Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Quantification of inaccurate diagnosis of COPD in primary care medicine: an analysis of the COACH clinical audit
Authors Abad-Arranz M, Moran-Rodríguez A , Mascarós Balaguer E , Quintana Velasco C, Abad Polo L, Núñez Palomo S , Gonzálvez Rey J, Fernández Vargas AM, Hidalgo Requena A , Helguera Quevedo JM, García Pardo M, Lopez-Campos JL
Received 23 December 2018
Accepted for publication 18 March 2019
Published 6 June 2019 Volume 2019:14 Pages 1187—1194
DOI https://doi.org/10.2147/COPD.S199322
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
María Abad-Arranz,1 Ana Moran-Rodríguez,2 Enrique Mascarós Balaguer,3 Carmen Quintana Velasco,4 Laura Abad Polo,5 Sara Núñez Palomo,6 Jaime Gonzálvez Rey,7 Ana María Fernández Vargas,8 Antonio Hidalgo Requena,9 Jose Manuel Helguera Quevedo,10 Marina García Pardo,11 Jose Luis Lopez-Campos on behalf of the COACH study investigators1,12
1Unidad Médico-Quirúrgica de Enfermedades Respiratorias, Instituto de Biomedicina de Sevilla (IBiS), Hospital Universitario Virgen del Rocío/Universidad de Sevilla, Seville, Spain; 2UGC-DCCU Bahía de Cádiz-La Janda, Cádiz, Spain; 3Centro de Salud Fuente de San Luis, Valencia, Spain; 4Centro de Salud Perpetuo Socorro, Huesca, Spain; 5Centro de Salud Illueca, sector Calatayud, Zaragoza, Spain; 6Centro de Salud Torrelaguna, Madrid, Spain; 7Centro de Salud Matamá, Vigo, Spain; 8Centro de Salud La Victoria, Málaga, Spain; 9Centro de Salud de Lucena, Córdoba, Spain; 10Centro de Salud Bajo Asón, Ampuero, Cantabria, Spain; 11Centro de Salud de Inca, Mallorca, Spain; 12Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES), Instituto de Salud Carlos III, Madrid, Spain
Background: Inaccurate diagnosis in COPD is a current problem with relevant consequences in terms of inefficient health care, which has not been thoroughly studied in primary care medicine. The aim of the present study was to evaluate the degree of inaccurate diagnosis in Primary Care in Spain and study the determinants associated with it.
Methods: The Community Assessment of COPD Health Care (COACH) study is a national, observational, randomized, non-interventional, national clinical audit aimed at evaluating clinical practice for patients with COPD in primary care medicine in Spain. For the present analysis, a correct diagnosis was evaluated based on previous exposure and airway obstruction with and without the presence of symptoms. The association of patient-level and center-level variables with inaccurate diagnosis was studied using multivariate multilevel binomial logistic regression models.
Results: During the study 4,307 cases from 63 centers were audited. The rate of inaccurate diagnosis was 82.4% (inter-regional range from 76.8% to 90.2%). Patient-related interventions associated with inaccurate diagnosis were related to active smoking, lung function evaluation, and specific therapeutic interventions. Center-level variables related to the availability of certain complementary tests and different aspects of the resources available were also associated with an inaccurate diagnosis.
Conclusions: The prevalence data for the inaccurate diagnosis of COPD in primary care medicine in Spain establishes a point of reference in the clinical management of COPD. The descriptors of the variables associated with this inaccurate diagnosis can be used to identify cases and centers in which inaccurate diagnosis is occurring considerably, thus allowing for improvement.
Keywords: COPD, clinical audit, primary care medicine, inaccurate diagnosis
Introduction
The adequacy of the diagnosis of COPD remains a challenge for clinicians and health-care managers. Recent epidemiological studies have reported a considerably high frequency of inadequate diagnosis,1 despite the well-known practice guidelines with clear recommendations for the diagnosis of the disease,2 resulting in two different clinical scenarios. On the one hand, under-diagnosis constitutes a problem already acknowledged by different studies in recent years.3–5 Receiving inadequate heath care6 or suffering from an uncontrolled disease that may also impact other comorbidities7 are some of the consequences of this under-diagnosis. On the other hand, over-diagnosis is another problem that frequently occurs in COPD. This over-diagnosis can impact several key aspects of the disease,8 including increased exposure to – pharmacological treatment that would not otherwise be needed as well as an increase in the use of health services by the wrong patients, and the performance of a number of diagnostic tests. Additionally, people may be urged to adapt their lifestyle for a disease they do not have, with regular unneeded monitoring which would finally label them as sick individuals. Finally, it clearly impacts the health system leading to potential extra costs.
