Back to Journals » Vascular Health and Risk Management » Volume 13
Questionable accuracy of home blood pressure measurements in the obese population – Validation of the Microlife WatchBP O3® and Omron RS6® devices according to the European Society of Hypertension-International Protocol
Authors Azaki A , Diab R , Harb A, Asmar R, Chahine MN
Received 1 November 2016
Accepted for publication 24 January 2017
Published 27 February 2017 Volume 2017:13 Pages 61—69
DOI https://doi.org/10.2147/VHRM.S126285
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Amudha Kadirvelu
Alaa Azaki,1,* Reem Diab,1,* Aya Harb,1,* Roland Asmar,1,2 Mirna N Chahine1,2
1Faculty of Medical Sciences, Lebanese University, Hadath, 2Foundation‑Medical Research Institutes (F-MRI®), Beirut, Lebanon
*These authors contributed equally to this work
Objective: Two oscillometric devices, the Microlife WatchBP O3® and the Omron RS6®, designed for self-blood pressure measurement were evaluated according to the European Society of Hypertension (ESH)-International Protocol (IP) Revision 2010 in the obese population.
Methods: The Microlife WatchBP O3 measures blood pressure (BP) at the brachial level and the Omron RS6 measures BP at the wrist level. The ESH-IP revision 2010 includes a total of 33 subjects. The difference between observers’ and device BP values was calculated for each measure. A total of 99 pairs of BP differences were classified into three categories (≤5, ≤10, and ≤15 mmHg). The protocol procedures were followed precisely in each of the two studies.
Results: Microlife WatchBP O3 and Omron RS6 failed to fulfill the criteria of the ESH-IP. The mean differences between the device and the mercury readings were: 0.3±7.8 mmHg and −1.9±6.4 mmHg for systolic BP and diastolic BP, respectively, for Microlife WatchBP O3, and 2.7±9.9 mmHg for SBP and 3.5±11.1 mmHg for diastolic BP for Omron RS6.
Conclusion: Microlife WatchBP O3 and Omron RS6 readings differing from the mercury standard by more than 5, 10, and 15 mmHg failed to fulfill the ESH-IP revision 2010 requirements in obese subjects. Therefore, the two devices cannot be recommended for use in obese subjects.
Keywords: Microlife WatchBP O3, Omron RS6, validation, blood pressure measurement, home blood pressure measurement, obese population, European Society of Hypertension, International Protocol
Introduction
Hypertension (HTN) is a common reason for office visits to physicians, as observed in daily clinical practice. Efforts are directed toward treatment of high blood pressure (BP) by pharmacologic and nonpharmacologic interventions to reduce the burden of the disease and its complications.1 Screening for HTN is fundamental in medical practice because subjects who are still normotensive at age 50 have a 90% time-life risk of developing HTN.2,3 Because HTN is only identified by measuring BP,4 accurate measurement is crucial for diagnosis and management.
The World Health Organization and the National Institutes of Health define subjects with a Body Mass Index (BMI) ≥25 kg/m2 as overweight and those with BMI ≥30 kg/m2 as obese.5 Accordingly, the prevalence of overweight and obesity is high in the general population.5 Moreover, obesity is an established risk factor for HTN,6,7 and the pathophysiologic mechanisms by which obesity predisposes to HTN are well-understood.8 This has made accurate BP measurements in such population of extreme importance.
Overweight and obese patients often require the use of large-sized cuffs.9 Unsuitable cuff sizes are associated with inaccurate measures of BP.10,11 The use of inappropriate cuff size is a common error in measuring BP, where small-sized cuffs lead to overestimation of BP.12 The need of a large-sized cuff is critical because data have shown that only the standard adult size is available in practice of Home BP measurements.13 In addition, a potential impact of adiposity on BP is underestimated in obese individuals.7 Obese people have arms that are shaped as an inverted cone, with the diameter at the top of the arm greater than that at the elbow, which adds to difficulty in choosing the proper cuff size.14 Therefore, BP measurement is problematic in obese for several reasons and present a number of bias such as the arm circumference and cuff-related bias, the subcutaneous adiposity thickness, compression of the artery, and modification of the BP signal, as well as the specific hemodynamic parameters impacting the BP signal.
