Back to Journals » Journal of Pain Research » Volume 16
Radiofrequency Ablation for Chronic Lumbar Zygapophyseal Joint Pain Using a V-Shaped Active Tip Needle: An Observational Retrospective Study
Authors Lo Bianco G , Misseri G, Stogicza AR, Cesare G, Li S , Day M, Kennedy DJ, Schatman ME
Received 1 February 2023
Accepted for publication 4 April 2023
Published 11 April 2023 Volume 2023:16 Pages 1243—1255
DOI https://doi.org/10.2147/JPR.S406714
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Dawood Sayed
Giuliano Lo Bianco,1,2 Giovanni Misseri,2 Agnes R Stogicza,3 Gregoretti Cesare,2,4 Sean Li,5 Miles Day,6 David J Kennedy,7 Michael E Schatman8,9
1Department of Biomedical and Biotechnological Sciences, University of Catania, Catania, Italy; 2Anesthesiology and Pain Department, Fondazione Istituto “G. Giglio”, Cefalù, Palermo, Italy; 3Anesthesia and Pain, Saint Magdolna Private Hospital, Budapest, Hungary; 4Department of Surgical, Oncological and Oral Science (Di.Chir.On.S.), University of Palermo, Palermo, Italy; 5National Spine and Pain Centers, Shrewsbury, NJ, USA; 6Pain Research, The Pain Center at Grace Clinic, Texas Tech University HSC, Lubbock, TX, USA; 7Department of PM&R, Vanderbilt University Medical Center, Nashville, TN, USA; 8Department of Anesthesiology, Perioperative Care, and Pain Medicine, NYU Grossman School of Medicine, New York, NY, USA; 9Department of Population Health – Division of Medical Ethics, NYU Grossman School of Medicine, New York, NY, USA
Correspondence: Giuliano Lo Bianco, Anesthesia and Pain Medicine Department, Fondazione Giglio Cefalù, Contrada Pietrapollastra, Via Pisciotto, Cefalù, Palermo, 90015, Italy, Tel +393289682219, Email [email protected]
Background: Lumbar zygapophyseal joint dysfunction represents one of the major sources of chronic low back pain. Radiofrequency ablation (RFA) using a V-shaped active tip needle may offer a larger lesion of the medial branch nerves, improving clinical outcome. The aim of our study is to evaluate the efficacy and the feasibility of RFA using V-shaped active tip needles.
Methods: This is a single-center observational retrospective study. Clinical records were screened and analyzed if they met the following inclusion criteria: adult patients (> 18 years), diagnosis of chronic lumbar zygapophyseal joint pain, failure of conservative treatments, ability to provide informed consent for data analysis and publication. Exclusion criteria: lumbar pain not related to zygapophyseal joints, previous spinal/lumbar surgery, incomplete data, absence or withdrawal of informed consent. The primary outcome of the study was a change in pain intensity at follow-up. The secondary outcomes were the evaluation of quality-of-life improvement, the occurrence of adverse events and the impact on post-procedural analgesic consumption. For these purposes, pre- and post-treatment numeric rating scale (NRS), neuropathic pain 4 questions (DN4), EuroQoL - EQ-5D-3L, EQ-VAS, EQ-index and North American Spine Society (NASS) index were retrieved and analysed.
Results: Sixty-four patients were included. 7.8% of patients at 1-month (CI95% 0.026, 0.173), 37.5% at 3-month (CI95% 0.257, 0.505), 40.6% at 6-month (CI95% 0.285, 0.536) and 35.9% at 9-month (CI95% 0.243, 0.489) follow-up reported a reduction of more than 80% in NRS Statistical analysis indicated a significant change in NRS, DN4, EQ-index and EQ-5D-VAS (p-value < 0.001) at the different time-points.
Conclusion: RFA using a V-shaped active tip needle might be a feasible and effective treatment for chronic lumbar zygapophyseal joint pain.
