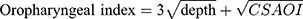Back to Journals » Nature and Science of Sleep » Volume 12
Quantitative Morphometric Measurements of the Oropharynx in Obstructive Sleep Apnea Syndrome Using a Laser Depth Measurement Module
Authors Kuo CFJ, Lin CS, Chuang CH, Lin CS, Chiu FS, Liu SC
Received 1 October 2020
Accepted for publication 26 November 2020
Published 14 December 2020 Volume 2020:12 Pages 1181—1190
DOI https://doi.org/10.2147/NSS.S284836
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Steven A Shea
Chung-Feng Jeffrey Kuo,1 Chun-Shu Lin,2 Cheng-Hsien Chuang,1 Chung-Shen Lin,3 Feng-Shiang Chiu,3 Shao-Cheng Liu3
1Department of Material Science & Engineering, National Taiwan University of Science and Technology, Taipei, Taiwan, Republic of China; 2Department of Radiation Oncology, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan, Republic of China; 3Department of Otolaryngology-Head and Neck Surgery Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan, Republic of China
Correspondence: Shao-Cheng Liu
Department of Otolaryngology-Head and Neck Surgery, Tri-Service General Hospital, National Defense Medical Center, No. 325, Sec. 2, Cheng-Gong Road, Neihu District, Taipei, Taiwan 114, Republic of China
Tel +886-2-8792-7192
Fax +886-2-8792-7193
Email [email protected]
Introduction: Current diagnostic routines in obstructive sleep apnea syndrome (OSAS), including drug-induced sleep endoscopy (DISE), provide qualitative data. Quantitative morphometric measurements of oropharyngeal structures remain challenging. This study aims to introduce a special linear laser projection device that can facilitate computer-assisted digitalized analysis and provide important quantitative information for OSAS prediction.
Materials and Methods: We used a single-wavelength green three-linear laser to provide the scaling reference, with one at an angle of 8.5 degrees with the other two which were parallel. The oropharyngeal images were divided into two groups: the non-OSAS and OSAS group, after polysomnography. A minimum of three evaluations were carried out to determine the maximum cross-sectional area of the oropharyngeal inlet (CSAOI) and the retropalatal depth.
Results: A total of 132 subjects were enrolled in this study, with 76 subjects in the non-OSAS group and 56 cases in the OSAS group. In the non-OSAS group, the CSAOI was significantly larger in males than in females. There was a trend toward deeper retropalatal region in men than in women (14.25 vs 11.76 mm). Correlation analysis revealed that retropalatal depth is significantly related to body height and the CSAOI. The body weight and BMI of patients with OSAS were significantly higher than those of participants without OSAS. The retropalatal depth and CSAOI were significantly decreased in OSAS patients as compared to those without OSAS. Our new parameter, the oropharyngeal index, showed the most outstanding discrimination by ROC analysis to predict OSAS.
Conclusion: Our innovative module can provide reference parameters, which make it possible to directly estimate the objective absolute values of relevant oropharyngeal structures. Our non-invasive approach can be used for outpatient screening, since it allows the identification of potential OSAS patients who should be referred for polysomnography, as many patients do not require DISE early in their evaluation.
Keywords: obstructive sleep apnea syndrome, quantitative endoscope, computer-aided diagnostic system, retropalatal depth, cross-sectional area, oropharyngeal inlet
Introduction
Obstructive sleep apnea syndrome (OSAS) is a serious and widespread health disorder characterized by intermittent repetitive partial or complete obstruction of the airway due to the narrowing of the respiratory passages.1 Techniques that are used to characterize the obstruction in OSAS, such as physical examination with Mallampati score or Friedman staging, fiber-optic nasal endoscopic evaluation with Muller maneuver, and drug-induced sleep endoscopy (DISE), provide only subjective, qualitative, or semi-quantitative data, with considerable inter-rater reliability.2 Cephalometric roentgenogram is cost-effective, but its use is limited due to the inherent ambiguity of locating landmarks and surfaces on the x-ray image.3 Computed tomography (CT) scanning and magnetic resonance imaging (MRI) provide better information in assessing the objective absolute value, but they are not widely available and are associated with high costs.4,5 Each of the above procedures has its unique advantages and disadvantages, but they are all time-consuming and patient-compliancable. Precisely identifying the sites of upper airway obstruction can potentially help to tailor surgical treatments, and a simpler, more objective, predictive, and even quantitative method is needed to improve surgical outcomes. Accordingly, a feasible assessment of upper airway obstruction is still evolving.