Three different protocols are used to validate the accuracy of BP measuring devices, such as the International protocol (IP) published by the working group on BP monitoring of the European Society of Hypertension (ESH),15 the British Hypertension Society16 protocols, and the Association for the Advancement of Medical Instrumentation protocol.17 Over the last 10 years, several automated devices have been successfully validated using these protocols,18 mostly in the general population. However, few studies10,19,20–23 have tested the accuracy of automated BP monitors in specific populations such as obese patients.
In 2010 and 2013, two BP devices, Microlife WatchBP O324 and Omron RS6,25 were validated in the general population following the ESH-IP. None of these BP devices was validated in the obese population. The objective of the study is to assess the accuracy of automatic oscillometric BP devices: the Microlife WatchBP O3 (at the brachial level) and the Omron RS6 (at the wrist level) in obese subjects according to the ESH-IP.
Materials and methods
Ethical information
This study was approved by the local ethical committee of the Faculty of Medical Sciences at the Lebanese University. Prior to any BP measurements, all eligible subjects included in this study signed an informed written consent.
Tested devices
Omron RS6
Omron RS6 (HEM-6221-E) is an automatic device for self-measurement of BP at the wrist level by using the oscillometric method. Inflation is automatic by a pump. Deflation is fast and automatic; 2×1.5 V “AAA” batteries are needed. It weighs 85 g without the batteries. Dimensions are 87 mm (L)×64 mm (H)×14 mm (D) without the bracelet. The wrist cuff circumference is suitable for a wrist circumference of 13.5–21.5 cm. The device has a digital liquid crystal display screen that displays the measured BP and pulse rate. The unit measures pressures from 0 to 299 mmHg and pulse from 40 to 180 beats/min.
Microlife WatchBP O3
Microlife WatchBP O3 (BP 3MZ1-1) is an automatic device for office, home, and ambulatory BP measurements taken at the arm level by using the oscillometric method. Inflation and deflation are automatic; 4×1.5 V “AA” batteries are needed. It weighs 385 g including the batteries. Dimensions are 150 mm (L)×100 mm (H)×50 mm (D). The device has a digital liquid crystal display screen that displays the measured BP and pulse rate. The unit measures pressures from 0 to 299 mmHg and pulse from 40 to 200 beats/min. Several cuff sizes of nylon and polyester are included with the Microlife WatchBP O3 and are applicable to different arm circumferences ranges; the only cuff used in our study is the one provided by the manufacturer, which is the large cuff for arm circumference of 32–42 cm.
Study protocol
According to the ESH-IP revision 2010, a total of 33 participants who fulfilled the age, gender, and entry BP requirements, with a BMI ≥30 kg/m2, were included in the validation of both devices, such as: age ≥25 years, with at least 10 men and 10 women, and 10–12 participants in each of the three BP recruitment ranges: 90–129, 130–160, and 161–180 mmHg for systolic BP (SBP) and 40–79, 80–100, and 101–130 mmHg for diastolic BP (DBP).
The arm circumference size of all participants was between 32 and 42 cm, and none of them had atrial fibrillation or any other arrhythmia. In these two validations, subjects were preselected from the outpatient clinics and from the inpatients at the Lebanese University affiliated hospitals (Mount-Lebanon, Lebanese Hospital Geitaoui, Rafik Hariri University Hospital, Governmental Hospital of Baabda, Rasoul el-Aazam Hospital, and Bahman Hospital).
For BP measurement, the validation team consisted of three persons: two observers trained in accurate BP measurement and a supervisor, all of whom completed training on the basis of a Compact Disc, read-only-memory specifically developed by the French Society of Hypertension for certification of observers involved in clinical studies and familiarized themselves with the use of the corresponding tested devices.
The gold standard instrument for BP measurement consisted of a mercury sphygmomanometer and a stethoscope. In our study, two parallel connected mercury sphygmomanometers and a teaching stethoscope were used by the two observers as a reference standard. The circumference of the arm was measured to ensure that the cuff size being used was adequate for the subject. The agreement between the two observers, blinded from each other’s result, was checked all over the evaluation period by the supervisor to make sure that the difference between the two observers was no more than 4 mmHg for SBP and DBP values. Otherwise, the measurement was repeated.