Keywords: arthralgia, chronic pain, zygapophyseal joint, pain management, radiofrequency ablation, neuromodulation, lumbar facet joint
Significance
The use of V-shaped active tip needles for radiofrequency ablation (RFA) procedures in the treatment of chronic lumbar zygapophyseal joint pain may have potential advantages compared to other approaches. The larger lesion size created by the V-shaped electrode might overcome the anatomical variability of the medial branch nerves, thus improving clinical outcomes. This study aims to evaluate the efficacy and the feasibility of RFA for lumbar chronic pain using this technique.
Introduction
Chronic lower back pain is a significant cause of disability, with increasing prevalence and an economic impact.1–3 Lumbar zygapophyseal joint (“z-joint”) pain represents a clinically burdensome source of chronic axial low back pain (LBP), estimated to affect between 10% and 15% of patients.4 Radiofrequency ablation (RFA) of the lumbar medial branch nerves is a common treatment modality for patients with z-joint mediated pain.5–8
RFA aims to prevent the conduction of nociceptive impulses through the use of an electric current that damages the medial branch nerves, which are the z-joints’ pain-conducting nerves. The effectiveness of radiofrequency denervation performed with rigorous standards and appropriate selection criteria has been demonstrated.9,10 However, medial branch nerves exhibit a wide range of anatomical variability, along with their small size and the inconsistency of their number.11 Therefore, the success of RFA is contingent upon creating a large enough lesion that overlaps the sensory nerve supplying the affected z-joint.2 Given this variability, a larger lesion should increase the possibility of capturing the target nerve. Additionally, this would potentially obviate the need to conduct numerous lesions, therein reducing procedure times.12–15
Monopolar RFA is the most commonly used technology, employing a single electrode probe inserted under fluoroscopic guidance adjacent to the target nerve. The use of a V-shaped needle, in which the electrode forks off from the active tip, has been demonstrated to result in increased lesion size,16 thus having the potential to compensate for the anatomical variability of the medial branches.
The aim of our study is to evaluate the effectiveness and feasibility of RFA procedures using a V-shaped active tip needle for chronic lumbar z-joint pain.
Methods
This is a single-center observational retrospective study of patients undergoing percutaneous RFA using a V-shaped active tip needle for chronic lumbar z-joint pain, conducted from September 2020 to January 2022. The study has been conducted at Fondazione Istituto “G. Giglio” – Cefalù, Palermo, after Local Ethics Committee (number and date of approval: 04/2022 – 13/04/2022) and Hospital Scientific Committee approval, in compliance with the Declaration of Helsinki.
Inclusion criteria were: Adult patients (>18 years), diagnosis of chronic lumbar zygapophyseal joint pain, failure of conservative treatments, and ability to provide informed consent for data analysis and publication. Exclusion criteria were: Lumbar pain not related to zygapophyseal joints, previous spinal/lumbar surgery, incomplete data, and absence or withdrawal of informed consent for data analysis and publication. One hundred and twenty (120) consecutive adult patients treated with this method were included. Data from 56 patients were omitted as they were incomplete (n = 50), due to follow-ups not reaching the minimal number of observations required for analysis, or because of consent withdrawal (n = 6).
For the remaining 64 patients, all medical records were independently screened by three investigators (G.L.B., G.M., C.G.). Patients’ characteristics including gender, age, diagnosis, timeline, and clinical presentation of chronic LBP were extracted from the records (Table 1).
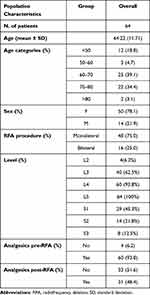 |
Table 1 Demographic and Baseline Characteristics of Patient’s Population |
We evaluated the pain level and their impacts on patients’ daily life assessing the NRS (scale 0–10),17 the DN4,18 the EuroQoL - EQ-5D-3L, EQ-VAS and the EQ-index (Figure 1).19 The EQ-5D-3L has been developed with the purpose to describe and value health. This is an instrument that includes a descriptive system questionnaire and a visual analogue scale. The EQ-VAS is a 0–100 scale on which respondents are asked to indicate their overall health on the day of questionnaire completion. However, in our study, we decided to rescale it from 0 to 10, with the aim of simplifying the phone-call questionnaire administration. The individual health status can also be expressed as a summary index value (EQ-index), obtained when the descriptive system profile is linked to a value set, which is a collection of index values for all possible EQ-5D health states. For the purpose of our study, UK value sets were used.