Digital images are composed of pixels, which are not easy to intuitively match with common measurement units. To convert the depth and area of an oropharynx into objective absolute values requires reliable scaling conversion references to understand the corresponding relationship between the image pixels and length unit. In this study, a computer-assisted digitalized analysis of endoscopy images with a new three-linear laser projection module was carried out to provide objective information on oropharyngeal structures. A special software program was used for quick and precise measurements. The oropharyngeal physiological parameters were digitalized, analyzed and compared with the participants’ age, gender, body height, body weight and body mass index (BMI). Our goal was to establish an early computer-aided assessment of clinical physical examination that can help direct treatment planning and serve as a new screening tool. This study aimed to set up a diagnostic system, by providing additional objective quantitative information, and to evaluate whether any predictability exists between our results and the polysomnography (PSG). Instead of the attempt to find a substitute for DISE, the performance of this type of outpatient screening is critical prior to obtaining the consenting of a patient for an invasive airway procedure.
Materials and Methods
Equipment
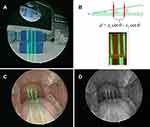
Our module comprised a rigid straight endoscope, three laser pointers and a grating sheet that were all commercially available and interchangeable. A single-wavelength green line laser was transformed and obtained from the dot laser (H435151D/R, 515nm) by the grating sheet (Figure 1A), to provide the scaling reference. The laser marking module was designed using 3D drawing software and constructed as a vehicle wrapping the endoscope as one piece (Figure 1B). The laser excitation apparatus was wrapped as a handle at the rear end to facilitate the holding of the endoscope and reduce oral discomfort. The laser guidance device (Figure 1C) is located at the front end. The line-generating green laser module provided a highly visible line projected onto a surface of oral mucosa. A total of three line laser beams, including one at an angle of 8.5 degrees with the other two which were parallel, were used to facilitate the calculation of the target depth (Figure 1D). After completion of the 3D drawings according to the design requirements, the rapid prototyping technology was used to make the prototype of the module.
Verification of the Laser Module
To verify the feasibility and reliability of the laser module, a series of experiments were conducted. First, lens correction was performed using Computer Vision Toolbox™ on Microsoft Windows 7. Lens correction is the process of estimating the characteristics of the image taken by an endoscope, acquiring the external and internal parameters of the lens, and using these parameters to evaluate the optical distortion and artifacts. It is an important process for the estimation of the distance between a fixed object and the lens, measurement of the size of the object in the image, and enhancement of the three-dimensional construction. The calibration pattern in this study is a fixed-size checkerboard image with 25-mm length per grid (Figure 2A). We analyzed 22 sets of images with the lens placed at different focusing distances and visual angles. Through multiple re-projections, the external parameters of the lens were calculated and the best interior parameters of the lens were obtained. After correction, the image lines were obviously flattened, and the area of each grid became even, which could reduce the distortion caused by the convex lens and make the projection of our module more accurate (Figure 2B). Second, to verify whether the marking module could accurately convert scaling of images, we used a mockup as the testing sample to simulate image capturing at varied positions, with different focusing distances and visual angles (Figure 2C–D). The result converted from the laser projection module was compared with the known mockup depth to calculate the accuracy of the device (Figure 3A–B).