Beyond these two situations, a number of other clinical scenarios can be identified in which a diagnosis of COPD is determined without confirming the diagnostic criteria, termed as inaccurate diagnosis. A recent review highlighted the possible clinical situations in which an inaccurate diagnosis of COPD could occur.9 Notably, the relevance of this inaccurate diagnosis has not been as exhaustively analyzed,10,11 with only few reports evaluating it, including a recent clinical audit on its prevalence in Wales.12
Accordingly, more information on the quantification of inaccurate diagnosis and the associated determinants would be of interest for clinicians and health-care managers. The Community Assessment of COPD Health Care (COACH) study was a national, observational, randomized, non-interventional, multicenter clinical audit aimed at evaluating clinical practice for patients with COPD in primary care medicine in Spain.13 The aim of the present study was to use the COACH data to evaluate the degree of inaccurate diagnosis in primary care medicine in Spain and evaluate the determinants associated with it.
Methods
The methodology of the COACH study has been extensively reported.13 Briefly, COACH was a national, observational, randomized, non-interventional, multicenter, clinical audit as result of a joint project between the Spanish Primary Care Respiratory Group (GRAP) and the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). The design was cross-sectional and recruitment of cases and gathering of information was retrospective by nature.
A randomization procedure was used for center selection, as previously explained13 using a Microsoft (Microsoft Corporation, Redmond, WA, USA) Excel spreadsheet, aiming to include 10% of all primary care centers (PCCs) in Spain, with replacement in case of refusal to participate.
The first draft of the databases was later discussed in a face-to-face kick-off meeting in Madrid, on September 13, 2014, and by email and teleconferences thereafter. The aim was to benchmark the results with the two most widely used guidelines in Spain: GOLD 201314 and the Spanish National Guideline for COPD (GesEPOC).15
Information was recorded in a centralized database, organized as a hierarchical web-based tool with different levels of data accessibility. Data were collected from January 1, 2015, to December 31, 2016. Since the data was retrospective, the doctors in charge of the patients were not informed that the audit was being carried out. During this period, each participating PCC was told to search the center’s medical records to select all cases with a diagnosis of COPD in no specific order. The local investigator consecutively reviewed each medical record to confirm that the diagnosis was established in the medical record and, if this was the case, the audit information was recorded. Because accuracy of COPD diagnosis was an outcome measurement of the audit, no diagnostic criteria were required for the case to be included in our study. The classification of the patient as having COPD in the medical record, was sufficient to include the case and perform the audit. No exclusion criteria were defined ad-hoc.
For descriptive purposes, age, gender and tobacco history expressed as pack-year were noted. The setting of the PCC (rural/urban) and comorbidities were also noted. Rural areas were defined as areas with a population of <25,000. Comorbidities were evaluated by comorbidity indexes including the Charlson index16 and the COPD-specific comorbidity test (COTE).17
For the present analysis, the evaluation of a correct diagnosis was done at two-time points: once upon establishing a COPD diagnosis as indicated in the medical record and again during the current audited visit. Diagnostic criteria were set according to the GOLD document.2 Two criteria were used to detect a correct diagnosis during the diagnostic visit: previous exposure to any noxious particles or gases (smoking, biomass and occupational origins) and the detection of an airflow limitation that is not fully reversible according to spirometry results. Although the aim was to assess lung function by using post-bronchodilator spirometry, most cases only had a pre-bronchodilator spirometry results recorded and available for assessment. In these cases, we decided to consider pre-bronchodilator spirometries. To detect a correct diagnosis in the audited visit we also considered the presence of respiratory symptoms. Dyspnea, cough, and expectoration were considered symptoms associated with COPD. Since chronic respiratory symptoms as a requirement for a correct diagnosis was included in GOLD 2017,2 which had not been released when the audit took place, we also studied diagnostic adequacy at the audited visit, with and without symptoms.