 |
Figure 1 Timeline of follow-up time points and variables analyzed. |
Pre-treatment (baseline) and post-treatment (follow-up) NRS, DN4, EQ-5D-3L, EQ-index and EQ-VAS were recorded and analyzed, as well as pre-treatment use of analgesic drugs (type and dose of analgesic in order to obtain a NRS <3). Follow-up was performed at 1-, 3-, 6-, and 9-months post-procedure (named, respectively, 1M, 3M, 6M and 9M). Type (unilateral or bilateral) and sites (lumbar and sacral levels) of RFA procedure were retrieved for all included patients. We screened clinical records for early and late-onset adverse events. While follow-up at 1 month was performed by the physician who carried out the procedure, re-evaluation at the different time points was conducted via a telephone call, to avoid unnecessary hospital access.20–23
The questionnaire was administered by an independent investigator, in order to avoid a potential bias, which might have influenced the evaluation of clinical results over time. At the last follow-up, patients were also asked to complete a NASS4-point patient satisfaction questionnaire.24 A score of 1 or 2 on the NASS patient satisfaction index was considered a successful response to the procedure. Patients were also asked whether use of post-procedural analgesics was resumed.
We adhered to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement.25
Technical Procedures
Screening Medial Branch Blocks
Needle placements for the screening medial branch blocks were conducted in accordance with the Spinal Intervention Society (SIS) Guidelines.13 Patients were positioned prone with C-arm fluoroscopy with an anteroposterior view of the appropriate level of the spine. After local anesthetic was administered at entry points, 22-gauge spinal needles were placed at the appropriate site. Lumbar z-joint pain was investigated through diagnostic blocks using 1% lidocaine. Patients with lidocaine-positive results were further studied using 0.25% bupivacaine on a separate occasion (3 to 4 weeks after the first injection). Following each block, the patient was examined and asked to perform previously painful movements. A positive response was defined as at least a 50% reduction of pain within 30 to 90 minutes post-procedure, lasting at least 2 hours when lidocaine was used and at least 3 hours in cases in which bupivacaine was used. Patients with a double-positive response to diagnostic blocks were scheduled for an RFA procedure.
Radiofrequency Ablation
The RFA procedures were performed in operating rooms under continuous monitoring of patients’ vital parameters.26
All patients were administered procedural sedation with short-acting intravenous agents (benzodiazepines, eg, midazolam), supplementing local anesthesia of the skin and underlying tissues. An 18-gauge 100 mm needle with a V-shaped active tip (Venom cannula, Stryker®) was introduced at each entry point, with RFA electrodes placed according to SIS guidelines for lumbar medial branch, L5 dorsal ramus, S1, S2, S3 lateral branches thermal radiofrequency neurotomy.13 Position of the cannula was checked on anteroposterior (AP) (Figure 2) and lateral views (Figure 3). The depth was adjusted until the cannula tip touched the bone (between the transverse vertebral process and the superior articular process) at the level of the target medial branch (Figure 4). The V-shaped active tip was placed parallel to the medial branch that was being treated. To improve the accuracy of needle positioning prior to lesioning, sensory and motor stimulation were performed (lower threshold range for detecting sensory stimulation at 50 Hz between 0.3 and 0.7 V; upper threshold range for ruling out motor stimulation at 2 Hz between 0.9 and 3 V). The presence of paresthesia at the site of patients’ usual pain was considered a positive sensory test, whereas the absence of contraction of major muscle groups was considered to be an acceptable motor test.27,28
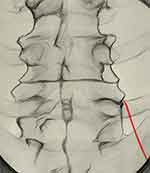 |
Figure 2 Antero-posterior view showing the cannula (red) crossing the ala of the sacrum and lying against the superior articular process of L5. |
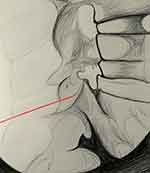 |
Figure 3 Lateral view showing the cannula (red) placed across the middle two-quarters of the neck of the superior articular process. |
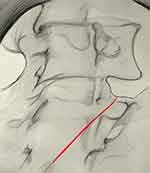 |
Figure 4 Oblique view showing the cannula (red) passing across the sulcus for the medial branch. |
Following the above-described test, 1 mL of lidocaine 1% was injected through the needle prior to the RFA procedure. RFA was conducted at 85°C for 90 seconds for each level. Prior to cannula removal, dexamethasone 2 mg and 1 mL ropivacaine 0.25% were injected for each treated level.
