Back to Journals » Journal of Pain Research » Volume 10
Quality of life during early radiotherapy in patients with head and neck cancer and pain
Authors Schaller A , Dragioti E , Liedberg GM, Larsson B
Received 28 March 2017
Accepted for publication 20 June 2017
Published 17 July 2017 Volume 2017:10 Pages 1697—1704
DOI https://doi.org/10.2147/JPR.S138113
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Anne Schaller,1 Elena Dragioti,1 Gunilla M Liedberg,2 Britt Larsson1
1Department of Medical and Health Sciences, Division of Community Medicine, Faculty of Health Sciences, Linköping University, Pain and Rehabilitation Centre, County Council of Östergötland, Linköping, 2Department of Social and Welfare Studies, Linköping University, Norrköping, Sweden
Background: Patients with head and neck cancer (HNC) have a potentially severe diagnosis and often suffer from tumor-related pain as well as from adverse side effects of treatment such as radiotherapy (RT). Knowledge about quality of life (QoL) during early RT in this group is limited and should be assessed in relation to diagnosis and treatment.
Purpose: The purpose of this cross-sectional study was to identify potential factors that may influence QoL in patients with HNC during the early stages of RT (no later than two weeks of ongoing RT). We hypothesized that pain intensity, pain interference, catastrophizing, and mood disturbances are associated with QoL during early RT.
Patients and methods: In this study, 54 patients (53% of eligible patients) diagnosed with HNC were consecutively recruited from the regular flow to the Pain and Rehabilitation Center at Linköping University. The patients completed self-reported questionnaires on sociodemographics, pain intensity, pain interference, anxiety, depression, pain catastrophizing, and QoL.
Results: The patients in this study scored high for QoL, low for pain intensity, and low for pain interference. The patients reported minor depressive symptoms and anxiety symptoms. Regression analyses showed that pain intensity and depressive symptoms negatively influenced QoL.
Conclusion: No later than two weeks of RT, pain intensity and depression negatively influenced QoL in patients with HNC. Early screening for pain and depression in a targeted preventive strategy might maintain QoL during the course of the RT for patients with HNC. This assumption needs to be further investigated.
Keywords: pain, quality of life, head and neck cancer, radiotherapy, cross-sectional study
Introduction
It is well known that patients diagnosed with head and neck cancer (HNC) often suffer from impaired quality of life (QoL).1,2 Patients with HNC who are receiving anticancer treatment experience extensive social consequences and psychological impacts such as anxiety and depression.3,4 As early as the treatment phase, patients with HNC have – a previous research has reported that they experience existential fear of death – a sense of meaninglessness and feelings of guilt.5
Patients with HNC have the highest prevalence of pain among the patients with cancer,6 and a pain prevalence of ~60% at diagnosis and 55% during treatment has been reported.7 Pain in patients with HNC may be related to tumor as well as to side effects of radiotherapy (RT), which is a common method of treatment for HNC.8
A cancer diagnosis in combination with pain negatively affects perceived health, including anxiety and depression.3,9 A study has shown that individuals with newly diagnosed cancer quite suffer from multiple symptoms associated with the disease itself as well as with the treatment.10 Another major concern is pain catastrophizing that magnifies the severity and impact of the pain.11 The occurrence of pain catastrophizing in HNC might increase the fear of treatment failure; moreover, catastrophizing has been positively related to pain and depression during RT.1
According to a concept of cancer pre-rehabilitation, it is important to identify and manage symptoms and impairments in patients recently diagnosed with cancer.12,13 Assessments of an individual’s needs and interventions tailored to these needs from the time between diagnosis and the start of cancer treatment can offer significant physical and psychological relief for patients.12,13 Patients with HNC have a potentially serious disease that might influence QoL even during the early stage of RT.14 At this stage of the disease, QoL might be possible to improve. Therefore, this study aimed to identify the potential factors that may influence the QoL in patients with HNC during the early stage of RT. We hypothesized that pain intensity, pain interference, mood disturbance, and catastrophizing impaired QoL during the early phase of RT, which in this study was no later than two weeks of ongoing RT.