The study observed the ethical requirements in Spain, including the Declaration of Helsinki, and the Spanish regulation on data protection and confidentiality (Spanish Organic Law 15/1999 of December 13 on the Protection of Personal Data). Main ethical approval was granted by the coordinating center’s Ethics Committee (Comité de Ética de Investigación de los hospitales universitarios Virgen Macarena-Virgen del Rocío, Seville, Spain, approval act 02/2014) and in every participating region according to local regional legislation. Medical records were anonymized in the database. Informed consent was waived due to the anonymization of the data, the lack of active therapeutic interventions, and the retrospective nature of the study. The Ethics Committee approved this procedure, which was clearly specified in the protocol.
Statistical analysis
Statistical analyses were done with IBM SPSS Statistics, version 20.0 (SPSS; IBM Corporation, Armonk, NY, USA). Descriptive analysis used the mean and SDs or absolute and relative frequencies, depending on the nature of the variable. The inter-regional range, representing the highest and lowest mean values from the participating centers at the regional level, was used to reflect variability. The significance of this variability was explored using the ANOVA test (using the Welch test in case of non-homogeneity of the variances) or chi-square test, according to the nature of the variable. The percentage of accurate diagnosis was obtained for each visit dividing the number of cases fulfilling the diagnostic criteria by the total number of audited cases. Accurate diagnosis was also calculated considering whether the patient had had an obstructive result during the diagnostic or audited visit. Once the cases with a correct diagnosis were identified, we evaluated variables associated with inaccurate diagnosis using the unpaired Student t test for numerical variables and chi-squared test for categorical variables.
Multivariate multilevel binomial logistic regression models were used to explore those patient-level and center-level variables found to be significantly associated with the presence of inaccurate diagnosis.18 We first constructed an empty model, which included only the hospital-cluster effect. Then, we estimated a second adjusted model, which added the variables at the patient-level (model 1). A final model 2 added the variables at the PCC level. The variables tested in the adjusted model were selected using a forward selection procedure based on the Wald test. The results are expressed as OR with 95% CI. P-value was set at 0.05.
Results
The audit included a total of 4,307 cases that were included from 63 PCC in 5 regions of Spain. The description of the audited cases is summarized in Table 1. This was a cohort of patients identified as being diagnosed with COPD, with a considerable number of active smokers and moderate lung function impairment.
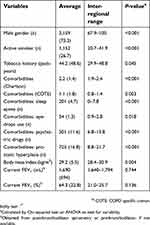 | Table 1 Characteristics of the 4,307 audited cases |
The accuracy of the diagnostic criteria at the time of the initial diagnosis and during the audited visit is summarized in Tables 2 and 3, respectively. Overall, the diagnosis (exposure + obstruction) was correctly established in 16.1% during the diagnostic visit and was correct during the audited visit in 5.0% and 3.6%, depending on symptom criteria. These figures presented a considerable variability. Considering the best possible scenario in which we require previous exposure and any obstructive spirometry at any visit, without full reversibility, the diagnosis was correct in 758 (17.6%) cases, with an inter-regional range from 9.8% to 23.2%. When we added symptoms to the equation as a necessary criterion for a correct diagnosis, the diagnosis would be correct in 369 (8.6%) cases with an inter-regional range from 0.7% to 14.0%. Therefore, taking these 758 cases as correctly diagnosed, the rate of inaccurate diagnosis in PCC in Spain was 82.4% (inter-regional range from 76.8% to 90.2%).