Human Data Acquisition and Processing
The oropharyngeal image samples were provided by the subjects present in our institution. They were divided into two groups: the non-OSAS and OSAS group, according to the result of the PSG. The non-OSAS group comprised healthy subjects or those who presented with clinical features other than OSAS and had normal PSG results. The patients in the OSAS group were all confirmed by PSG, with apnea hypopnea index (AHI) >5 and oxygen desaturation index (ODI) ≥3%. The mean AHI was 30.3 (range 6.1–75.6) and the mean ODI was 36.6 (ODI range 3–105.5). The exclusion criteria included edentulous jaws, pain from the craniomandibular system, recent upper airway disease, history of oropharyngeal trauma or tumor, and history of radiotherapy or any kind of neck operations; asthmatic patients on inhalation medication were also excluded. To acquire the oropharyngeal images, we asked each subject to open their mouth widely, and a tongue depressor was used only in the anterior one-third of the tongue without eliciting a gag reflex, while leaving the tongue base in its natural position. During this procedure, the subject was also instructed to breathe normally through the nose. A minimum of three evaluations were carried out so the observer could acquire and determine the maximum cross-sectional area of the oropharyngeal inlet (CSAOI), which is bounded by the uvula, soft palate and palatopharyngeal fold (Figure 3C). The images were captured and processed by MATLAB. The retropalatal depth and the CSAOI were determined and calculated (Figure 3D). Our new parameter was defined as the following equation:
Ethical Considerations
The research protocol (NO: 1–108-05-132) was reviewed and approved by the Institutional Review Board of the Tri-Service General Hospital, Taipei, Taiwan. All methods were performed in accordance with the relevant guidelines and regulations. All patients (including the healthy volunteers) provided written informed consents prior to participation, and this study complied with the Declaration of Helsinki.
Statistical Analysis
Data were presented as the mean ± standard error of the mean (SEM). All statistical analyses were performed using Student’s t test on GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA, USA); P < 0.05 was considered statistically significant. We used logistic regression to assess the association between these two parameters (BMI, oropharyngeal index) and OSAS. ROC analysis was used to calculate the cutoff values in order to detect patients with a high risk of OSAS.
Results and Validation
To verify the reliability and feasibility of the laser projection device, we first captured images of a 3D printing mockup (Figure 3A) with a depth of 30mm and verified them by shooting multiple angles and different distances from it. The average depth of the mockup converted by the projection device was 30.39 mm, and the average error range was around 1.29%. This confirmed the reliability of our laser projection markers.
A total of 132 subjects were enrolled in this study. The non-OSAS group was composed of 76 subjects and the OSAS group 56. The age range for all subjects was 20 to 75 years old. The mean age of patients with OSAS and those without OSAS was 42.18±14.06 and 49.39±15.21, respectively. The non-OSAS group comprised 44 males and 32 females, with mean age of 46.82±17.21 and 45.81±12.45 (p > 0.05), respectively. Men were obviously taller and weightier than women (all p values <0.001), but there was no significant difference in BMI between both genders (25.59 vs 23.95 kg/m2, p=0.228) (Table 1). The CSAOI was significantly larger in males than in females (269.22 vs 192.06 mm2, p < 0.05). There was a trend toward deeper retropalatal region in men than in women (14.25 vs 11.76 mm), but no statistical significance was found. We used correlation analysis to assess the association between age, body height, weight, BMI, retropalatal depth and CSAOI. The retro-palatal depth was found to have a strong relationship with body height (p=0.007) and CSAOI (p=0.041). All the other variables were hardly related to each other.
 |
Table 1 Characteristics and the Anthropometric Measurements of the Control Group |
In the OSAS group, there were 34 males and 22 females with mean age 41.35±14.31 and 43.45±14.25 (p > 0.05), respectively. No significant gender difference was noted as regard BMI, although men were obviously taller and weightier than women (Table 2). There was a trend, but without statistical significance, toward deeper retropalatal region and larger CSAOI in men than in women.
 |
Table 2 Characteristics and the Anthropometric Measurements of the OSAS Group |
The mean age of males (41.35±14.31) and females (43.45±14.25) with OSAS revealed no significant difference as compared to the mean of those without OSAS (46.82±17.21 and 45.81±12.45, respectively). Body weight and BMI of both female and male patients with OSAS were significantly higher than those of subjects without OSAS (all p values <0.001). For body height, there was no significant difference between the OSAS and non-OSAS group. The retropalatal depth was significantly decreased in both female and male patients with OSAS (9.07±0.87 and 8.12±1.44 mm, respectively) as compared to those without OSAS (14.25±1.36 and 11.76±1.41 mm, respectively) (all p values <0.05). Men with OSAS had significantly smaller CSAOI than men without OSAS (213.49±18.44 vs 269.22±30.89 mm2, p values <0.05). A similar trend was observed in females (192.06±20.72 vs 176.36±28.27 mm2) but without statistical significance.