Patients and methods
This cross-sectional study on HNC patients was performed at the Pain and Rehabilitation Centre, Linkoping University Hospital, Linkoping, Sweden.
Participants and study procedure
Patients with HNC referred to the Pain and Rehabilitation Centre, Linkoping University Hospital, because of anticipated impending pain related to RT were invited to participate in the study. Ideally, the patients should have been included in the study before the start of RT. Because Swedish law restricts access to these patients before being referred to the Pain and Rehabilitation Centre, including these patients in our study before the start of RT was not feasible. Therefore, our sample was restricted to patients during their early stage of treatment that is no later than two weeks of ongoing RT.
The recruitment procedure consecutively followed the ordinary flow of patients from January 2015 to August 2016. Inclusion criteria were 18 years of age or older, enrollment for RT with curative intent, and ability to read, write, and understand Swedish. Verbal and written information about the study was delivered to all eligible patients by a trained research nurse in connection to a scheduled RT treatment session. After approximately one week, the presumptive participants were contacted by telephone. If the patient decided to participate, a written consent was signed before inclusion in the study. The patients completed self-reported questionnaires on sociodemographics, pain and psychological symptoms, and QoL. To supplement information on the diagnosis, medical records were reviewed by one of the authors (AS). The study was approved by the regional ethical committee of Linköping University (diary number: 2014/356-31). The Code of Ethics of the World Medical Associations (Declaration of Helsinki) was applied throughout the study.
Questionnaires
A survey questionnaire including five validated patient-reported outcome measurements15–21 was used in this study.
Euro QoL-5 dimensions
The Euro QoL-5 dimension questionnaire (EQ-5D) assesses health outcome and perceived state of health. The questionnaire comprises five items: mobility, self-care, usual activities, pain and discomfort, and anxiety and depression. Each dimension has three levels: no problems, some problems, and extreme problems. The answers are coded on a scale of 1–3. The final individual score was calculated by an algorithm developed for EQ-5D; the EQ-5D score has a range from −0.5 to 1; negative values mean low QoL and 1 means no reduction in QoL. The EQ-5D scores were determined by applying scores from standard population values.
Euro quality of life vertical visual analog scale (EQ VAS)
The EQ VAS measures a respondent’s health on a vertical visual analog line with 100 scale steps with the endpoints labeled “best imaginable health state” and “worst imaginable health state”.
Brief pain inventory (BPI)
BPI measures how pain interferes with daily activities (seven items), and pain intensity (four items) was rated on a 0–10 Likert scale. The scores were summed, and a mean value of the seven pain items of pain interference was calculated. This was also the case for the four pain intensity items.
Hospital anxiety and depression scale
The hospital anxiety and depression scale (HADS) addresses anxiety (seven items) and depression (seven items), both with scores ranging from 0 to 21. Each item uses a four-point Likert scale (ranging from 0 to 3) and the responses are summed. Higher scores indicate likelihood of anxiety or depressive symptoms. A score of ≤7 indicates a non-case, a score of 8–10 indicates a doubtful case, and a score of ≥11 indicates a definite case.
Pain catastrophizing scale (PCS)
PCS is based on 13 items assessing the incidence of catastrophizing in relation to how individuals experience pain. Each item is rated on a five-point scale (0=not at all; 4=all the time). The maximum score is 52; a high score represents a worse situation.
Statistics
IBM Statistical Package for the Social Sciences version 23 was used for statistical analysis. p-value <0.05 was set as the level of significance. Continuous data are presented as mean and standard deviation (SD) and the categorical data are presented as n (%). For comparisons between groups, Student’s t-tests and one-way analysis of variance (ANOVA) were performed. Pearson correlation test was used for bivariate correlation between the dependent variables (EQ-5D and EQ VAS) and independent variables (BPI, PCS, and HADS). Data from these analyses are presented as p-values and correlation coefficients.
Multivariable linear regression models were (furthermore undertaken) also used to investigate the possible associations between the dependent variables and independent variables. These results are presented as unstandardized (regression coefficients with 95% confidence intervals) and standardized regression coefficients (with p-values). Multicollinearity was assessed by examining the Pearson correlation coefficients between the examined variables. Bivariate correlation coefficients ≥0.7 indicate risk of collinearity.