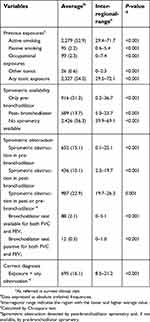 | Table 2 Validation of the initial diagnostic criteria upon diagnosis |
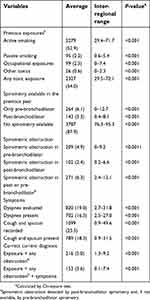 | Table 3 Validation of the diagnostic criteria in the audited visit |
Variables associated with inaccurate diagnosis in the crude bivariate analysis are summarized in
 | Table 4 Multivariable multilevel regression analysis showing factors associated with COPD inaccurate diagnosis |
Discussion
The present study analyzes the inaccurate diagnosis of COPD in primary care medicine in Spain, quantifying the degree of inaccurate diagnosis and evaluating the number of factors at the patient and PCC level that are associated with this inaccurate diagnosis. As a result, variables related to smoking status, lung function evaluation and specific interventions were considered important at the patient level. Additionally, the availability of complementary tests and different aspects of the resources available including the presence of primary care trainees, the availability of a tobacco cessation unit or home nebulized therapy were also associated with an inaccurate diagnosis.
The main strengths of this study include the national coverage and evaluation of the different diagnostic criteria both separately and in a single composite index. However, in order to be able to interpret our results correctly, there are some methodological aspects that should be taken into consideration. First and most importantly, an audit aims to assess clinical performance, not the results thereof. Thus, the focus of the audit is to have an idea of the practitioner’s clinical performance, rather than the clinical situations of the patients. Second, the clinical audit data is collected from the contents of medical records and therefore, undocumented interventions are not evaluated. Accordingly, the estimates presented here may have underestimated the real values. Our results further support the need to record every aspect of clinical practice in the medical record.19 Third, the application and interface to access the data differed depending on the region of the country, which may have influenced the accessibility of the data. Therefore, our first step in the analysis was to evaluate data that was extreme, missing, or inconsistent. This was corrected by the investigators upon request. Fourth, the audit evaluated the medical record at two specific time points, ie, upon diagnosis and during the current visit. It is possible that the visits in between may offer relevant information that has not been considered here.
Although the diagnostic criteria for COPD are quite clear in the current recommendation documents, the adequate diagnosis of this disease is far from being optimal in real life. There are likely several factors that have a complementary influence on this situation. First, the impact of epidemiological studies on the prevalence of the disease may have played an important role. These studies, generally based on population, study the prevalence of COPD by performing spirometry in the population without considering other diagnostic criteria. As a result, prevalence studies often report the prevalence of chronic airflow obstruction rather than COPD, contributing to the confusion.20,21 Secondly, considering the clinical context, the next key element for diagnosis is the performance of spirometry. This spirometry should be done in a stable clinical situation, at rest, and with a bronchodilator test. The performance of spirometry after the bronchodilator test is essential to confirm the diagnosis, not because of the potential broncho-reversibility that may present, but rather because it is spirometry after bronchodilation that establishes the functional diagnostic criterion.22 Unfortunately, the verification of this diagnostic criterion in primary care medicine is far from optimal in Spain. A study conducted in Spain evaluated the availability and frequency of performing spirometry in primary care medicine in Spain,23 revealing that most health centers had a spirometer. However, the frequency of performing spirometries was 5.6 per week with an inter-regional range between 2.0 and 8.8 spirometries per week for the study of airway diseases. Considering the population prevalence of COPD, asthma and bronchiectasis – the three main chronic airway diseases by frequency – it can clearly be concluded that the frequency of spirometry in primary care medicine is insufficient. The present study reflects this situation, since the performance of spirometry was the most lacking diagnostic criterion in our cohort. In this regard, there are some recent initiatives to try to improve lung function evaluation in primary care medicine with different devices beyond formal spirometry such as the Piko-6,24 the COPD-6,25 micro-spirometry26 and the peak-flow meter.27 Additionally, several questionnaires have also been evaluated.28,29 Third, another aspect that may have contributed to the confusion in the diagnosis of the disease is the inclusion of the symptoms.30 Through the 2016 version, the GOLD document was not clearly limited in defining whether symptoms should be a diagnostic criterion in establishing the COPD label. In the 2017 version,2 GOLD changed to include symptoms in the concept and the diagnostic criteria. This new approach is accompanied by another debate regarding which symptoms should be considered indicative of the disease. In the present work, we have selected dyspnea, cough and chronic expectoration because they symptoms most frequently evaluated by the clinician, who is familiar with their measurement. However, the clinical expression of the disease is more extensive and symptoms such as fatigue31 or limitation of daily activities32,33 constitute areas of the disease that must be borne in mind.