Measurements with the mercury sphygmomanometer were performed according to the “same arm, consecutive measurements” supported at the heart level. Measurements by the Microlife WatchBP O3 device were on the same arm supported at the heart level, whereas the ones by the Omron RS6 device were at the wrist level, as recommended by the manufacturers, with the subjects asked to relax for 5–10 min, while making sure that they were seated with their legs uncrossed and back supported. The only cuff used in our study for the standard mercury sphygmomanometer measurements was the same one provided by the manufacturer with the Microlife WatchBP O3 device, that is, the large cuff for arm circumference of 32–42 cm. In total, for each device, nine consecutive BP measurements were performed in each patient using the mercury sphygmomanometers (five times) and the tested device (four times), and were recorded as follows:
BPA entry BP, observers 1 and 2 each with the mercury standard
BPB device detection BP, supervisor
BP1 observers 1 and 2 with mercury standard
BP2 supervisor with the test instrument
BP3 observers 1 and 2 with mercury standard
BP4 supervisor with the test instrument
BP5 observers 1 and 2 with mercury standard
BP6 supervisor with the test instrument
BP7 observers 1 and 2 with mercury standard
However, since every subject was participating in the validation of both BP devices, 13 consecutive BP measurements were performed in each patient using the mercury sphygmomanometers (five times) and both of the tested devices (four times ×2).
Data analysis
Results were analyzed and expressed according to the ESH-IP requirements to conclude if the device passed or failed to pass the validation protocol. The statistical analysis was realized by using specific analysis software developed by the International Society for Vascular Health. For each subject, the device measurements BP2, BP4, and BP6 were first compared to the observer measurements BP1, BP3, and BP5, respectively, and then to the observer measurements BP3, BP5, and BP7, respectively. Comparisons more favorable to the device were used. BP1, BP3, BP5, and BP7 were the means of the two observer measurements. Briefly, differences between tested device and control measurements were classified according to whether their values lied within 5, 10, or 15 mmHg. Differences were calculated by subtracting the observer measurement from the device measurement; they were classified separately in this way for both SBP and DBP. Data were expressed as mean ± standard deviation.
Bland–Altman plots, used to show the deviations and linear correlation coefficients, were drawn to analyze the relationship between Delta SBP (device – reference) and Mean SBP (device and reference) or Delta DBP (device – reference) and Mean DBP (device and reference). The Pearson’s correlation coefficient “r” was calculated from r2; then p was determined by software using “r” and the total number of subjects “n”, following a two-tailed probability. The p value was considered statistically significant when p<0.05.
Results
Participants
A total of 72 subjects were screened and 34 participants were recruited following the three different BP ranges as follows: 11 subjects in the range 90–129 mmHg, 11 subjects in the range 130–160 mmHg, 10 subjects in the range 161–180 mmHg, and in 2 subjects >180 mmHg, for SBP; and 11 subjects in the range 40–79 mmHg, 12 subjects in the range 80–100 mmHg, and 11 subjects in the range 101–130 mmHg for DBP. The participants were distributed as follows: there were 18 males and 16 females; the mean age of the participants was 48±12 years; their wrist circumference ranged from 16 to 20 cm and their arm circumference ranged from 32 to 41 cm. The mean recruitment SBP was 146.1±26 mmHg (108–197 mmHg) and the mean recruitment DBP was 88.1±16 mmHg (56–120 mmHg).
BP measurements
Microlife WatchBP O3
The difference between the two observers was 0.0±2.2 and −0.3±1.9 mmHg for SBP and DBP, respectively (−4 to +4 mmHg). The mean differences between the observers and the tested device were +0.3±7.8 mmHg for SBP and −1.9±6.4 mmHg for DBP. The numbers of measurements differing from the mercury standard by 5, 10, and 15 mmHg or less are shown in Table 1. Bland–Altman plots of the differences between BP measurements obtained with the Microlife WatchBP O3 and the sphygmomanometer are shown for SBP (Figure 1A) and for DBP (Figure 1B). These results are in discordance with the requested criteria of the IP. Thus, the Microlife WatchBP O3 device failed to fulfill the validation criteria of the ESH-IP Revision 2010.
