Back to Journals » Patient Preference and Adherence » Volume 9
Quality of life and type 1 diabetes: a study assessing patients’ perceptions and self-management needs
Authors Alvarado-Martel D , Velasco R, Sánchez-Hernández RM, Carrillo A, Nóvoa FJ, Wägner AM
Received 24 April 2015
Accepted for publication 3 July 2015
Published 14 September 2015 Volume 2015:9 Pages 1315—1323
DOI https://doi.org/10.2147/PPA.S87310
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Johnny Chen
Dácil Alvarado-Martel,1,2 Rebeca Velasco,1 Rosa M Sánchez-Hernández,1,2 Armando Carrillo,1,2 Francisco Javier Nóvoa,1,2 Ana María Wägner1,2
1Servicio de Endocrinología y Nutrición, Complejo Hospitalario Universitario Insular Materno-Infantil de Gran Canaria, 2Instituto Universitario de Investigaciones Biomédicas y Sanitarias, Universidad de Las Palmas de Gran Canaria, Las Palmas de Gran Canaria, Spain
Purpose: The main objective of this study was to assess quality of life (QoL) and treatment satisfaction in a group of patients with type 1 diabetes (T1D) and explore their needs regarding and their perception of QoL living with diabetes.
Materials and methods: Patients with type 1 diabetes attending the outpatient endocrinology clinics of a reference hospital were invited to participate in a cross-sectional study. Clinical and sociodemographic data were obtained (interview and clinical records), and diabetes-related QoL was assessed using a standardized questionnaire. In 67 participants, satisfaction with treatment was also assessed, and an open interview was performed, assessing the impact of diabetes, long-term worries, flexibility, restrictions, and self-perception of QoL. Descriptive statistical analysis, bivariate analysis, and multivariate analysis were performed in order to find factors associated with QoL. Interviews were analyzed and summarized questionwise.
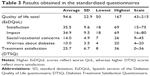
Results: Mean patient age was 31.4±11.6 years, diabetes duration 14.2±9.3 years, and glycated hemoglobin (HbA1c) 8.5%±1.9% (69±20.8 mmol/mol International Federation of Clinical Chemistry [IFCC]). The questionnaires showed good average QoL scores (94.6+22.9) and treatment satisfaction scores (25.7±6.7). QoL worsened with increasing HbA1c, female sex, severity of complications, and lower education (r2=0.283, P<0.005). In the open interview, 68.5% of the patients reported that diabetes had changed their lives, 83.5% identified complications as their most important long-term concern, and 59.7% said that they needed more training to manage the disease.
Conclusion: Poor glycemic control, lower education, complications, and female sex are associated with worse QoL. Semi-structured interviews identified aspects not included in the standardized questionnaires.
Keywords: type 1 diabetes, qualitative, quality of life, self-management, patients’ perceptions
Introduction
Treatment of type 1 diabetes (T1D) consists of multiple insulin injections and a high degree of self-management, in order to prevent complications of the disease. The Diabetes Control and Complications Trial (DCCT) proved that intensive insulin treatment, where the patient plays an active role (multiple insulin injections and blood glucose measurements, carbohydrate quantification), allows patients to achieve better glycemic control and reduces the risk of complications.1 Indeed, since the publication of its results, intensified insulin treatment has become the standard of care in T1D. However, achieving good glycemic control is not easy, and even during the DCCT, patients in the intervention group were at increased risk of severe hypoglycemia and weight gain.1
The Canary Islands have the highest incidence of childhood T1D described in Spain, with 23.2 cases/100,000 persons/year in the 1990s2 and 31.6/100,000 at the start of the present century.3 Furthermore, the high incidence of ketoacidosis4 calls for immediate preventive measures.