Results
Description of the patients
Of the 102 HNC patients who were invited to participate, 54 (53%) agreed to take part in the study. The only reason reported for declining to participate in the study was poor health. Forty-five of the 54 patients were diagnosed with HNC and informed (in mean 6.7 weeks before inclusion in the study) on the curative intent. These 45 patients were continuously denominated as “newly diagnosed”.
Nine patients out of 54 patients were diagnosed with HNC and informed (in mean 160 weeks before inclusion in the study) on the curative intent. These nine patients were continuously denominated as “non-newly diagnosed”. Of the nine non-newly diagnosed patients, seven patients had undergone surgery once between 2013 and 2015, one patient had undergone surgery in 2001, 2008, and 2015, and one patient had received RT in 2010. The types of HNC are presented in Table 1. The patients completed the questionnaires in a mean of 6 days (SD 3 days) after the start of RT.
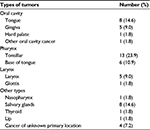  | Table 1 Types of tumors found among the 54 patients |
Sociodemographic data
Sociodemographics of the participants are listed in Table 2. About half the participants were older adults and the majority were men. Most patients cohabitated, and a majority were former smokers or smokers and a minority had a university degree (defined as a high education level in this study).
  | Table 2 Sociodemographic characteristics of the 54 patients |
Description of the dependent and independent variables
The mean values for QoL were high and the mean values for pain intensity and pain interference were low (Table 3). Likewise, low mean values were found for HAD-anxiety, HAD-depression, and pain catastrophizing. Differences in all examined variables with respect to sociodemographic characteristics are also summarized in Table 3. Only HAD-depression (HAD-D) differed significantly in relation to education level (p<0.05).
  | Table 3 Distribution of mean values (±standard deviations) and comparisons among the examined variables in relation to the sociodemographic characteristics of the 54 patients Notes: aStudent’s t-tests were used for the comparisons; bone-way analysis of variance was used for the comparisons. Data presented as mean (SD). Abbreviations: SD, standard deviation; EQ-5D, Euro QoL-5 dimension questionnaire; EQ-VAS, the European quality of life vertical25 visual analog scale; BPI, brief pain inventory; HAD, hospital anxiety and depression scale; PCS, pain catastrophizing scale. |
Regression analyses
Before the regression analysis, multicollinearity was observed for BPI interference and BPI intensity (i.e., bivariate correlation >0.7), and only pain intensity was entered into the regression models because it provides a stronger relationship with the dependent variables (EQ-5D and EQ VAS). In the regression model with EQ-5D as a dependent variable, pain intensity (BPI intensity) and depression (HAD-D) were significant regressors (Table 4). These associations remained significant after adjustments for age, gender, living status, education, smoking habits, and weeks from diagnosis to inclusion in the study (newly diagnosed and non-newly diagnosed). In the regression model with EQ VAS as dependent variable, pain intensity (BPI intensity) and depression (HAD-D) also were significant regressors (Table 5). Likewise, adjustments from the earlier variables did not alter the results. Hence, the regression models showed that pain intensity and depression negatively influenced both dimensions of QoL.
Discussion
This study found that patients diagnosed with HNC and who recently began RT reported preserved QoL, low pain intensity and pain interference, and minor depressive or anxiety symptoms. The results of the regression analysis showed that only pain intensity and depression negatively influenced QoL as measured by the EQ-5D and EQ VAS.
The EQ-5D score was equal to the general Swedish population; the total EQ-VAS was slightly deteriorated.22,23 This inconsistency might be because the EQ-5D covers physical symptomatology such as mobility and psychological symptomatology that are not particularly affected in the early stage of the disease, while the EQ-VAS expresses a more general concept of perceived health. Our patients reported preserved QoL compared to international general populations.24 A global network of scientists reported highest self-expression values, meaning a high level of trust in Sweden.25 Hence, the high QoL obtained in this study might be due to the trust in the Swedish social welfare and health care system.