A recent clinical audit has evaluated the degree of inaccurate diagnosis in PCCs in Wales.12 These authors evaluated 48,105 patients and found that 13.9% of evaluated cases had compatible post-bronchodilator spirometry. Therefore, by only considering functional criteria, the degree of inaccuracy in this diagnosis was high. Additionally, this figure would likely decrease if documented previous exposure and symptoms were considered diagnostic criteria in the analysis.
Several key aspects at the patient level were associated with inaccurate diagnosis. Active smoking34 and certain features associated with severity, ie, the use of double bronchodilation, lung function evaluation and the use of home-based therapies are likely expected findings. Interestingly, milder COPD was associated with an increased probability of a correct diagnosis, likely because these mild patients need spirometry to be detected.35 The use of long-term oxygen therapy and the availability of computed tomography reflects contact with respiratory clinics, and it is therefore associated with a confirmed diagnosis. Similarly, the association with health-status evaluation may indicate a specific primary care clinic devoted to COPD or feedback from a respiratory clinic, since health status evaluation is relatively uncommon in clinical practice.13 Comorbidities were not associated with inaccurate diagnosis. This suggests that the presence of other comorbid conditions is not associated with an erroneous diagnosis. However, they can be associated with under-diagnosis, since other diseases can also explain respiratory symptoms.36,37
In summary, the present study analyzes the prevalence of inaccurate COPD diagnosis in primary care medicine in Spain. The prevalence data establishes a point of reference in the clinical management of COPD at this level of care. The descriptors of the variables associated with this inaccurate diagnosis can be used to identify cases and centers in which inaccurate diagnosis is occurring considerably. In this way, we can establish targeted interventions that can improve the detection of COPD cases by making health care more efficient.
Acknowledgments
The coordinators of the project are grateful to all the local investigators in each participating PCC for the hard work and implication in gathering data with enthusiasm and dedication. This project has been possible with the collaboration of an unrestricted grant from Boehringer Ingelheim.
Disclosure
Dr Enrique Mascarós Balaguer reports personal fees from Pfizer, personal fees from GSK, personal fees from Novartis, grants and personal fees from GRAP, personal fees from semFYC, personal fees from SEPAR, personal fees from Chiesi, personal fees from AstraZeneca, personal fees from TEVA, personal fees from Live-Med Spain, personal fees from Orion, and personal fees from Bial, outside the submitted work. Dr Jaime Gonzálvez Rey reports personal fees from Boehringer Ingelheim, personal fees from ROVI, personal fees from AstraZeneca, personal fees from GSK, personal fees from GRAP, personal fees from 1aria, personal fees from semFYC, personal fees from SEMG, personal fees from Novartis, personal fees from Fundación Colegio Médico Pontevedra, non-financial support from Separ, grants from IPCRG, grants from ISCIII, and grants from Pfizer, outside the submitted work. Dr Antonio Hidalgo Requena reports personal fees from Esteve, personal fees from AstraZeneca, personal fees from GSK, personal fees from Boehringer Inguelheim, personal fees from Menarini, personal fees from Novartis, personal fees from Pfeizer, personal fees from Mundifarma, personal fees from Teva, and personal fees from Rovi, outside the submitted work. Dr Jose Manuel Helguera Quevedo reports personal fees from GSK, non-financial support from Zambon, personal fees from Mundipharma, and personal fees from Novartis, outside the submitted work. Dr Jose Luis Lopez-Campos reports grants, personal fees, non-financial support from Boehringer, grants, personal fees, non-financial support from Novartis, grants and personal fees from AstraZeneca, grants and personal fees from Chiesi, personal fees from Esteve, personal fees from Ferrer, grants and personal fees from GSK, grants, personal fees and non-financial support from Grifols, grants, personal fees and non-financial support from Menarini, and personal fees and non-financial support from Rovi, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Hangaard S, Helle T, Nielsen C, Hejlesen OK. Causes of misdiagnosis of chronic obstructive pulmonary disease: a systematic scoping review. Respir Med. 2017;129:63–84. doi:10.1016/j.rmed.2017.05.015
2. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Arch Bronconeumol. 2017;53(3):128–149. doi:10.1016/j.arbres.2017.02.001
3. Casas Herrera A, Montes de Oca M, Lopez VMV, et al. COPD underdiagnosis and misdiagnosis in a high-risk primary care population in four Latin American Countries. a key to enhance disease diagnosis: the PUMA study. PLoS One. 2016;11(4):e0152266.
