Back to Journals » Clinical Interventions in Aging » Volume 12
R2(GFR)CHADS2 and R2(GFR)CHA2DS2VASc schemes improved the performance of CHADS2 and CHA2DS2VASc scores in death risk stratification of Chinese older patients with atrial fibrillation
Authors Fu S, Zhou S, Luo L, Ye P
Received 30 March 2017
Accepted for publication 4 May 2017
Published 8 August 2017 Volume 2017:12 Pages 1233—1238
DOI https://doi.org/10.2147/CIA.S138405
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Shihui Fu,1,2,* Shanjing Zhou,3,* Leiming Luo,1 Ping Ye1
1Department of Geriatric Cardiology, 2Department of Cardiology and Hainan Branch, 3Department of Traditional Chinese Medicine and Hainan Branch, Chinese People’s Liberation Army General Hospital, Beijing, People’s Republic of China
*These authors contributed equally to this work
Background: This analysis was carried out to refine the CHADS2 and CHA2DS2VASc scores by combining creatinine clearance (CrCl) and glomerular filtration rate (GFR) and evaluate the performance of CrCl-based and GFR-based schemes in death risk stratification of Chinese older patients with atrial fibrillation (AF).
Methods: There were 219 older patients with AF, and all-cause mortality was assessed during the follow-up of 1.11 years. Renal function was evaluated using the CrCl formula and different GFR (Modification of Diet in Renal Disease [MDRD], Chinese MDRD [CMDRD], Mayo Clinic Quadratic [Mayo] and Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI]) formulas, and five kinds of R2CHADS2 and R2CHA2DS2VASc schemes were generated by combining CrCl and GFR with CHADS2 and CHA2DS2VASc scores.
Results: In Cox regression multivariate analysis, CrCl <60 mL/min was moderately associated with death risk (P=0.122 and P=0.144). When MDRD, CMDRD, CKD-EPI and Mayo formulas were used to ascertain the GFR, GFR <60 mL/min/1.73 m2 was significantly associated with death risk (P<0.001 for all). In the models with CHADS2 and CHA2DS2VASc scores as the linear covariates, CrCl and GFR as the continuous variables were significantly associated with death risk (P<0.05 for all). C-statistics of CrCl-based schemes – R2(CrCl)CHADS2 and R2(CrCl)CHA2DS2VASc – moderately exceeded that of CHADS2 and CHA2DS2VASc scores (P=0.081 and 0.082). C-statistics of GFR-based schemes – R2(GFR)CHADS2 and R2(GFR)CHA2DS2VASc – significantly exceeded that of CHADS2 and CHA2DS2VASc scores (P<0.05 for all).
Conclusion: Chinese older patients with AF with lower levels of GFR and GFR <60 mL/min/1.73 m2 had a significantly high death risk, and those with lower levels of CrCl or CrCl <60 mL/min had a significantly or modestly high death risk. There was significantly better performance of GFR-based schemes and moderately better performance of CrCl-based schemes in death risk stratification compared with CHADS2 and CHA2DS2VASc scores.
Keywords: atrial fibrillation, CHADS2, CHA2DS2VASc, older patients, creatinine clearance, glomerular filtration rate
Background
As the most common arrhythmia, atrial fibrillation (AF) has a clear increase in prevalence with an increasing age and is obviously associated with death risk.1–3 A key step in reducing the death risk for patients with AF is an effective stratification of death risk. Various clinical features have been identified to stratify the death risk. However, there is no mature and practical scheme developed for stratifying the death risk in patients with AF, and some researchers like Nakagawa et al have successfully applied the CHADS2 score, commonly used for assessing the thromboembolic events, to effective stratification of death risk in patients with AF.4 Although this transition has an important value for clinical practice, the currently available schemes, such as CHADS2 and CHA2DS2VASc scores, are largely derived from dated populations and have a limited discriminatory ability.5,6 Renal function has emerged as a risk factor for mortality in patients with AF, and thus, Piccini et al has made attempts to combine creatinine clearance (CrCl) with the CHADS2 score. However, as a standard index of renal function, glomerular filtration rate (GFR) has not been systematically analyzed in the clinical studies, and nobody knows if it is appropriate or even better to combine GFR with these schemes, including CHADS2 and CHA2DS2VASc scores.7 Moreover, ongoing validations of these schemes in other populations with AF from different ethnic and age groups will confirm their true value. The objectives of this analysis were to refine the CHADS2 and CHA2DS2VASc scores by combining not only CrCl but also GFR and evaluate the performance of new CrCl-based and GFR-based schemes in death risk stratification of Chinese older patients with AF.