A study has reported higher QoL in early anticancer therapy compared to the end stage of treatment, including HNC patients,26 and these findings are partly in line with our results. Several studies display results contradictory to ours. Impairment of QoL has been found to be related to worries about the diagnosis and treatment in patients with recently discovered oral cancer.27 QoL has been reported to be impaired at the time of diagnosis as well as one month after diagnosis in patients with HNC.28
It is possible that our patients experienced less average pain and less interference by pain because they were not suffering from the common side effects that often result from RT by the end of the second week.29
Another explanation for the preserved QoL might be the dilemma that patients experienced in admitting the potential severity of cancer diagnosis. According to a review, the prevalence of denial of cancer diagnosis has been found to exist in 47% of patients.30
In addition, the review studied whether denial influenced QoL and concluded that this issue could only be partially answered as denial might be well adaptive in severely ill cancer patients. It has also been shown that patients with cancer minimized negative emotions, attributing them to a normal cancer response to create the impression that the situation was under control and hoping to avoid disease progression, a mechanism that might have been present also in this study.31 The great majority of patients had been informed about the restorative treatment recently, and this may have contributed to the preserved QoL. When patients asked for details on treatment results (this was rare), they were informed explicitly that no guarantees for health recovery could be given.
Internet access among the Swedish population is ~92%,32 and it is easy for patients to discover that the five-year overall survival rate of HNC is about 60%.33 This information and more detailed figures and information are quite easily accessed on the Internet via several national cancer information sites such as the Swedish Cancer Society and the National Cancer Strategy,34,35 two organizations that health care providers often recommend to patients. Searching the Internet for information on cancer is associated with factors such as being a younger female, having a higher income, having a higher level of education, and being married.36 In our study, the great majority of the participants were older men and a minority had a high level of education, so it is likely that these participants did not use the Internet to gain information. This lack of Internet use might contribute to the high QoL during early treatment. However, we do not have any data on such information and we do not know the potential significance of this knowledge.
Many studies have shown that both the EQ-5D and EQ VAS have good validity and reliability in cancer patients37 and this is also the case for the other instruments used in this study.38–40
An advantage of the EQ5D is that it addresses only a few items and provides a few alternative answers, but this instrument fails to address the issues of a specific disease. Other commonly used measures for HNC patients are the European Organization for Research and Treatment of Cancer (EORTC) QoL questionnaire and the University of Washington QoL scale.41,42 The advantages of these instruments are that they can measure disease-specific aspects; however, their usage is limited due to their complicated context, which consequently increases the risk of missing data. Thus, it is difficult to make appropriate comparisons regarding QoL in HNC because of the existence of heterogeneity on measurements. Another possible explanation regarding differences in pain and psychological burden of disease compared to previous research might be the differences in health care systems across studied populations.43–46
Despite the low levels of physical and psychological impairment, we found a statistically significant negative association between QoL, pain intensity, and depressive symptoms. Such negative associations also have been found in previous research.47 Our regression models accounted for 35%–70% of the total variance of QoL, indicating that pain intensity and depression play an important role in the variation of QoL. The other examined variables did not show this association. The pain catastrophizing score was low in our study and not associated with QoL. A complex interplay between pain intensity depression and catastrophizing has been described by Sullivan et al.48 According to our findings, the levels of depressive and catastrophizing symptoms that will probably be impaired in patients with HNC during RT might be related to the predictable increase of pain during the treatment. Our findings indicate that individually tailored pre-rehabilitation programs13 targeting pain and depressive symptomatology delivered during the initial stages of HNC RT treatment might maintain a patient’s QoL.
Our study agrees with previous studies on HNC: HNC is nearly twice as common among men as among women and it is diagnosed most often among individuals over 50 years of age and among smokers/ex-smokers. The representativeness of our sample thus was good regarding these sociodemographics. Limitations of this study include the small sample size and cross-sectional design. The cross-sectional design made it difficult to assess causal relations.