Receiver operating characteristic (ROC) curves were used to determine the cutoff values in order to detect patients with a high risk of OSAS. High AUC values suggested that the BMI was “good” at classifying a high risk for OSAS (Figure 4), while our new parameter, the oropharyngeal index, demonstrated outstanding discrimination (Figure 5). Females with BMI greater than 24.18 kg/m2 (sensitivity=91%) and males with BMI greater than 26.06 kg/m2 (sensitivity=82%) were found to be at a high risk for OSAS. Both females and males with oropharyngeal index greater than 15.46 were also found to be at a high risk for OSAS, with the highest AUC value (sensitivity=79%, AUC=0.91).
 |
Figure 4 ROC curve of BMI for (A) males and (B) females to predict OSAS. |
 |
Figure 5 ROC curve of our new parameter, the oropharyngeal index, to predict OSAS. Abbreviation: CSAOI, cross-sectional area of the oropharyngeal inlet. |
Discussion
To our knowledge, this study is the first report with a standardized and computer-based quantitative analysis of the actual size of oropharyngeal structures, including the retropalatal depth and CSAOI, which are of great clinical importance in practical medicine. In the past, numerous examinations were used for the evaluation of the degree or level of obstruction of the upper airway, such as cephalometry, CT, MRI and DISE. DISE is widely performed now with its advantage over the other methods to dynamically observe not only the cross-sectional area, but also volumetric changes in the upper airway. Nevertheless, its disadvantages include invasiveness and unignorable inter-observer variation.6 Upper airway morphologic analysis with DISE is still dominated by the examiner’s judgment, which is largely subjective. Computer-assisted evaluation has been conducted before, which brought a new perspective and highlighted its clinical usefulness.7,8 However, due to lack of scaling references, those studies only provided qualitative or semi-quantitative data.9 The lack of this entity inspired our attempt to develop a new device that can determine the absolute value of the upper airway. Accordingly, we performed an endoscopic morphometric measurement study using a laser projection marking module to provide the reference scaling. Oropharyngeal endoscopy can easily be performed to directly observe pharyngeal dynamics, and automatic measurement of airway cross-sectional area can be achieved with our laser module. Obstruction of the upper airways in OSAS is usually located at multiple levels, frequently requiring multilevel surgery. Precise identification can potentially be used to tailor surgical treatments and improve surgical outcomes.