Methods
Study participants
The current analysis was made up of 219 patients older than 60 years with AF. All of them had the medical history, clinical symptoms and electrocardiograph records showing AF. The Chinese People’s Liberation Army General Hospital was their designated hospital and had their integrated long-term medical and final death records, which made it easier for us to follow up these patients effectively and judge the end point accurately. The study protocol was approved by ethics committee of the Chinese People’s Liberation Army General Hospital (Beijing, China). Each participant provided written informed consent to be included in the study.
Risk stratification schemes
CHADS2 score awards 1 point each for the presence of congestive heart failure, hypertension, age ≥75 years and diabetes mellitus and 2 points for prior stroke or transient ischemic attack (TIA). CHA2DS2VASc score awards 1 point each for the presence of congestive heart failure, hypertension, vascular diseases, diabetes mellitus and female sex; 2 points for prior stroke or TIA and 0, 1 or 2 points depending on age. For each patient, the current analysis obtained the additional risk schemes by combining the CHADS2 and CHA2DS2VASc scores with an additional 2 points for CrCl <60 mL/min and GFR <60 mL/min/1.73 m2 and designated them as R2CHADS2 and R2CHA2DS2VASc schemes.
Risk factor definition
Patients with mean systolic blood pressure ≥140 mmHg, mean diastolic blood pressure ≥90 mmHg or medications for the treatment of hypertension were defined as having hypertension. Mean systolic and diastolic blood pressures were taken as the average of five separate measurements. Patients with fasting glucose concentration ≥7.0 mmol/L or treatment with oral hypoglycemic agents/insulins were defined as having diabetes mellitus. Standard echocardiogram was performed, and left ventricular ejection fraction was measured by the Simpson’s method.8 Stroke was defined as the new, sudden focal neurological deficit resulting from a presumed cerebrovascular cause that persisted >24 hours rather than other identifiable causes such as tumor or seizure. Events that involved the symptoms that lasted <24 hours were considered as TIA. Myocardial infarction and peripheral artery disease were combined as a single variable termed vascular diseases. Serum creatinine concentration was measured using an enzymatic method, and the calibration formula of Jaffe’s kinetic method was as follows:9
|
The enzymatic method of serum creatinine was used in the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula, and the Jaffe’s kinetic method of serum creatinine was used in the other four formulas. CrCl and GFR of all participants were evaluated with different formulas as follows:
- Cockcroft–Gault formula:10
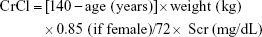
- Modification of Diet in Renal Disease (MDRD) formula:11
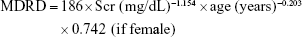
- Chinese MDRD (CMDRD) formula:12
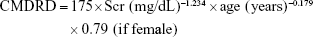
- Mayo Clinic Quadratic (Mayo) formula:13
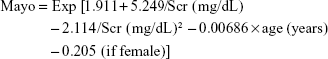
- CKD-EPI formula:14
If female and if Scr ≤0.7 mg/dL:
If female and if Scr >0.7 mg/dL: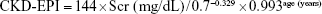
If male and if Scr ≤0.9 mg/dL: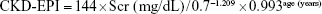
If male and if Scr >0.9 mg/dL: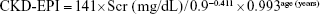
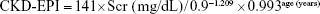
End point ascertainment
Given the priority of all-cause mortality in the outcome studies, as well as the high prevalence of multiple organ failure in the elderly, the primary end point in the current analysis was all-cause mortality. The current analysis performed the follow-up to assess the all-cause mortality during the mean time of 1.11 years (406 days; median: 313 days; interquartile range: 199–532 days) and had the survival status for all these patients. Death was determined from death records, a legal document including time, site and other information.
Statistical analysis
Baseline characteristics were summarized as frequencies for categorical variables and median values with interquartile range for continuous variables. Cox regression model was used to explore whether renal function is a significant factor associated with death risk after adjusting for CHADS2 and CHA2DS2VASc scores or their components during the follow-up. C-statistic was calculated to assess the discriminatory ability of these schemes for primary outcome. Two-sided P-value <0.05 was considered significant. Statistics analysis was performed using Statistical Package for the Social Sciences version 17 (SPSS Inc., Chicago, IL, USA) and MedCalc 11.6 for Windows (MedCalc Software bvba, Mariakerke, Belgium).