Omron RS6
The difference between the two observers was 0.0±2.2 and −0.3±1.9 mmHg for SBP and DBP, respectively (−4 to +4 mmHg). The mean differences between the observers and the tested device were 2.7±9.9 mmHg for SBP and 3.5±11.1 mmHg for DBP. The numbers of measurements differing from the mercury standard by 5, 10, and 15 mmHg or less are shown in Table 2. Bland–Altman plots of the differences between the BP measurements obtained with the Omron RS6 and the sphygmomanometer are shown for SBP (Figure 2A) and for DBP (Figure 2B). These results are in discordance with the requested criteria of the IP. Thus, the Omron RS6 device failed to fulfill the validation criteria of the ESH-IP Revision 2010.
Discussion
This study provides information on the accuracy of two devices for home BP measurement in the obese population. Each of the two specified devices was previously successfully validated in the general population.24,25 We compared the BP values obtained by the gold standard mercury sphygmomanometer with those obtained by each of the two devices. The results of the study showed that Microlife WatchBP O3 and Omron RS6 failed to meet the requirements of ESH-IP 2010 in the obese population.
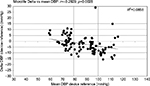
In addition to the analysis required by the ESH-IP protocol, and in order to evaluate the difference according to the BP baseline values, assessment of the determinants of the differences observed between each device and the mercury sphygmomanometer may provide further information. For Microlife WatchBP O3, the difference between values from the Microlife and the mercury sphygmomanometer devices according to the mean BP level did not show significant correlation between the averaged differences and the baseline values for SBP (r=−0.074; p=0.45), but showed significant correlation for DBP (Figure 3; r=−0.292; p=0.002), which may be interpreted by a higher underestimation of the DBP (in diastolic pressure levels >80 mmHg) by the Microlife WatchBP O3 device. For the Omron RS6, the difference between values of the device and the mercury sphygmomanometer according to the mean BP level did not show significant correlations between the averaged differences and the baseline values for both SBP (r=−0.144; p=0.148) and DBP (r=+0.174; p=0.07).
Important points related to both this specific population and the validation protocol need to be discussed: despite the efforts of the manufacturers to improve the quality of BP measuring devices, both the cuff and wrist characteristics of devices remain a point of weakness in this specific population. In obese people, using appropriate cuff size in BP measurement is substantial, where “miscuffing” is a serious source of error for BP readings. Indeed, BP measurement in some of our obese subjects presented some difficulties related to their arms shaped more as a cone than a cylinder; the diameter at the top of the arm is larger than the diameter of the arm in the region of the brachial artery. This shape results in a poor fit over the brachial artery and also results in inaccurate measurements.14 In addition, recent findings have demonstrated that despite using the appropriate cuff size, SBP appeared to be higher in those with bigger arms,26 meaning a bigger limb may require greater pressure simply because there is more tissue to compress and not necessarily because there is fatty tissue. Indeed, they also observed that those with larger, more muscular arms were more likely to be misclassified as prehypertensive or hypertensive compared to those with smaller arms, whereas those with smaller arms may be misclassified as normal despite having elevated BP.26 This study recommended that a further correction factor for arm size may be needed even when using the correct cuff size.26 Nonetheless, in our study, we did not try to classify each obese participant per se, rather every participant was his own control, such as one measurement recorded with the device vs the reference measurement recorded by the sphygmomanometer; in order to limit this particular bias in our study, we just used one cuff (the one provided by the manufacturer Microlife) suitable for both the device and the sphygmomanometer.
Concerning the wrist characteristics, it also presents accuracy issues for BP measurements; therefore, it may not only be related to the arm circumference, but also to the BP signal itself. Wrist monitors appear to be more attractive for the obese population because of their large arms, where subjects experience more comfort using a device applied at their relatively small-sized wrists; for the above-mentioned reasons, more validation studies in the obese population are highly needed. Actually, there are only four studies that have established the validation of oscillometric BP devices in the obese population or those with large arms. AlTunkan et al20,21 have validated two BP devices, the Omron M6 (HEM-7001-E) at the brachial level and the Omron 637IT at the wrist level, according to ESH-IP. El Feghali et al22 validated the Omron M7 (HEM-780-E) BP measuring device in people requiring large cuffs according to ESH-IP. In addition, Masiero et al23 validated the Microlife WatchBP Office Ankle Brachial Index according to ESH-IP in people requiring large/extra large cuff. Thus, considering the high percentage of obesity and large circumference arms as risk factors for HTN, more validation studies of BP devices in the obese population are needed.
