Back to Journals » Cancer Management and Research » Volume 10
Quality of life of young Chinese breast cancer patients after adjuvant chemotherapy
Authors Yeo W, Mo FKF , Pang E, Suen JJS, Koh J , Yip CHW, Yip CCH, Li L , Loong HHF, Liem GS
Received 25 August 2017
Accepted for publication 21 November 2017
Published 22 February 2018 Volume 2018:10 Pages 383—389
DOI https://doi.org/10.2147/CMAR.S149983
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lu-Zhe Sun
Winnie Yeo,1,2 Frankie KF Mo,1,2 Elizabeth Pang,1,2 Joyce JS Suen,1 Jane Koh,1,2 Claudia HW Yip,1 Christopher CH Yip,1 Leung Li,1 Herbert HF Loong,1,2 Giok S Liem1
1Department of Clinical Oncology, Sir YK Pao Centre for Cancer, Prince of Wales Hospital, 2Hong Kong Cancer Institute, State Key Laboratory of Oncology in South China, Faculty of Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong
Introduction: Understanding of quality of life (QoL) of young Chinese breast cancer patients after adjuvant cytotoxic chemotherapy is limited. This study aims to assess the QoL of premenopausal Chinese breast cancer women after receiving adjuvant chemotherapy.
Patients and methods: Eligibility criteria included stage I–III breast cancer, premenopausal and age ≤45 years at cancer diagnosis and having received adjuvant chemotherapy within 3–10 years before entry to the present study. Patients’ background demographics at the time of breast cancer diagnosis, together with tumor characteristics and anticancer treatments, were collected. At the time of study entry, the menopausal status based on menstrual history, body mass index, and QoL (assessed using Functional Assessment of Cancer Therapy-Breast +4) were recorded.
Results: Two hundred and eighty patients were recruited. Ninety-five patients (33.9%) underwent breast-conserving surgery, and nearly all (98.6%) underwent axillary dissection. For adjuvant therapies, 249 patients (88.9%) received anthracycline-containing chemotherapy and 79 (28.2%) received taxane-containing chemotherapy, while 68 (24.3%) received both. One hundred and eighty six patients (66.4%) received adjuvant radiotherapy, and 214 (76.4%) received adjuvant tamoxifen. The median time from breast cancer diagnosis to study entry was 5.01 years. QoL assessment at study entry revealed that older patients had worse social well-being (SWB; mean scores for age ≤40, 41–45, 46–50 and >50 years were 22.0, 19.3, 19.1 and 18.1, respectively, P=0.0442). Patients who underwent axillary dissection had worse scores for breast cancer subscale (BCS; mean score 22.2 vs. 28.3, P=0.0212). Patients who underwent taxane-containing chemotherapy had worse scores for arm subscale (mean score 13.8 vs. 15.3, P=0.0053).
Conclusion: At a median follow-up of 5 years post-diagnosis, patients who were younger had fewer disturbances in their SWB. Patients who had axillary dissection had worse BCS scores, while those who received taxane had worse scores for arm subscale. Further studies are warranted for breast-specific QoL to address the specific issues encountered by breast cancer patients.
Keywords: cytotoxic, QoL, FACT-B+4, cancer survivors breast, premenopausal
Introduction
Breast cancer is one of the most common female malignancies. In Hong Kong, the incidence of breast cancer has been increasing. Based on the most recent data from 2014, among the 4,397 newly diagnosed breast cancer patients, >80% had early-stage disease.1 On the other hand, a recent report on 7,152 patients entered into the Hong Kong Breast Cancer Registry has showed that while over 40% were diagnosed at age 40–49 years, only 1% were diagnosed at age 20–29 years, and another 13% were diagnosed at age 30–39 years.2 Women with early-stage breast cancer are treated with curative intent with surgery followed by adjuvant chemotherapy, radiation therapy and/or endocrine therapy. It has been noted that a higher proportion of younger breast cancer patients were subjected to cytotoxic chemotherapy as part of the adjuvant treatments.2 Anticancer treatment, especially cytotoxic chemotherapy, is associated with immediate- as well as long-term toxicities which may affect the quality of life (QoL) and well-being of cancer survivors.3–8
In our recent report on women aged younger than 45 years who received adjuvant chemotherapy for their breast cancer,9 over 90% developed chemotherapy-related amenorrhea within the first year of chemotherapy; further, nearly 50% developed chemotherapy-related menopause upon protracted follow-up, with over 60% having developed menopause at the age of <45 years as a result of premature ovarian failure. In addition, a significant proportion of these patients were detected to have increased body mass index (BMI), hypertension and dyslipidemia during follow-up.10 Women with cessation of ovarian function encounter physiological and emotional changes that may affect their QoL. Young patients have been reported to have poorer QoL following breast cancer diagnosis when compared with their older counterparts due to the distinct effect on their physical and psychosocial aspects.11–13 As such, QoL among breast cancer patients may be affected by adjuvant cytotoxic chemotherapy. There have been studies assessing long-term QoL after initial breast cancer treatment; however, data on younger breast cancer patients have been limited.11
In this prospective cross-sectional study, young Chinese premenopausal women with early-stage breast cancer who underwent adjuvant chemotherapy were assessed. The objectives were to assess the QoL of these patients during follow-up and to identify potential factors associated with a lower QoL after adjuvant chemotherapy.