The deteriorated health of non-participants, which led to the possible exclusion of individuals with severe pain, may constitute a selection bias toward an overestimation of QoL. That is, this study probably underestimates pain and psychological symptoms.
Conclusion
In patients with HNC, pain intensity and depression negatively influenced QoL as measured by the EQ-5D and EQ VAS during the early stage of RT. Early screening for pain and depression in a targeted preventive strategy might maintain a good level of QoL during the course of RT for patients with HNC. This assumption needs to be further investigated.
Acknowledgment
The authors state that this work was presented at the Annual Meeting of MASCC/ISOO Washington, DC, USA, June 22–24, 2017.
Disclosure
The authors report no other conflicts of interest in this work.
References
Sawada NO, De Paula JM, Sonobe HM, Zago MMF, Guerrero GP, Nicolussi AC. Depression, fatigue, and health-related quality of life in head and neck cancer patients: a prospective pilot study. Support Care Cancer. 2012;20(11):2705–2711. | ||
Rogers SN, Heseltine N, Flexen J, Winstanley HR, Cole-Hawkins H, Kanatas A. Structured review of papers reporting specific functions in patients with cancer of the head and neck: 2006–2013. Br J Oral Maxillofac Surg. 2016;54(6):e45–e51. | ||
Fischer DJ, Villines D, Kim YO, Epstein JB, Wilkie DJ. Anxiety, depression, and pain: differences by primary cancer. Suppor Care Cancer. 2010;18(7):801–810. | ||
Verdonck-de Leeuw IM, de Bree R, Keizer AL, et al. Computerized prospective screening for high levels of emotional distress in head and neck cancer patients and referral rate to psychosocial care. Oral Oncol. 2009;45(10):e129–e133. | ||
Schaller A, Liedberg GM, Larsson B. How relatives of patients with head and neck cancer experience pain, disease progression and treatment: a qualitative interview study. Eur J Oncol Nurs. 2014;18(4):405–410. | ||
van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437–1449. | ||
van der Molen L, van Rossum MA, Ackerstaff AH, Smeele LE, Rasch CR, Hilgers FJ. Pretreatment organ function in patients with advanced head and neck cancer: clinical outcome measures and patients’ views. BMC Ear, Nose, Throat Disord. 2009;9:10. | ||
Lalla RV, Saunders DP, Peterson DE. Chemotherapy or radiation-induced oral mucositis. Dent Clin North Am. 2014;58(2):341–349. | ||
Kroenke K, Theobald D, Wu J, Loza JK, Carpenter JS, Tu W. The association of depression and pain with health-related quality of life, disability, and health care use in cancer patients. J Pain Symptom Manage. 2010;40(3):327–341. | ||
Cheng KK, Yeung RM. Impact of mood disturbance, sleep disturbance, fatigue and pain among patients receiving cancer therapy. Eur J Cancer Care. 2013;22(1):70–78. | ||
Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother. 2009;9(5):745–758. | ||
Silver JK. Cancer rehabilitation and prehabilitation may reduce disability and early retirement. Cancer. 2014;120(14):2072–2076. | ||
Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. 2013;92(8):715–727. | ||
Haisfield-Wolfe ME, McGuire DB, Soeken K, Geiger-Brown J, De Forge B, Suntharalingam M. Prevalence and correlates of symptoms and uncertainty in illness among head and neck cancer patients receiving definitive radiation with or without chemotherapy. Supportive Care Cancer. 2012;20(8):1885–1893. | ||
EuroQoL Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. | ||
Fayers PM, Machin D. Quality of Life: The Assessment, Analysis and Interpretation of Patient-Reported Outcomes. New Jersey: John Wiley & Sons; 2013. | ||
Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23(2):129–138. | ||
Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277–284. | ||
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. | ||
Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. | ||
Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524–532. | ||
Burstrom K, Johannesson M, Diderichsen F. Swedish population health-related quality of life results using the EQ-5D. Qual Life Res. 2001;10(7):621–635. | ||