4. Fu SN, Yu WC, Wong CK, Lam MC. Prevalence of undiagnosed airflow obstruction among people with a history of smoking in a primary care setting. Int J Chron Obstruct Pulmon Dis. 2016;11:2391–2399. doi:10.2147/COPD.S106306
5. Llordes M, Jaen A, Almagro P, et al. Prevalence, risk factors and diagnostic accuracy of COPD among smokers in primary care. Copd. 2015;12(4):404–412. doi:10.3109/15412555.2014.974736
6. Soriano JB, Zielinski J, Price D. Screening for and early detection of chronic obstructive pulmonary disease. Lancet. 2009;374(9691):721–732. doi:10.1016/S0140-6736(09)61290-3
7. Putcha N, Drummond MB, Wise RA, Hansel NN. Comorbidities and chronic obstructive pulmonary disease: prevalence, influence on outcomes, and management. Semin Respir Crit Care Med. 2015;36(4):575–591. doi:10.1055/s-0035-1556063
8. Colak Y, Afzal S, Nordestgaard BG, et al. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med. 2017;5(5):426–434. doi:10.1016/S2213-2600(17)30119-4
9. Fernandez-Villar A, Soriano JB, Lopez-Campos JL. Overdiagnosis of COPD: precise definitions and proposals for improvement. Br J Gen Pract. 2017;67(657):183–184. doi:10.3399/bjgp17X690389
10. Fernandez-Villar A, Lopez-Campos JL, Represas Represas C, et al. Factors associated with inadequate diagnosis of COPD: on-sint cohort analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:961–967. doi:10.2147/COPD.S79547
11. Enright P. Patients are hurt by a false diagnosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(2):229. doi:10.1164/rccm.201306-1150OC
12. Fisk M, McMillan V, Brown J, et al. Inaccurate diagnosis of COPD: the Welsh National COPD Audit. Br J Gen Pract. 2019;69(678):e1–e7. doi:10.3399/bjgp18X700385
13. Abad-Arranz M, Moran-Rodriguez A, Mascaros Balaguer E, et al. Community assessment of COPD health care (COACH) study: a clinical audit on primary care performance variability in COPD care. BMC Med Res Methodol. 2018;18(1):68.
14. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi:10.1164/rccm.201204-0596PP
15. Miravitlles M, Soler-Cataluna JJ, Calle M, et al. Spanish guideline for COPD (GesEPOC). Update 2014. Arch Bronconeumol. 2014;50(Suppl 1):1–16. doi:10.1016/S0300-2896(14)70070-5
16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
17. Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. doi:10.1164/rccm.201201-0034OC
18. Merlo J. Multilevel analytical approaches in social epidemiology: measures of health variation compared with traditional measures of association. J Epidemiol Community Health. 2003;57(8):550–552.
19. Lee TM, Tu K, Wing LL, Gershon AS. Identifying individuals with physician-diagnosed chronic obstructive pulmonary disease in primary care electronic medical records: a retrospective chart abstraction study. NPJ Prim Care Respir Med. 2017;27(1):34.