Patients and methods
Between September 2008 and February 2011, 286 patients were enrolled into this study. Eligibility criteria included female of Chinese ethnicity, stage I–III breast cancer, premenopausal and age younger than 45 years at breast cancer diagnosis and having received adjuvant chemotherapy within 3–10 years after breast cancer diagnosis before recruitment to the present study. Patients who received ovarian ablation as part of the endocrine therapy and those who had hysterectomy prior to breast cancer diagnosis were excluded from the study. Patients who came for their follow-up visit at the Prince of Wales Hospital provided their written informed consent for the study. After written consent, they were asked to fill in the Functional Assessment of Cancer Therapy-Breast (FACT-B) +4 questionnaire to assess their QoL, and to recall their menstruation history; the latter was conducted with the assistance of a research nurse. Patients’ demographics and details of their breast cancer characteristics and anticancer treatments at diagnosis were recorded. The study was approved by the Joint CUHK-NTEC Clinical Research Ethics Committee of the Chinese University of Hong Kong and Hong Kong Hospital Authority.
QoL assessment using FACT-B+4
FACT-B+4 is a breast cancer-specific multidimensional QoL assessment instrument. FACT-B+4 version consists of 41 items which are divided into six subscales assessing physical well-being (PWB), emotional well-being (EWB), social well-being (SWB), functional well-being (FWB), breast cancer subscale (BCS) and arm subscale. Each item is rated on a 5-point Likert scale. Negatively worded items were recorded such that a higher score indicated a better QoL. Scores from PWB, EWB, SWB, FWB and BCS yielded a FACT-B total score, with higher scores reflecting better QoL.14,15
Statistical analysis
Statistical analysis was performed using SAS version 9.3. Clinical characteristics data were summarized as patient number (n) and percentage (%) for categorical variables, and mean and standard deviation for continuous variables.
The t-test or analysis of variance test for mean comparison was performed to identify any factors associated with impairment in QoL. All statistical tests were two-sided, and a P-value of <0.05 was regarded as significant.
Results
Patients’ background demographics and breast cancer characteristics
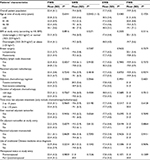
A total of 280 breast cancer patients were eligible and consented to participate in this study. Table 1 shows the patients’ background demographics. Seventeen percent had primary school education, 67% received secondary school education and 16% had tertiary or higher level of education. With regard to marital status, 15.4% were single, 77.1% were married or had partner, 5.4% were either divorced or separated and the remaining 2.1% were widowed. Concerning monthly family income, 9.6% of patients had income less than HK$5,000, 54% had income between HK$5,000 and 25,000, 30% had income between HK$25,000 and 50,000 and 7% had income that exceeded HK$50,000.
The median age at breast cancer diagnosis was 41 (range: 24–45) years; at the time of breast cancer diagnosis, 41 patients were ≤35 years, 82 were aged 36–40 years and 157 were aged 41–45 years. Eighty-eight had stage I, 165 had stage II and 27 had stage III breast cancer. Adjuvant chemotherapy regimens included taxane-containing (28.2%; including 26.4% who also had anthracycline) and non-taxane-containing regimens (71.8%; including 65.7% who had anthracyclines with or without other cytotoxic agents). Two hundred and fourteen patients also received adjuvant tamoxifen; at the time of the study, 99 patients had completed tamoxifen, while 115 were still on the drug. No patient received adjuvant aromatase inhibitors. Table 1 shows the patients’ treatments received at breast cancer diagnosis, as well as age, BMI and menopausal status at study entry. The median time from breast cancer diagnosis to study entry was 5.04 (range: 2.96–9.94) years.