Statistical Analysis
Data are expressed as mean ± standard deviation (SD) or as median and interquartile range (IQR), where appropriate. Categorical variables are expressed as proportions (frequencies and percentages). Normality of data distribution was verified through the Shapiro–Wilk normality test. Considering the non-normal distribution of the variables, comparisons over different time-points were performed using the Friedman test with post-hoc analysis using the pairwise Wilcoxon signed-rank test. P-values were adjusted according to the Bonferroni multiple testing correction method. Fisher’s exact test was used to determine whether there was a significant association between the type of RFA procedure applied (unilateral or bilateral) and the use of post-procedural analgesics. P-values <0.05 were considered to be statistically significant. The statistical analysis was performed using R software.29
Results
Of the 64 patients (50 women and 14 men, mean age: 64.22 years ± 11.71) included in the study, patients between ages 60–70 years were the most represented group (25; 39.1%). Monolateral RFA procedures were performed on 48 patients (75%). Analgesics consumption was present in 60 patients (93.8%; CI95% 0.848, 0.983) prior to the RFA procedure in 31 patients (48.4%; CI95% 0.358, 0.613) at the final follow-up, while 33 patients (51.6%; CI95% 0.387, 0.642) confirmed that they resumed their use at 9 months following the procedure. Table 1 illustrates the baseline characteristics of the studied population.
The median baseline NRS was 8.00 (IQR 6.00, 8.00). Follow-ups post-RFA procedures demonstrated a reduction of pain score, with a NRS decreasing from 5.50 (IQR 3.75, 7.00) at 1M to 2.00 (IQR 1.00, 4.00) at 9M follow-up (Table 2). Considering the percentage of pain reduction at the different post-procedural time-points (Table 3), relief of greater than 80% in NRS was experienced by 7.8% of patients at 1M (CI95% 0.026, 0.173), 37.5% at 3M (CI95% 0.257, 0.505), 40.6% at 6M (CI95% 0.285, 0.536) and 35.9% at 9M follow-up (CI95% 0.243, 0.489). At 6M follow-up, 78.1% (CI95% 0.66, 0.875) of patients reported a reduction of at least 40% to 60% of symptoms. Only 1 patient experienced exacerbation of symptoms at 1-month post-RFA procedure, while no exacerbations were subsequently observed at the rest of the time-points.
 |
Table 2 Pain Scores (NRS) and Quality-of-Life Assessment (EQ-Index) of Patients at Baseline and at Different Times of Follow-Up, with Friedman Test and Pairwise Comparisons Results |
According to the NASS index, 47 subjects (73.4%) reported a successful outcome (NASS score of 1 or 2) and 17 (26.6%) reported an unsuccessful outcome (NASS score of 3 or 4). When NRS modifications over time are stratified as either success or failure, patients with a NASS score of 1 or 2 demonstrated a −2 point (IQR −4.50, 0.00) reduction in their NRS pain scores at 1M, −6 (IQR −7.00, −4.50), at 3M, −6 (IQR −7.00, −5.00) at 6M and −6 (IQR −7.00, −4.00) and at 9M (Table 4).
When considering repeated measures over the different time-points, the analysis indicated a statistically significant change in NRS (Friedman chi-squared = 175.13, df = 4, p-value <0.001), DN4 (Friedman chi-squared = 117.9, df = 4, p-value <0.01), EQ-index (Friedman chi-squared = 132.8, df = 4, p-value <0.001), and EQ-5D-VAS (Friedman chi-squared = 129.71, df = 4, p-value <0.001) at the different time-points (Table 2).