Our morphological measurements in healthy subjects revealed significant correlation between retropalatal depth and body height. However, there were no significant relationships found between this depth and body weight, BMI, age or sex. Many previous studies indicated the significant impact of BMI on OSAS. Since the body height does not change a lot as an adult, a high BMI means overweight, and, therefore, obesity features are important risk factors for OSAS development.10,11 Various anthropometric measurements targeted at obesity, including neck circumference, waist circumference, and waist–hip ratio, are used throughout the follow-up of OSAS patients.12 On the other hand, we surprisingly found that body height was an independent predictor for retropalatal depth, which implies that a taller person has a deeper retropalatal region, and this finding may help to prevent upper airway obstruction. Although there is not much that can be done by most adults to increase their height, there are still several ways a parent can influence the height of their child, such as nutritional supplements or growth hormones, so as to decrease OSAS development in the future. We also found significant correlation between retropalatal depth and CSAOI. In the upper airway, the retropalatal region and oropharyngeal inlet are the most collapsible areas.13 A study by Gao et al found that the volume of the retropalatal region increased with aging.14 The authors hypothesized that the aging effect could reduce the tone of the soft palate muscle, increase the soft palate length and shrink the tonsils. In the current study, the oropharyngeal size showed no significant relationship with age. Meanwhile, men had significantly larger CSAOI and deeper retropalatal region than women had, and both the body weight and height of men were greater than those of women. The correlation analysis revealed no significant relationship between the upper airway parameters and body weight or gender. Instead, the truly determining factor remained the body height. Although adult males are typically taller than adult females and have deeper retropalatal region, females are more likely to bother with dieting and weight loss than males. The idea of what constitutes an ideal body shape differs between both genders. Men show a desire to gain weight while the great majority of women want to lose weight.15 Therefore, our finding was still consistent with those of previous literatures that severe OSAS is more common in obese males.16,17
We found significant differences in BMI, retropalatal depth, and CSAOI between the OSAS and non-OSAS groups. Svensson et al indicated that the degree and level of collapsibility might differ according to the BMI.18 Individuals with a BMI > 28 are 8 to 10 times more likely to suffer OSAS. Our results demonstrate a significantly increased risk of OSAS in both males and females in the event of high BMI and weight. The OSAS cutoff values were measured to be 24.18 kg/m2 in women and 26.06 kg/m2 in men in our study (Figure 4). For the Korean population, OSAS cutoff values were stated to be 23.05 and 24.95 kg/m2 in women and men, respectively.19 The impact of obesity on OSAS has been investigated extensively. Instead of an increased overall body fat ratio, an increase in the central region is suggested to have a pivotal role in OSAS.11 Upper airway changes, such as redundant soft palate, elongated uvula, enlarged tonsils, and oropharyngeal crowding, have also been reported to be associated with OSAS.20 However, morphological measurements, including retropalatal depth and CSAOI, which were difficult to carry out in the past, have not been clearly established. In our study, retropalatal depth and CSAOI proved to be very important predictors, showing a significant decrease in OSAS patients, especially the males. Our results showed a moderately negative correlation between OSAS and CSAOI, and a stronger negative correlation between OSAS and retropalatal depth in the awake state. This can be explained by the fact that increased BMI aggravates the narrowing of the upper airway and consequently exacerbates the severity of sleep apnea. The thickness of the lateral pharyngeal muscular walls and the enlargement of the parapharyngeal fat pads are both reported to be the predominant anatomical factors that contribute to airway narrowing in OSAS patients.21 In addition, patients with OSAS have an increased mobility of soft tissue in the velo- and gloss-opharynx, which results in recurrent obstruction of the pharyngeal airway. Increased BMI often has a parallel effect on fat accumulated over the ventral tongue and soft palate, which pushes the uvula backward and consequently decreases the retropalatal depth and increases oropharyngeal crowding. The clinical implication may suggest that adequate uvula-palatoplasty and reduction of the wide volume of the tongue base should be considered in higher AHI or BMI patients.
Another novel finding of the study is that, for the first time, we demonstrated the role of the oropharyngeal index in predicting OSAS development. Previous predisposing factors, including the BMI and airway dimensions, were interpreted as oropharyngeal index in our result, and should be considered as independent factors for predicting OSAS. In the absence of scaling references, previous upper airway endoscopic measurements were usually qualitative. For example, endoscopy with the Muller maneuver (MM) for the evaluation of OSAS has been widely reported in the literature.8 It is a simple method with limited use. Even with computer-assisted evaluation, most studies have yielded only subjective or semi-quantitative data for calculating the relative ratio of change in upper airway dimensions.22 With the laser marking module in our study, these parameters were easily measured, and the cutoff value of our oropharyngeal index was found to be 15.457, which might play an essential role in OSAS prediction. In this context, the determination of the oropharyngeal index will allow the identification of potential OSAS patients who need referral to sleep centers for polysomnography. The measurement of the oropharyngeal index provides an insight into anatomical changes in the upper airway of OSAS patients. It provides a new perspective for further research. However, our study was limited by the use of static awake endoscopy, a method that falls short of evaluating the dynamic changes in the upper airway during sleep. In addition, our measurement of the oropharyngeal index was not assessed in terms of OSAS prognosis.