The median age at study entry was 46.5 (range: 28–54) years; eight patients were ≤35 years, 26 were aged 36–40 years, 76 were aged 41–45 years, 146 were aged 46–50 years and 24 were aged >50 years. At study entry, 112 patients remained premenopausal (40.0%), 31 were perimenopausal (11.1%) and 137 (48.9%) were postmenopausal. According to the BMI for Asian population,16 3.9% were underweight, 44.0% were normal weight, 22.1% were overweight and the remaining 30.0% were obese.
Analysis for QoL at study entry
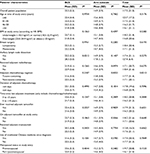
The mean scores and standard deviation for PWB, EWB, SWB and FWB were 23.4 (4.1), 19.4 (6.0), 17.7 (4.2) and 19.9 (5.4), respectively; details are listed in Table 2. Higher scores were attained for PWB, while relatively lower scores were attained for EWB.
With respect to potential factors that could be associated with QoL, older patients had significantly worse SWB score compared to younger patients; the mean SWB scores for age <40, 41–45, 46–50 and >50 years were 22.0, 19.3, 19.1 and 18.1, respectively (P=0.0442). SWB was not associated with BMI, menopausal status, other types of anticancer treatments and use of traditional Chinese medicine.
The mean scores for BCS and arm subscales and FACT-B total score were 22.3 (5.3), 14.9 (3.9) and 102.6 (18.8), respectively; details are listed in Table 3. Internal consistency was checked using Cronbach’s alpha; the alpha coefficient of FACT-B total score was found to be 0.81, reflecting good reliability. On assessing the BCS, patients who had undergone axillary lymph node dissection had significantly worse BCS scores compared to those who did not have the surgery; the mean scores were 22.2 and 28.3, respectively (P=0.0212). Assessment of the arm subscale revealed that patients who had taxane-containing chemotherapy had significantly worse score; the median scores were 13.8 and 15.3, respectively (P=0.0053).
On assessment of PWB, EWB, FWB and the FACT-B total scores, none of the clinical and treatment factors were found to affect these aspects of QoL (Tables 2 and 3).
Discussion
Patients with breast cancer may have their QoL affected to different extent according to their disease status. It has been shown that while patients with metastatic disease suffer mostly from symptom burden and impairment in QoL, women with early-stage disease were not spared.17 Oncologists had previously focused on survival and early detection of cancer recurrence during follow-up visits of breast cancer survivors. With the improvement in breast cancer treatments, patients now have better outcomes with longer survival, and long-term toxicities associated with cancer treatments have become more evident. It is well known that women with breast cancer may have their QoL profoundly affected by the cancer diagnosis per se as well as the associated anticancer treatment in a protracted manner. In a recent study reported by Hamer et al,17 early-stage breast cancer patients who received chemotherapy had more symptoms burden and lower QoL when being assessed 2–10 years posttreatment. Apart from overall QoL scores, PWB, EWB, and FWB were affected. Indeed, the lower QoL may be associated with prolonged fatigue among women after chemotherapy.18
The impact on QoL may differ between different age groups of cancer survivors. For premenopausal patients, endocrine changes may affect fertility and menopausal aspects,19,20 which may adversely affect QoL outcomes. Estrogen reduction as a result of ovarian failure results in a number of common vasomotor symptoms including hot flashes, night sweats, as well as vaginal dryness, dyspareunia and weight changes. Women who become menopausal from treatment have been noted to experience more severe menopausal symptoms compared to those who undergo a natural transition of menopause.19
The present study was undertaken to specifically assess the QoL issues among Chinese young breast cancer patients who were aged <45 years at the time of their breast cancer diagnosis and undergoing adjuvant chemotherapy. Women in this age group represent a unique social situation as they may be at the peak of their career with full-time jobs, may be in more recent marriages and are still active in child rearing. The current data indicate that even in this overall group of young patients, those who were relatively older experienced more impairment in QoL; specifically, they had lower SWB scores. Although other aspects of QoL were not found to be different, menopausal symptoms, which could have affected QoL, were not specifically addressed in this study.
Patients who had undergone axillary lymph node dissection were found to have worse BCS scores than those who did not have axillary dissection. With regard to chemotherapy, it is noted that the majority of patients (nearly 90%) who received taxane-containing as well as non-taxane-containing chemotherapy also received anthracyclines. However, only patients who received taxane-containing chemotherapy were found to have worse scores for the arm subscale than those who did not, which may have been related to the well-known adverse neurological and musculoskeletal effects of this class of agents.