Results
For all patients, median age was 86 years, median CHADS2 score was 3.0 and median CHA2DS2VASc score was 4.0; 14.6% of patients were female. Over the median follow-up of 1.11 years, 24.2% of patients died. Baseline characteristics of all patients according to death occurrence are shown in Table 1.
In Cox regression univariate analysis (Table 2), not only lower levels of CrCl, MDRD-GFR, CMDRD-GFR, EPI-GFR and Mayo-GFR but also CrCl <60 mL/min, MDRD-GFR <60 mL/min/1.73 m2, CMDRD-GFR <60 mL/min/1.73 m2, EPI-GFR <60 mL/min/1.73 m2 and Mayo-GFR <60 mL/min/1.73 m2 were significantly associated with death risk (P<0.05 for all). After accounting for all the factors that constituted the CHADS2 and CHA2DS2VASc scores in the Cox regression multivariate analysis, CrCl <60 mL/min was modestly associated with death risk (P=0.122 and P=0.144). When MDRD, CMDRD, CKD-EPI and Mayo formulas were used to ascertain the GFR, GFR <60 mL/min/1.73 m2 was significantly associated with death risk after adjusting for the components of CHADS2 and CHA2DS2VASc scores (P<0.001 for all). In the models developed with CHADS2 and CHA2DS2VASc scores used as the linear covariates, CrCl, MDRD-GFR, CMDRD-GFR, EPI-GFR and Mayo-GFR as the continuous variables were significantly associated with death risk (P<0.05 for all).
As provided in Table 3, C-statistics of R2(CrCl)CHADS2 and R2(CrCl)CHA2DS2VASc schemes moderately exceeded that of CHADS2 and CHA2DS2VASc scores (P=0.081 and P=0.082). C-statistics of R2(MDRD-GFR)CHADS2, R2(CMDRD-GFR)CHADS2, R2(EPI-GFR)CHADS2 and R2(Mayo-GFR)CHADS2 and R2(MDRD-GFR)CHA2DS2VASc, R2(CMDRD-GFR)CHA2DS2VASc, R2(EPI-GFR)CHA2DS2VASc and R2(Mayo-GFR)CHA2DS2VASc schemes significantly exceeded that of CHADS2 and CHA2DS2VASc scores (P<0.05 for all). There were no differences in C-statistics between R2(MDRD-GFR)CHADS2, R2(CMDRD-GFR)CHADS2, R2(EPI-GFR)CHADS2 and R2(Mayo-GFR)CHADS2 and between R2(MDRD-GFR)CHA2DS2VASc, R2(CMDRD-GFR)CHA2DS2VASc, R2(EPI-GFR)CHA2DS2VASc and R2(Mayo-GFR)CHA2DS2VASc.
Discussion
As a frequent arrhythmia in clinical practice, AF increases in prevalence with age and accounts for an increased death risk.1–3 Nakagawa et al have manifested that impaired renal function was related to an increased mortality among 387 Japanese patients with AF.4 The current analysis validated that GFR as the continuous variable and GFR <60 mL/min/1.73 m2 were significantly and independently associated with death risk, whereas the CrCl as the continuous variable or CrCl <60 mL/min was significantly or modestly associated with death risk during the follow-up of older patients with AF. Meanwhile, the current analysis evaluated the renal function with not only the CrCl formula but also different GFR formulas and refined five different kinds of R2CHADS2 and R2CHA2DS2VASc schemes by combining CrCl and GFR with CHADS2 and CHA2DS2VASc scores. Moreover, the addition of GFR to CHADS2 and CHA2DS2VASc scores fared significantly better than that to CHADS2 and CHA2DS2VASc scores, whereas the addition of CrCl to CHADS2 and CHA2DS2VASc scores fared marginally better than that to CHADS2 and CHA2DS2VASc scores.