During validation with a mockup, our device revealed a maximum of 1.3% error in average quality. The reasons why we could not reach 100% accuracy might be due to errors in the process while defining the center of the laser lines, which might have biased the scaling references. Enhancing laryngoscopy resolution with a full HD (high-definition) video capture device and picture processing with at least 300 pixels per inch may reduce this error. Our study was performed while the patients were seated and awake, and this situation probably did not reflect the anatomical abnormality during sleep. However, we have provided strong evidence that computer-based evaluation of oropharyngeal images could play a greater role in OSAS prediction in the near future by offering better insight into oropharyngeal pathophysiology. Our screening tool is not a substitute for DISE or PSG, but it may be crucial in early assessment, as many patients do not require surgery or DISE early in the evaluation. In summary, we recommend this approach as a good and cost-effective method because of its simplicity and predictability. Further work is now needed to validate the approach in a prospective clinical setting.
Conclusion
This is the first report on truly objective morphometric measurement of oropharyngeal structures. Our innovative module can provide reference parameters, which make it possible to directly estimate the objective absolute values of relevant oropharyngeal structures, which have been interpreted as the oropharyngeal index. Our non-invasive approach allows the identification of potential OSAS patients who need referral to sleep centers for polysomnography. In the future, we can extend its clinical application to measure subtle oropharyngeal changes in OSAS patients after surgical intervention, which are often difficult to objectively quantify.
Data Sharing Statement
The datasets generated from this study are available from the corresponding author on reasonable request.
Ethical Considerations
The research protocol (NO: 1-108-05-132) has been reviewed and approved by the Institutional Review Board of Tri-Service General Hospital.
Consent for Publication
Written informed consent to publish has been obtained from all participants (including the healthy volunteers), and this study complied with the Declaration of Helsinki.
Acknowledgments
The research was supported by Tri-Service General Hospital, National Defense Medical Center-National Taiwan University of Science and Technology Joint Research Program (TSGH-D-109054, TSGH-NTUST-107-01).
Author Contributions
All authors have agreed on the journal to which the article will be submitted.
All authors have reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage.
All authors agree to take responsibility and be accountable for the contents of the article.
The specific role of each author are as follows:
1. Chung-Feng Jeffrey Kuo, PhD: study design, critical article review/editing.
2. Chun-Shu Lin: data analysis and interpretation, article and images review/editing.
3. Cheng-Hsien Chuang: acquisition of data, image editing, article drafting
4. Chung-Shen Lin, MD: acquisition of data, critical article review/editing.
5. Feng-Shiang Chiu: acquisition of data, critical article review/editing.
6. Shao-Cheng Liu, MD, PhD: study design, data collection, literature search, image editing, article drafting, article submission.
Funding
We have no sources of funding.
Disclosure
The authors report no conflicts of interest for this work and have no interests that might be perceived to influence the results and/or discussion reported in this paper.
References
1. Semelka M, Wilson J, Floyd R. Diagnosis and treatment of obstructive sleep apnea in adults. Am Fam Physician. 2016;94(5):355–360.
2. Dijemeni E, Kotecha B. Drug-Induced Sedation Endoscopy (DISE) DATA FUSION system: clinical feasibility study. Eur Arch Otorhinolaryngol. 2018;275(1):247–260.
3. Neelapu BC, Kharbanda OP, Sardana HK, et al. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: A systematic review and meta-analysis of cephalometric studies. Sleep Med Rev. 2017;31:79–90.
4. Kim WY, Hong SN, Yang SK, et al. The effect of body position on airway patency in obstructive sleep apnea: CT imaging analysis. Sleep Breath. 2019;23(3):911–916.
5. Nguyen HT, Magalang U, Abduljalil A, et al. MRI-based methodology to monitor the impact of positional changes on the airway caliber in obstructive sleep apnea patients. Magn Reson Imaging. 2019;61:233–238.