One of the limitations of the present study was that QoL assessment was only conducted using the FACT-B+4 tool. QoL of the studied patient population could have been further assessed by the World Health Organization Quality of Life-BREF questionnaire, and subsequent comparison with FACT-B+4 could have been valuable and would have strengthened the QoL information of the current study. Further, among patients who developed menopause after adjuvant chemotherapy, the potential burden of menopausal symptoms such as hot flashes and dyspareunia were not assessed. Nonetheless, the current study provides a glimpse into the QoL aspects of young Chinese breast cancer patients after adjuvant chemotherapy. Despite potentially curative therapies, the QoL of these women could be impaired by sequelae of cytotoxic chemotherapy as well as other anticancer therapies. Further research into QoL in a larger patient population, along with assessment of menopausal symptoms and other symptom burden, may help clinicians to further understand the true impact on QoL of the patients.
Acknowledgments
This study was supported in part by Hong Kong Cancer Fund and Madam Diana Hon Fun Kong Donation for Cancer Research. The authors thank Rita Ng for her administrative support to this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Hong Kong Cancer Registry, Hospital Authority (2016). Available from: http://www3.ha.org.hk/cancereg/. Accessed December 26, 2016. | ||
Yeo W, Lee HM, Chan A, et al. Risk factors and natural history of breast cancer in younger Chinese women. World J Clin Oncol. 2014;5(5):1097–1106. | ||
Schover LR. Premature ovarian failure and its consequences: vasomotor symptoms, sexuality, and fertility. J Clin Oncol. 2008;26:753–758. | ||
Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE. Breast cancer in younger women: reproductive and late health effects of treatment. J Clin Oncol. 2003;21(22):4184–4193. | ||
Leining MG, Gelber S, Rosenberg R, Przypyszny M, Winer EP, Partridge AH. Menopausal-type symptoms in young breast cancer survivors. Ann Oncol. 2006;17(12):1777–1782. | ||
Avis NE, Crawford S, Manuel J. Quality of life among younger women with breast cancer. J Clin Oncol. 2005;23(15):3322–3330. | ||
Pandey M, Thomas BC, SreeRekha P, et al. Quality of life determinants in women with breast cancer undergoing treatment with curative intent. World J Surg Oncol. 2005;3:63. | ||
Parulekar WR, Day AG, Ottaway JA, et al. Incidence and prognostic impact of amenorrhea during adjuvant therapy in high-risk premenopausal breast cancer: analysis of a National Cancer Institute of Canada Clinical Trials Group Study-NCIC CTG MA.5. J Clin Oncol. 2005;23(25):6002–6008. | ||
Liem GS, Mo FK, Pang E, et al. Chemotherapy-related amenorrhea and menopause in young Chinese breast cancer patients: analysis on incidence, risk factors and serum hormone profiles. PLoS One. 2015;10(10):e0140842. | ||
Yeo W, Mo FKF, Pang E, et al. Profiles of lipids, blood pressure and weight changes among premenopausal Chinese breast cancer patients after adjuvant chemotherapy. BMC Women’s Health. 2017;17(1):55. | ||
Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(5):386–405. | ||
Wenzel LB, Fairclough DL, Brady MJ, et al. Age-related differences in the quality of life of breast carcinoma patients after treatment. Cancer. 1999;86(9):1768–1774. | ||
Kroenke CH, Rosner B, Chen WY, Kawachi I, Colditz GA, Holmes MD. Functional impact of breast cancer by age at diagnosis. J Clin Oncol. 2004;22(10):1849–1856. | ||
Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol. 1997;15(3):974–986. | ||
FACT-G: Functional Assessment of Cancer Therapy–General. Available from: http://www.facit.org/facitorg/questionnaires. Accessed December 26, 2016. | ||
World Health Organization, International Association for the Study of Obesity, International Obesity Task Force. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney: Health Communications; 2000. | ||
Hamer J, McDonald R, Zhang L, et al. Quality of life (QOL) and symptom burden (SB) in patients with breast cancer. Support Care Cancer. 2017;25(2):409–419. | ||
Goedendorp MM, Andrykowski MA, Donovan KA, et al. Prolonged impact of chemotherapy on fatigue in breast cancer survivors: a longitudinal comparison with radiotherapy-treated breast cancer survivors and noncancer controls. Cancer. 2012;118(15):3833–3841. | ||
Rosenberg SM, Partridge AH. Premature menopause in young breast cancer: effects on quality of life and treatment interventions. J Thorac Dis. 2013;5 Suppl 1:S55–S61. | ||
Marino JL, Saunders CM, Emery LI, Green H, Doherty DA, Hickey M. How does adjuvant chemotherapy affect menopausal symptoms, sexual function, and quality of life after breast cancer? Menopause. 2016;23(9):1000–1008. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.