Burstrom K, Sun S, Gerdtham UG, et al. Swedish experience-based value sets for EQ-5D health states. Quality Life Res. 2014;23(2):431–442. | ||
Szende A, Janssen B, Cabases J, editors. Self-Reported Population Health: An International Perspective Based on EQ-5D. Berlin: Springer; 2014. | ||
World Values Survey. Online data analysis. Available from: www.worldvaluessurvey.org/WVSOnline.jsp. Accessed June 6, 2017. | ||
Loorents V, Rosell J, Salgado Willner H, Borjeson S. Health-related quality of life up to 1 year after radiotherapy in patients with head and neck cancer (HNC). SpringerPlus. 2016;5(1):669. | ||
Lee HF, Liu HE. Prospective changes of the quality of life for patients newly diagnosed with oral cancer during the acute stage. Eur J Oncol Nurs. 2010;14(4):310–315. | ||
Hammerlid E, Bjordal K, Ahlner-Elmqvist M, et al. A prospective study of quality of life in head and neck cancer patients. Part I: at diagnosis. Laryngoscope. 2001;111(4 Pt 1):669–680. | ||
Epstein JB, Thariat J, Bensadoun RJ, et al. Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin. 2012;62(6):400–422. | ||
Vos MS, de Haes JC. Denial in cancer patients, an explorative review. Psychooncology. 2007;16(1):12–25. | ||
Ryan H, Schofield P, Cockburn J, et al. How to recognize and manage psychological distress in cancer patients. Eur J Cancer Care. 2005;14(1):7–15. | ||
Statistics Sweden. Use of computers and the internet by private persons in 2014. Available from: http://www.scb.se/statistik/_publikationer/le0108_2014a01_br_it01br1402.pdf. Accessed December 2, 2016. | ||
Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371(9625):1695–1709. | ||
The Swedish Cancer Society. Available from: www.cancerfonden.se. Accessed January 3, 2017. | ||
Regionala Cancercentrum. Available from: www.cancercentrum.se. Accessed January 3, 2017. | ||
Waters EA, Wheeler C, Hamilton JG. How are information seeking, scanning, and processing related to bliefs about the roles of genetics and behavior in cancer causation? J Health Commun. 2016;21(Suppl 2):6–15. | ||
Pickard AS, Wilke CT, Lin HW, Lloyd A. Health utilities using the EQ-5D in studies of cancer. PharmacoEconomics. 2007;25(5):365–384. | ||
Wu JS, Beaton D, Smith PM, Hagen NA. Patterns of pain and interference in patients with painful bone metastases: a brief pain inventory validation study. J Pain Symptom Manage. 2010;39(2):230–240. | ||
Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O’Neill E. Factor structure, reliability, and validity of the pain catastrophizing scale. J Behav Med. 1997;20(6):589–605. | ||
Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: a systematic review of assessment instruments. J Natl Cancer Inst. 2009;101(21):1464–1488. | ||
Pfister DG, Ang KK, Brizel DM, et al; National Comprehensive Concer Network. Head and neck cancers. J Natl Compr Cancer Netw. 2011;9(6):596–650. | ||
Rogers SN, Gwanne S, Lowe D, Humphris G, Yueh B, Weymuller EA Jr. The addition of mood and anxiety domains to the University of Washington quality of life scale. Head Neck. 2002;24(6):521–529. | ||
Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893–1907. | ||
Lazenby M, Sebego M, Swart NC, Lopez L, Peterson K. Symptom burden and functional dependencies among cancer patients in Botswana suggest a need for palliative care nursing. Cancer Nurs. 2016;39(1):E29–E38. | ||
Alsirafy SA, Al-Alimi KA, Thabet SM, Al-Nabhi A, Aklan NA. Voluntary reporting to assess symptom burden among Yemeni cancer patients: common symptoms are frequently missed. J Community Support Oncol. 2016;14(3):117–121. | ||
Gandhi AK, Roy S, Thakar A, Sharma A, Mohanti BK. Symptom burden and quality of life in advanced head and neck cancer patients: AIIMS study of 100 patients. Indian J Palliat Care. 2014;20(3):189–193. | ||
Duenas M, Ojeda B, Salazar A, Mico JA, Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res. 2016;9:457–467. | ||
Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17(1):52–64. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