How to implement the effective stratification of death risk and accordingly decrease the death risk in patients with AF are of great concern. Various clinical factors have the ability to predict the death risk. However, there was no reliable schema developed for stratifying the death risk in patients with AF, and some researchers like Nakagawa et al have successfully applied the CHADS2 score, generally used for appraising the thromboembolic likelihood, to stratifying the death risk in patients with AF.4 This transition had an important value for clinical practice, but the current schemes including the CHADS2 and CHA2DS2VASc scores have a limited discriminatory ability.5,6 Renal function is a powerful risk factor for mortality in patients with AF, and thus, Piccini et al have made attempts to combine CrCl with the CHADS2 score. However, as a standard index of renal function, GFR has not been adequately assessed, and nobody knows if it is appropriate or even better to combine GFR with these scoring systems (CHADS2 and CHA2DS2VASc scores).7 The current analysis certified that GFR-based schemes – R2(GFR)CHADS2 and R2(GFR)CHA2DS2VASc – refined by four different formula-calculated GFR provided a significant improvement of predictive ability for death risk in older patients with AF, but with the addition of CrCl to CHADS2 and CHA2DS2VASc scores – R2(CrCl)CHADS2 and R2(CrCl)CHA2DS2VASc, there was only a modest improvement in death risk stratification. We are unaware of other published studies that have used GFR to refine the CHADS2 and CHA2DS2VASc scores and verified that GFR-based schemes performed better than original versions in death risk stratification of Chinese older patients with AF.
The current analysis has some limitations. First, as the current analysis was made up of 219 Chinese older patients with AF, to validate the current conclusion in a larger study population will be more valuable and very necessary. Second, due to the priority of all-cause mortality in the outcome studies, as well as the high prevalence of multiple organ failure in the elderly, all-cause mortality rather than cardiovascular/stroke-related mortality was considered in the current analysis.
Conclusion
The current analysis confirmed that Chinese older patients with AF with lower levels of GFR and GFR <60 mL/min/1.73 m2 had a significantly high death risk and those with lower levels of CrCl or CrCl <60 mL/min had a significantly or modestly high death risk. To aid the death risk scoring, the current analysis evaluated the renal function using not only CrCl formula but also different GFR formulas and then generated five different kinds of R2CHADS2 and R2CHA2DS2VASc schemes by combining CrCl and GFR with CHADS2 and CHA2DS2VASc scores. Meanwhile, the current analysis provided evidence for the significantly better performance of GFR-based schemes – R2(GFR)CHADS2 and R2(GFR)CHA2DS2VASc – and the moderately better performance of CrCl-based schemes – R2(CrCl)CHADS2 and R2(CrCl)CHA2DS2VASc – in death risk stratification compared with other published schemes without considering renal function (CHADS2 and CHA2DS2VASc scores). To our knowledge, it is the first time that GFR was applied to refine the CHADS2 and CHA2DS2VASc scores, and GFR-based schemes – R2(GFR)CHADS2 and R2(GFR)CHA2DS2VASc – were testified to be superior to original versions in the risk stratification of Chinese older patients with AF.
Acknowledgment
This work was supported by grants from the National Key Basic Research Project (2012CB517503 and 2013CB530804), Health Special Scientific Research Project of Chinese People’s Liberation Army (12BJZ34 and 14BJZ12) and Sanya Medical and Health Science and Technology Innovation Project (2016YW21).
Disclosure
The authors report no conflicts of interest in this work.
References
Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285(18):2370–2375. | ||
Kistler PM, Sanders P, Fynn SP, et al. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol. 2004;44(1):109–116. | ||
Kamanth S, Lip GY. Atrial fibrillation in the elderly: anticoagulation strategies and indications in the very elderly. Am J Geriatr Cardiol. 2002;11(6):357–364. | ||
Nakagawa K, Hirai T, Takashima S, et al. Chronic kidney disease and CHADS(2) score independently predict cardiovascular events and mortality in patients with nonvalvular atrial fibrillation. Am J Cardiol. 2011;107(6):912–916. | ||
Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–2870. | ||
Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137(2):263–272. | ||
Piccini JP, Stevens SR, Chang Y, et al; ROCKET AF Steering Committee and Investigators. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (rivaroxaban once-daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation) and ATRIA (AnTicoagulation and risk factors in atrial fibrillation) study cohorts. Circulation. 2013;127(2):224–232. | ||
Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocardiogr. 1989;2(5):358–367. | ||
Zhang L, Zuo L, Xu G, et al. Community-based screening for chronic kidney disease among populations older than 40 years in Beijing. Nephrol Dial Transplant. 2007;22(4):1093–1099. | ||
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. | ||
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470. | ||
Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944. | ||
Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141(12):929–937. | ||
Levey AS, Stevens LA, Schmid CH, et al; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