6. Altintaş A, Yegin Y, Çelik M, Kaya KH, Koç AK, Kayhan FT. Interobserver consistency of drug-induced sleep endoscopy in diagnosing obstructive sleep apnea using a VOTE classification system. J Craniofac Surg. 2018;29(2):e140–e143.
7. Santaolalla Montoya F, Iriondo Bedialauneta JR, Aguirre Larracoechea U, Martinez Ibargüen A. The predictive value of clinical and epidemiological parameters in the identification of patients with obstructive sleep apnoea (OSA): a clinical prediction algorithm in the evaluation of OSA. Eur Arch Otorhinolaryngol. 2007;264(6):637–643.
8. Ko MT, Su CY. Computer-assisted quantitative evaluation of obstructive sleep apnea using digitalized endoscopic imaging with Muller maneuver. Laryngoscope. 2008;118(5):909–914.
9. Terris DJ, Hanasono MM, Liu YC. Reliability of the Muller maneuver and its association with sleep-disordered breathing. Laryngoscope. 2000;110(11):1819–1823.
10. Unal Y, Ozturk DA, Tosun K, Kutlu G. Association between obstructive sleep apnea syndrome and waist-to-height ratio. Sleep Breathing. 2019;23(2):523–529. doi:10.1007/s11325-018-1725-4
11. Saint Martin M, Roche F, Thomas T, Collet P, Barthélémy JC, Sforza E. Association of body fat composition and obstructive sleep apnea in the elderly: a longitudinal study. Obesity. 2015;23(7):1511–1516. doi:10.1002/oby.21121
12. Borel A-L, Coumes S, Reche F, et al. Waist, neck circumferences, waist-to-hip ratio: which is the best cardiometabolic risk marker in women with severe obesity? The SOON cohort. PLoS One. 2018;13(11):e0206617. doi:10.1371/journal.pone.0206617
13. Trudo FJ, Gefter WB, Welch KC, Gupta KB, Maislin G, Schwab RJ. State-related changes in upper airway caliber and surrounding soft-tissue structures in normal subjects. Am J Respiratory Critical Care Med. 1998;158(4):1259–1270. doi:10.1164/ajrccm.158.4.9712063
14. Gao F, Li YR, Xu W, et al. Upper airway morphological changes in obstructive sleep apnoea: effect of age on pharyngeal anatomy. J Laryngology Otology. 2020;134(4):354–361. doi:10.1017/S0022215120000766
15. Lee Y. Slender women and overweight men: gender differences in the educational gradient in body weight in South Korea. Int J Equity Health. 2017;16(1):202.
16. Wu MF, Chen YH, Chen HC, Huang WC. Interactions among obstructive sleep apnea syndrome severity, sex, and obesity on circulatory inflammatory biomarkers in patients with suspected obstructive sleep apnea syndrome: a retrospective, cross-sectional study. Int J Environ Res Public Health. 2020;17(13):4701.
17. Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383(9918):736–747.
18. Svensson M, Holmstrom M, Broman JE, Lindberg E. Can anatomical and functional features in the upper airways predict sleep apnea? A population-based study in females. Acta Otolaryngol. 2006;126(6):613–620.
19. Kang HH, Kang JY, Ha JH, et al. The associations between anthropometric indices and obstructive sleep apnea in a Korean population. PLoS One. 2014;9(12):e114463.
20. Sundman J, Fehrm J, Friberg D. Low inter-examiner agreement of the Friedman staging system indicating limited value in patient selection. Eur Arch Otorhinolaryngol. 2018;275(6):1541–1545.
21. Sanner BM, Heise M, Knoben B, et al. MRI of the pharynx and treatment efficacy of a mandibular advancement device in obstructive sleep apnoea syndrome. Eur Respir J. 2002;20(1):143–150.
22. Huang JF, Chen GP, Wang BY, et al. Assessment of upper-airway configuration in obstructive sleep apnea syndrome with computed tomography imaging during Müller maneuver. Respir Care. 2016;61(12):1651–1658.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.