For the purpose of the secondary outcome, subjects were stratified into 2 groups according to pain reduction at the different time-points (greater vs less than 50% when compared to baseline evaluation) and quality of life assessed using EQ-5D-3L (Table 5). Patients with greater than 50% pain reduction at 6M and 9M referred a fair (46% at 6M; 42.2% at 9M) to optimal (54% at 6M; 57.8% at 9M) quality of life when compared to pre-procedural assessment. When considering patients with less than 50% pain reduction, only 14.3% and 10.5% (at 6M and 9M, respectively) reported a worsened post-procedural quality of life.
There was no significant statistical association between the type of RFA procedure applied (monolateral or bilateral) and the use of analgesics after the RFA procedure (p-value= 0.7758) or between the post-procedural use of analgesics and NASS index assessment (p-value= 0.779). No adverse events were observed or reported by patients during follow-up.
Discussion
There are few conditions in interventional pain medicine as controversial as lumbar z-joint pain treatment. Despite facet joint interventions representing the second most common pain management procedures in the USA, the safety and efficacy of RFA for the treatment of chronic LBP has yet to be well substantiated.30,31
Patients who were included in our study demonstrated a reduction in pain scores, with a peak reduction of pain occurring at 6 months following the procedure. Up to 78.1% (CI95% 0.66, 0.875) of the patients experienced a reduction of NRS of at least of 40–60% at 6 months post-RFA for z-joint chronic lumbar pain. Despite the re-emergence of pain within 6–9 months of the procedure, the symptoms reported were significantly reduced when compared to baseline assessment (−6 points in NRS after 6 and 9 months within the success group; 46 patients with NRS ≤ 3 at 9-month). In addition, when considering the EQ-index scores between the different time points analyzed, the impact on the re-emergence of pain on daily life seems to have been contained. Our results are in line with extant published data. It is generally accepted that relief following radiofrequency denervation typically lasts for between 6 and 12 months,6 although it has been reported to provide relief for greater than 2 years in some cases. Repeating RFA up to 3 times has demonstrated to possibly relieve z-joint pain and maintain results over time.32 However, RFA treatment success remains inconstant, and a small proportion of patients do not experience any pain relief or only a very time-limited benefit from the procedure. When analyzing our study cohort, patients undergoing an unsuccessful procedure (26.6%) reported a limited median reduction of NRS as compared to subjects undergoing a successful RFA procedure (73.4%) using the same technique, with a significant difference between these 2 groups at 9-month follow-up (Table 2 and Table 3). The rate of nerve regeneration and subsequent return of previous LBP symptoms are thought to be related to the failure of procedure technique (failure of direct nerve coagulation or minimal ablation of a limited section of the target nerve) and whether correct anatomical placement of the RFA electrode is achieved. As reported by Bogduk,33 nerve regeneration following coagulation requires a longer period of time than the 1 mm per month observed in nerve transection injuries. For this reason, post-RFA pain relief might last longer when a larger area of the nerve is contacted, and a greater length of the nerve segment is coagulated.34 Different techniques have been described with the purpose of increasing the likelihood of success rates and durations of pain relief following RFA for chronic z-joint related LBP.35 The two-needle RFA technique was developed to heat a wider volume of tissue and minimize technical failure due to incomplete coagulation. This approach uses a dual needle placement of two 10 mm active tip RFA cannulas separated by 6 mm.36 Some of the major limiting factors of the two-needle RFA technique are improper needle placement, difficulties in their correct positioning and the time for the operator to accurately place the active tips. As a result, the costs and time constraints and the limited availability of long-term clinical results of this procedure have hampered its widespread adoption. As demonstrated by Cedeno et al,16 the use of V-shaped active tip needles provides an additional 0.6×0.6 mm lesion size when compared with standard monopolar RFA needles, using equivalent settings. Thus, V-shaped active tip RFA may provide a wider lesion using a single needle, with additional cost and time savings. It is important to recognize that a technical limitation is that achieving the maximum lesion generated at the target location requires that the V-shaped electrode be placed in the correct plane parallel to the nerve (Figures 2–4). Endoscopic techniques might overcome this limitation, allowing for more accurate denervation because of the direct visualization of the facet joint and the dorsal medial ramus to treat.37