20. Echazarreta AL, Arias SJ, Del Olmo R, et al. Prevalence of COPD in 6 urban clusters in Argentina: the EPOC.AR study. Arch Bronconeumol. 2018;54(5):260–269. doi:10.1016/j.arbres.2017.09.018
21. Santurtun A, Rasilla DF, Riancho L, Zarrabeitia MT. Relationship between chronic obstructive pulmonary disease and air pollutants depending on the origin and trajectory of air masses in the north of Spain. Arch Bronconeumol. 2017;53(11):616–621. doi:10.1016/j.arbres.2017.03.017
22. Miravitlles M, Soler-Cataluna JJ, Calle M, et al. Spanish guidelines for management of chronic obstructive pulmonary disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch Bronconeumol. 2017;53(6):324–335. doi:10.1016/j.arbres.2017.03.018
23. Lopez-Campos JL, Soriano JB, Calle M. Encuesta de Espirometria en Espana P. A comprehensive, national survey of spirometry in Spain: current bottlenecks and future directions in primary and secondary care. Chest. 2013;144(2):601–609. doi:10.1378/chest.12-2690
24. Hidalgo Sierra V, Hernandez Mezquita MA, Palomo Cobos L, et al. Usefulness of the piko-6 portable device for early COPD detection in primary care. Arch Bronconeumol. 2018. doi:10.1016/j.arbr.2018.07.008
25. Kjeldgaard P, Lykkegaard J, Spillemose H, Ulrik CS. Multicenter study of the COPD-6 screening device: feasible for early detection of chronic obstructive pulmonary disease in primary care? Int J Chron Obstruct Pulmon Dis. 2017;12:2323–2331. doi:10.2147/COPD.S136244
26. Schermer TR, Vatsolaki M, Behr R, et al. Point of care microspirometry to facilitate the COPD diagnostic process in primary care: a clustered randomised trial. NPJ Prim Care Respir Med. 2018;28(1):17.
27. Thorat YT, Salvi SS, Kodgule RR. Peak flow meter with a questionnaire and mini-spirometer to help detect asthma and COPD in real-life clinical practice: a cross-sectional study. NPJ Prim Care Respir Med. 2017;27(1):32. doi:10.1038/s41533-017-0036-8
28. Sogbetun F, Eschenbacher WL, Welge JA, Panos RJ. A comparison of five surveys that identify individuals at risk for airflow obstruction and chronic obstructive pulmonary disease. Respir Med. 2016;120:1–9. doi:10.1016/j.rmed.2016.09.010
29. Spyratos D, Haidich AB, Chloros D, et al. Comparison of three screening questionnaires for chronic obstructive pulmonary disease in the primary care. Respiration. 2017;93(2):83–89. doi:10.1159/000453586
30. Izquierdo JL, Miravitlles M, Esquinas C, et al. Characteristics of COPD patients managed in respiratory medicine departments in Spain, according to GOLD groups and GesEPOC clinical phenotypes. Arch Bronconeumol. 2018. doi:10.1016/j.arbr.2018.09.006
31. Kouijzer M, Brusse-Keizer M, Bode C. COPD-related fatigue: impact on daily life and treatment opportunities from the patient‘s perspective. Respir Med. 2018;141:47–51. doi:10.1016/j.rmed.2018.06.011
32. Soler-Cataluna JJ, Puente Maestu L, Roman-Rodriguez M, et al. Creation of the SAQ-COPD questionnaire to determine physical activity in COPD patients in clinical practice. Arch Bronconeumol. 2018. doi:10.1016/j.arbr.2018.07.003
33. Rabinovich RA. Physical activity in COPD. Significance, prognosis, measurement and therapeutic interventions. Arch Bronconeumol. 2018;54(9):449–450. doi:10.1016/j.arbres.2018.01.011
34. Carrion Valero F, Paulos Dos Santos S, Celli BR. Smoking in COPD patients: a new clinical phenotype? Arch Bronconeumol. 2018;54(5):249–250. doi:10.1016/j.arbres.2017.10.021
35. Rossi A, Butorac-Petanjek B, Chilosi M, et al. Chronic obstructive pulmonary disease with mild airflow limitation: current knowledge and proposal for future research – a consensus document from six scientific societies. Int J Chron Obstruct Pulmon Dis. 2017;12:2593–2610. doi:10.2147/COPD.S132236
36. Hanlon P, Nicholl BI, Jani BD, et al. Examining patterns of multimorbidity, polypharmacy and risk of adverse drug reactions in chronic obstructive pulmonary disease: a cross-sectional UK Biobank study. BMJ Open. 2018;8(1):e018404. doi:10.1136/bmjopen-2017-018404
37. Divo MJ, Celli BR, Poblador-Plou B, et al. Chronic Obstructive Pulmonary Disease (COPD) as a disease of early aging: evidence from the EpiChron cohort. PLoS One. 2018;13(2):e0193143.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