The IP used in this study was published in 2002 by the Working Group on Blood Monitoring of the ESH and was then revised in 2010.15 The aim of this protocol was to simplify the earlier protocols (British Hypertension Society16 and Association for the Advancement of Medical Instrumentation17) without violating their integrity. The main advantage of ESH-IP is that it requires a smaller sample size (N=33) compared to the two other protocols (N=85). However, it has some disadvantages and limitations.
Limitations
First, the specific requirements needed for the sample size concern the age of the subjects. It must be above 25 years, which, therefore, excludes children and young adults from being studied, thereby omitting data from the obese population aged 18–25 years. In addition, the gender distribution must include at least ten males and ten females; this particular point makes studies in pregnant women, for example, questionable. Furthermore, the adult population is a part of a larger heterogeneous population affected by HTN; therefore, the results of these validation studies in a selected population cannot be extrapolated to a more specific one, and may be dangerous and may negatively affect the clinical practice. Essentially, the IP concerns the general population only, with no specific requirements in specific population such as children, pregnant women, obese subjects, arrhythmic patients, and so on.
Second, following the IP, subjects are not recruited according to their arm circumference. This may lead to the use of large-sized cuff in only minority of patients in any study where the majority of the included subjects require a standard sized cuff. Thus, the results of such studies may not be applicable to obese people with large arm circumference. This issue should be taken into consideration knowing that the prevalence of obesity is growing overtime and obese people are at increased risk of developing HTN to a more extent than the general population. This poses a challenge to have an accurate BP measurement in this specific population. Therefore, it is highly recommended to perform more validations studies in specific populations.
Third, the IP does not specify the number of validation studies needed to approve the device accuracy, although there is an agreement among experts that a device should be validated in at least two different centers separately. Therefore, since both the tested devices were previously successfully validated in the general population24,25 and failed to pass with obese subjects, it is highly recommended to check the accuracy of BP measuring devices in a specific population, such as obese people with large arm circumference, as a mandatory step in the validation process.
Conclusion
The results of the present study are of importance since they showed that the two tested devices, the Microlife WatchBP O3 that measures BP at the brachial level and the Omron RS6 that measures BP at the wrist level, failed to meet the requirements of ESH-IP 2010 in the obese population, despite their validation in the general population. Therefore, extrapolation of the results of validation studies in the general population to a more specific one may be risky and may affect the clinical practice negatively. In addition, the ESH-IP should stress on validating the BP devices in specific populations by publishing explicit criteria for such validation in these populations. Furthermore, it would be highly recommended to assess the accuracy of these two devices in other specific populations such as pregnant women, elderly subjects, arrhythmic patients, and so on.
Acknowledgments
We are grateful to the staff and the clinical research unit of the Foundation-Medical Research Institutes (Lebanese Hospital Geitaoui) as well as the people from the other academic hospitals affiliated with the Lebanese University, such as Mount-Lebanon, Lebanese Hospital Geitaoui, Rafik Hariri University Hospital, Governmental Hospital of Baabda, Rasoul el-Aazam Hospital, and Bahman Hospital, who assisted us to contact the patients to participate in the study, as well as the outpatient obese subjects.
Disclosure
The authors report no conflicts of interest in this work.