As is the case with any procedure involving damage to the peripheral nervous system,38 RFA poses a risk of post-procedural neuropathic pain. Corticosteroids might have a beneficial impact, although their concomitant administration during RFA procedures at the level-sites treated remains controversial. To date, only a randomized controlled pilot study36 has identified a potential protective effect of dexamethasone injections against post-procedural neuropathic pain emergence. All patients included in our retrospective study underwent local injections of dexamethasone for each site treated. Considering DN4 assessment at 6 (1; 0.00–3.00) and 9 months (2; 0.00, 3.00) after the procedure, one may speculate an influence of dexamethasone administration in avoiding post-procedural neuropathic pain development. However, these results are of limited value and require further studies in order to determine whether corticosteroids might be helpful in containing post-RFA adverse events. There are several limitations to our study, most of which are inherent in any non-controlled retrospective observational study with a small sample size. Although demonstrating that RFA using V-shaped active tip needles is effective, a larger prospective RCT comparing the conventional monopolar technique to V-shaped active tip needle RFA is necessary in order to clarify the efficacy and safety of this novel technique. Second, the accuracy of a diagnostic block is contingent on several technical, anatomical, and psychological factors. As a result, there exists the possibility of false-positive responses, which undermine RFA treatment results over time. Furthermore, a bias might be introduced by telephone-questionnaire administration during follow-up; even if interviews and data analysis were performed by an independent investigator, patients may be reluctant to report poor outcomes due to their desires to please medical professionals. Third, we did not consider potential co-intervention (eg, physical therapy, spinal manipulation, educational or psychological therapies) which might have interfered with the results of the analyzed treatment. In addition, some of the improvements seen may be attributable to spontaneous relief and/or placebo effect.
Conclusion
Radiofrequency ablation for chronic lumbar z-joint pain using a V-shaped active tip needle is a feasible and effective technique. However, the relative efficacy of this technique compared to conventional RFA following rigorous criteria remains unclear. Accordingly, the results of our study should be considered as preliminary, and we believe that future randomized controlled trials building on our reported results will further clarify the overall benefits of this novel approach.
Acknowledgement
We would like to thank the artist Andrea Lino for the drawings.
Author Contributions
Giuliano Lo Bianco: conceptualization, study design, execution, acquisition of data, writing (original draft); Giovanni Misseri: conceptualization, methodology, study design, data analysis and interpretation, writing (original draft); Agnes R Stogicza: methodology, data interpretation, writing (substantial and critical revision of the manuscript); Cesare Gregoretti: study design, writing (critical revision of the manuscript); Sean Li: methodology, writing (critical revision of the manuscript); Miles Day: data analysis and interpretation, writing (critical revision of the manuscript); David J Kennedy: study design, methodology, writing (substantial and critical revision of the manuscript); Michael E Schatman: methodology, data analysis and interpretation, writing (critical revision of the manuscript). All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
Dr Giuliano Lo Bianco reports consulting for Stryker, outside the submitted work. Dr Gregoretti Cesare reports personal fees from Philips, during the conduct of the study. Dr Sean Li reports grants, consultant, research grant from Abbott, Avanos, Averitas, Biotronik, Boston Scientific, Nevro, PainTeq, SPR Therapeutics; consultant, research grant, minority options from Nalu; consultant for Vertos and Pria Health; speaker bureau from Silex Pharma; grants from Ethos Laboratory, during the conduct of the study. Dr Michael E Schatman reports Research consultant for Modoscript and on Scientific Steering Committee for Collegium Pharmaceutical, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Manchikanti L, Singh V, Datta S, et al. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician. 2009;12:E35–E70. doi:10.36076/ppj.2009/12/E35
2. Linton SJ, Hellsing AL, Halldén K. A population-based study of spinal pain among 35–45-year-old individuals: prevalence, sick leave, and health care use. Spine. 1998;23:1457–1463. doi:10.1097/00007632-199807010-00006
3. Leggett LE, Soril LJJ, Lorenzetti DL, et al. Radiofrequency ablation for chronic low back pain: a systematic review of randomized controlled trials. Pain Res Manag. 2014;19(5):e146–e153. doi:10.1155/2014/834369
4. Cohen SP, Raja SN. Pathogenesis, diagnosis and treatment of lumbar zygapophysial (facet) joint pain. Anesthesiology. 2007;106:591–614. doi:10.1097/00000542-200703000-00024
5. Boswell MV, Colson JD, Sehgal N, Dunbar EE, Epter R. A systematic review of therapeutic facet joint interventions in chronic spinal pain. Pain Physician. 2007;10(1):229–253. doi:10.36076/ppj.2007/10/229
6. Cohen SP, Huang JH, Brummett C. Facet joint pain – advances in patient selection and treatment. Nat Rev Rheumatol. 2013;9(2):101–116. doi:10.1038/nrrheum.2012.198
7. Walter SG, Schildberg FA, Rommelspacher Y. Endoscopic sacrolumbar facet joint denervation in osteoarthritic and degenerated zygapophyseal joints. Arthrosc Tech. 2018;7(12):e1275–e1279. doi:10.1016/j.eats.2018.08.014
8. van Zundert J, Hartrick C, Lataster A, Huygen F, Mekhail N, van Kleef MPJ. Evidence-Based Interventional Pain Practice: According to Clinical Diagnoses. Oxford, UK: Wiley-Blackwell; 2011.
