Wood TF, Allegra D, et al. Cryosurgical ablation and radiofrequency ablation for unresectable hepatic malignant neoplasms: a proposed algorithm. Arch Surg. 2000;135(6):657–662. | ||
Meric F, Patt YZ, Curley SA, et al. Surgery after downstaging of unresectable hepatic tumors with intra-arterial chemotherapy. Ann Surg Oncol. 2000;7(7):490–495. | ||
Mcgahan JP, Brock JM, Tesluk H, Gu WZ, Schneider P, Browning PD. Hepatic ablation with use of radio-frequency electrocautery in the animal model. J Vasc Interv Radiol. 1992;3(2):291–297. | ||
Buscarini E, Savoia A, Brambilla G, et al. Radiofrequency thermal ablation of liver tumors. Eur Radiol. 2005;15(5):884–894. | ||
Kuvshinoff BW, Ota DM. Radiofrequency ablation of liver tumors: influence of technique and tumor size. Surgery. 2002;132(4):605–612. | ||
Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221(1):159–166. | ||
Oshowo A, Gillams A, Harrison E, Lees WR, Taylor I. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg. 2003;90(10):1240–1243. | ||
Livraghi T, Solbiati L, Meloni F, Ierace T, Goldberg SN, Gazelle GS. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the “test-of-time approach”. Cancer. 2003;97(12):3027–3035. | ||
Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg. 2006;141(5):460–466. | ||
Leblanc F, Fonck M, Brunet R, Becouarn Y, Mathoulin-Pélissier S, Evrard S. Comparison of hepatic recurrences after resection or intraoperative radiofrequency ablation indicated by size and topographical characteristics of the metastases. Eur J Surg Oncol. 2008;34(2):185–190. | ||
Kim KH, Yoon YS, Yu CS, Cs Y, et al. Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J Korean Surg Soc. 2011;81(1):25. | ||
Hur H, Ko YT, Min BS, et al. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg. 2009;197(6):728–736. | ||
Zhai Z, Gu J. Influence of tumor size on the prognosis in patients with colon cancer. Zhonghua Wei Chang Wai Ke Za Zhi Chin J Gastrointest Surg. 2012;15:495–498. | ||
Chen CH, Hsieh MC, Hsiao PK, Lin EK, Lu YJ, Wu SY. A critical reappraisal for the value of tumor size as a prognostic variable in rectal adenocarcinoma. J Cancer. 2017;8(10):1927–1934. | ||
van Duijnhoven FH, Jansen MC, Junggeburt JM, et al. Factors influencing the local failure rate of radiofrequency ablation of colorectal liver metastases. Ann Surg Oncol. 2006;13(5):651–658. | ||
Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371(9617):1007–1016. | ||
Gillams AR, Lees WR. Five-year survival following radiofrequency ablation of small, solitary, hepatic colorectal metastases. J Vasc Interv Radiol. 2008;19(5):712–717. | ||
Machi J, Oishi AJ, Sumida K, et al. Long-term outcome of radiofrequency ablation for unresectable liver metastases from colorectal cancer: evaluation of prognostic factors and effectiveness in first- and second-line management. Cancer J. 2006;12(4):318–326. | ||
Ruers T, Punt C, Van Coevorden F, et al; EORTC Gastro-Intestinal Tract Cancer Group, Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO) and the National Cancer Research Institute Colorectal Clinical Study Group (NCRI CCSG). Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol. 2012;23(10):2619–2626. | ||
Knudsen AR, Kannerup AS, Mortensen FV, Nielsen DT. Radiofrequency ablation of colorectal liver metastases downstaged by chemotherapy. Acta Radiol. 2009;50(7):716–721. | ||
Gillams AR, Lees WR. Survival after percutaneous, image-guided, thermal ablation of hepatic metastases from colorectal cancer. Dis Colon Rectum. 2000;43(5):656–661. | ||
Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239(6):818–827. | ||
Tricoli JV, Blair DG, Anders CK, et al. Biologic and clinical characteristics of adolescent and young adult cancers: Acute lymphoblastic leukemia, colorectal cancer, breast cancer, melanoma, and sarcoma. Cancer. 2016;122(7):1017–1028. | ||
Bleyer A, Barr R, Hayes-Lattin B, et al; Biology and Clinical Trials Subgroups of the US National Cancer Institute Progress Review Group in Adolescent and Young Adult Oncology. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer. 2008;8(4):288–298. | ||
Hubbard JM, Grothey A. Adolescent and young adult colorectal cancer. J Natl Compr Canc Netw. 2013;11(10):1219–1225. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.