References
Seyedmazhari M. Pharmacological and non- pharmacological treatment of hypertension: a review article. ARYA Atheroscler J. 2012;8(Special Issue in National Hypertension Treatment):S217–S221. | ||
Kaplan NM, Victor RG. Hypertension in the population at large. In: Kaplan’s Clinical Hypertension. 11th ed. Philadelphia, PA: Wolter’s Kluwer; 2015:1–17. | ||
James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520. | ||
Thomas G. Pickering, MD. Principles and techniques of blood pressure measurement. Cardiol Clin. 2010;28(4):571–586. | ||
Wolk R, Shamsuzzaman AS, Somers VK. Obesity, sleep apnea, and hypertension. Hypertension. 2003;42(6):1067–1074. | ||
Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–1727. | ||
Kotchen TA. Obesity-related hypertension?: weighing the evidence. Hypertension. 2008;52(5):801–802. | ||
Landsberg L, Aronne LJ, Beilin LJ, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of the obesity society and the American Society of Hypertension. J Clin Hypertens (Greenwich). 2013;15(1):14–33. | ||
Palatini P, Parati G. Blood pressure measurement in very obese patients: a challenging problem. J Hypertens. 2011;29(3):425–429. | ||
Masiero S, Saladini F, Benetti E, Palatini P. Accuracy of the Microlife large-extra large-sized cuff (32–52 cm) coupled to an automatic oscillometric device. Blood Press Monit. 2011;16(2):99–102. | ||
Graves JW, Bailey KR, Sheps SG. The changing distribution of arm circumferences in NHANES III and NHANES 2000 and its impact on the utility of the “standard adult” blood pressure cuff. Blood Press Monit. 2003;8(6):223–227. | ||
Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1, Blood pressure measurement in humans. Circulation. 2005;111(5):697–716. | ||
Smith L. New AHA Recommendations for Blood Pressure Measurement. Am Fam Physician. 2005;1;72:1391–1398. | ||
McFarlane J. Blood pressure measurement in obese patients. Critical Care Nurse. 2012;32:70–73 | ||
O’Brien E, Atkins N, Stergiou G, et al: Working Group on Blood Pressure Monitoring of the European Society of Hypertension. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010;15(1):23–38. | ||
O’Brien E, Petrie J, Littler W, et al. The British Hypertension Society Protocol for the evaluation of blood pressure measuring devices. J Hypertens. 1993;11(Suppl 2):S43–S63. | ||
Association for the Advancement of Medical Instrumentation; 2013. ANSI/AAMI/ISO 11137-2:2013. Sterilization of health care products — Radiation — Part 2: Establishing the sterilization dose. Arlington, VA, USA. Available from: http://my.aami.org/aamiresources/previewfiles/1113702_1307_preview.pdf. | ||
DABL Educational Trust. Devices for blood pressure measurement. Available from: http://www.dableducational.org. Accessed December 12, 2012. | ||
Alpert BS. Validation of the Welch Allyn Spot Vital Signs blood pressure device according to the ANSI/AAMI SP10: 2002. Accuracy and cost-efficiency successfully combined. Blood Press Monit. 2007;12(5):345–347. | ||
Altunkan S, Ilman N, Kayatürk N, Altunkan E. Validation of the Omron M6(HEM-7001-E) upper-arm blood pressure measuring device according to the International Protocol in adults and obese adults. Blood Press Monit. 2007;12(4):219–225. | ||
Altunkan S, Oztaş K, Altunkan E. Validation of the Omron 637IT wrist blood pressure measuring device with a position sensor according to the International Protocol in adults and obese adults. Blood Press Monit. 2006;11(2):79–85. | ||
El Feghali RN, Topouchian JA, Pannier BM, El Assaad HA, Asmar RG. Validation of the OMRON M7 (HEM-780-E) blood pressure measuring device in a population requiring large cuff use according to the International Protocol of the European Society of Hypertension. Blood Press Monit. 2007;12(3):173–178. | ||
Masiero S, Saladini F, Benetti E, Palatini P. Accuracy of the Microlife large-extra large-sized cuff (32–52 cm) coupled to an automatic oscillometric device. Blood Press Monit. 2011;16(2):99–102. | ||
Ragazzo F, Saladini F, Palatini P. Validation of the Microlife WatchBP O3 device for clinic, home and ambulatory blood pressure measurement, according to the International Protocol. Blood Press Monit. 2010;15(1):59–62. | ||
Takahashi H, Yoshika M, Yokoi T. Validation of Omron RS8, RS6, and RS3 home blood presssure monitoring devices, in accordance with the European Society of Hypertension International Protocol revision 2010. Vascu Health Risk Manag. 2013;9:265–272. | ||
Loenneke JP, Loprinzi PD, Abe T, et al. Arm circumference influences blood pressure even when applying the correct cuff size: is a further correction needed? Int J Cardiol. 2016;202:743–744. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.