9. MacVicar J, Borowczyk JM, MacVicar AM, Loughnan BM, Bogduk N. Lumbar medial branch radiofrequency neurotomy in New Zealand. Pain Med. 2013;14(5):639–645. doi:10.1111/pme.12000
10. Schneider BJ, Doan L, Maes MK, Martinez KR, Gonzalez Cota A, Bogduk N; Standards Division of the Spine Intervention Society. Systematic review of the effectiveness of lumbar medial branch thermal radiofrequency neurotomy, stratified for diagnostic methods and procedural technique. Pain Med. 2020;21(6):1122–1141. doi:10.1093/pm/pnz349
11. Bogduk N, Macintosh J, Marsland A. Technical limitations to the efficacy of radiofrequency neurotomy for spinal pain. Neurosurgery. 1987;20(4):529–535. doi:10.1227/00006123-198704000-00004
12. Cosman ER, Dolensky JR, Hoffman RA. Factors that affect radiofrequency heat lesion size. Pain Med. 2014;15:2020–2036. doi:10.1111/pme.12566
13. Bogduk N, ed. Practice Guidelines for Spinal Diagnostic and Treatment Procedures. San Francisco, CA: International Spine Intervention Society; 2004.
14. Lord SM, McDonald GJ, Bogduk N. Review article: percutaneous radiofrequency neurotomy of the cervical medial branches – a validated treatment for cervical zygapophysial joint pain. Neurosurg Q. 1998;8:288–308. doi:10.1097/00013414-199812000-00004
15. Cohen SP, Bhaskar A, Bhatia A, et al. Consensus practice guidelines on interventions for lumbar facet joint pain from a multispecialty, international working group. Reg Anesth Pain Med. 2020. doi:10.1136/rapm-2019-101243
16. Cedeño DL, Vallejo A, Kelley CA, Tilley DM, Kumar N. Comparisons of lesion volumes and shapes produced by a radiofrequency system with a cooled, a protruding, or a monopolar probe. Pain Physician. 2017;6:E915–E922. doi:10.36076/ppj.20.5.E915
17. Shafshak TS, Elnemr R. The visual analogue scale versus numerical rating scale in measuring pain severity and predicting disability in low back pain. J Clin Rheumatol. 2021;27(7):282–285. doi:10.1097/RHU.0000000000001320
18. Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114:29–36. doi:10.1016/j.pain.2004.12.010
19. EuroQol Research Foundation. EQ-5D-Y user guide; 2020. Available from: https://euroqol.org/publications/user-guides.
20. Lo Bianco G, Papa A, Schatman ME, et al. Practical advices for treating chronic pain in the time of COVID-19: a narrative review focusing on interventional techniques. J Clin Med. 2021;10(11):2303. doi:10.3390/jcm10112303
21. Lo Bianco G, Di Pietro S, Mazzuca E, et al. Multidisciplinary approach to the diagnosis and in-hospital management of COVID-19 infection: a narrative review. Front Pharmacol. 2020;11:572168. doi:10.3389/fphar.2020.572168
22. Cappelleri G, Fanelli A, Ghisi D, et al. The role of regional anesthesia during the SARS-CoV2 pandemic: appraisal of clinical, pharmacological and organizational aspects. Front Pharmacol. 2021;12:574091. doi:10.3389/fphar.2021.574091
23. Lopes N, Vernuccio F, Costantino C, et al. An Italian guidance model for the management of suspected or confirmed COVID-19 patients in the primary care setting. Front Public Health. 2020;8:572042. doi:10.3389/fpubh.2020.572042
24. Daltroy LH, Cats-Baril WL, Katz JN, Fossel AH, Liang MH. The North American spine society lumbar spine outcome assessment instrument: reliability and validity tests. Spine. 1996;21(6):741–749. doi:10.1097/00007632-199603150-00017
25. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi:10.1016/S0140-6736(07)61602-X
26. Lo Bianco G, Tinnirello A, Papa A, et al. Interventional pain procedures: a narrative review focusing on safety and complications. PART 2 interventional procedures for back pain. J Pain Res. 2023;16:761–772. doi:10.2147/JPR.S396215
27. Leoni MLG, Schatman ME, Demartini L, Lo Bianco G, Terranova G. Genicular nerve pulsed dose radiofrequency (PDRF) compared to intra-articular and genicular nerve PDRF in knee osteoarthritis pain: a propensity score-matched analysis. J Pain Res. 2020;13:1315–1321. doi:10.2147/JPR.S240138
28. Papa A, Di Dato MT, Lo Bianco G, et al. Intraarticular STP radiofrequency for painful osteoarthritis in the knee: a retrospective single center analysis. J Pain Res. 2021;14:2441–2447. doi:10.2147/JPR.S317569
29. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. Available from: https://www.R-project.org/.
30. Manchikanti L. The growth of interventional pain management in the new millennium: a critical analysis of utilization in the medicare population. Pain Physician. 2004;7(4):465–482. doi:10.36076/ppj.2004/7/465
31. Vigneri S, Sindaco G, La Grua M, et al. Electrocatheter-mediated high-voltage pulsed radiofrequency of the dorsal root ganglion in the treatment of chronic lumbosacral neuropathic pain: a randomized controlled study. Clin J Pain. 2020;36(1):25–33. doi:10.1097/AJP.0000000000000766
32. Schofferman J, Kine G. Effectiveness of repeated radiofrequency neurotomy for lumbar facet pain. Spine. 2004;29(21):2471–2473. doi:10.1097/01.brs.0000143170.47345.44
33. Bogduk N. Evidence-informed management of chronic low back pain with facet injections and radiofrequency neurotomy. Spine J. 2008;8(1):56–64. doi:10.1016/j.spinee.2007.10.010
34. Lau P, Mercer S, Govind J, Bogduk N. The surgical anatomy of the lumbar medial branch neurotomy. Pain Med. 2004;5:289–298. doi:10.1111/j.1526-4637.2004.04042.x
35. Cosman ER, Gonzalez CD. Bipolar radiofrequency lesion geometry: implications for palisade treatment of sacroiliac joint pain. Pain Pract. 2011;11(1):3–22. doi:10.1111/j.1533-2500.2010.00400.x
36. Shustorovich A, AlFarra T, Arel AT, Singh JR, Roemmich RT, Chhatre A. Dexamethasone effectively reduces the incidence of post-neurotomy neuropathic pain: a randomized controlled pilot study. Pain Physician. 2021;24(8):517–524.
37. Walter SG, Struwe C, Scheidt S, et al. Endoscopic facet joint denervation for treatment of chronic lower back pain. Clin Neurol Neurosurg. 2020;195:105904. doi:10.1016/j.clineuro.2020.105904
38. Lo Bianco G, Papa A, Gazzerro G, et al. Dorsal root ganglion stimulation for chronic postoperative pain following thoracic surgery: a pilot study. Neuromodulation. 2021;24(4):774–778. doi:10.1111/ner.13265
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