Patient behavior will, to a great extent, determine the outcome of diabetes,5 and current care has progressively become more patient-centered. People with T1D have to cope with many factors that affect everyday disease management. The study of quality of life (QoL) in these patients is somewhat different from other populations, since T1D requires a high degree of patient involvement and frequent decision making (frequent glucose monitoring, insulin injection and dose adjustment, carbohydrate estimation, planning of therapeutic adjustments to physical activity, etc). Indeed, an Australian guideline on the assessment of diabetes education programs recommended the inclusion of not only knowledge-based evaluations but also self-management, QoL, and psychological well-being.6
QoL in T1D has been assessed before7–11 mostly in association with the presence of chronic complications,12–14 glycemic control,15–18 and duration of the disease.19 A review of instruments used to measure QoL in diabetes20 drew attention to the excessive simplification of the term QoL, which often included other aspects, such as treatment satisfaction and psychological and health-related well-being. Indeed, several diabetes-specific instruments have been developed:21 Appraisal of Diabetes Scale, Diabetes 39, Audit of Diabetes-Dependent Quality of Life (ADDQoL), Diabetes Quality of Life Measure (DQoL), eDiabetes Health Profile, Diabetes Quality of Life Clinical Trial Questionnaire, Barriers to Physical Activity in Diabetes, Diabetes Obstacles Questionnaire, Diabetes Treatment Satisfaction Questionnaire (DTSQ), Diabetes Treatment Satisfaction Questionnaire for Inpatients, Diabetes Symptom Checklist-Revised, Diabetes Computerized Adaptive Testing, Diabetes Impact Survey, Insulin Treatment Satisfaction Questionnaire, Diabetes Empowerment Scale, and Diabetes Specific Quality of Life Questionnaire and Satisfaction with Oral Anti-Diabetic Agent Scale. However, only two questionnaires with good psychometric properties, specifically evaluating QoL, have been validated in Spain: DQoL and ADDQoL.
The aim of this study was to assess QoL and treatment satisfaction in patients with T1D, as well as to explore their needs, before starting an educational intervention.
Materials and methods
Study design and study population
Patients were consecutively seen in the diabetes outpatient endocrinology clinic at a reference hospital and invited to participate as they arrived to their routine clinical appointments. This was done once a week (the day when a higher number of patients with T1D were expected) between March 2010 and March 2011. They all signed a written, informed consent before entering the study, which had been previously presented to and approved by the CEIC Complejo Hospitalario Universitario Insular-Materno Infantil de Las Palmas Ethics Committee.
A total of 100 patients with T1D (>6-month duration) were individually seen by one investigator (DA-M), who was independent of care provision. All participants completed a diabetes-specific QoL questionnaire, and 67 also completed a treatment satisfaction questionnaire, as well as an open, semi-structured interview. Most of the participants did this while they waited for their scheduled appointment.
Methods
Clinical and sociodemographic information
Clinical and sociodemographic information was obtained by interviewing the patients and by reviewing their clinical records. Data were extracted (RV) and verified (RMS-H) by clinicians who were blind to the interview information. Chronic complications of diabetes were defined following American Diabetes Association criteria.22 In addition, they were classified into mild–moderate or severe, according to the following ad hoc criteria: blindness or significantly reduced sight, and predialysis or dialysis. Cardiovascular risk factors (hypertension, dyslipidemia, smoking, and obesity) were also identified.
Quality of life
QoL was assessed using the Spanish version of the Diabetes Quality of Life questionnaire (EsDQoL).23 It was created for the DCCT, to assess the impact of intensive insulin treatment on lives of people with T1D, by the DCCT Research Group in 1988. It was validated24 and used to evaluate QoL during DCCT and Epidemiology of Diabetes Interventions and Complications.1,14,25 It is one of the most frequently used tools to measure patients’ perception about their QoL and has been translated into and validated in several languages.20 It comprises 43 items in four dimensions: life satisfaction (15 items), diabetes impact (17 items), social/vocational concerns (seven items), and worries about diabetes (four items). Each item can be given 1–5 points on a Likert scale. A lower score reflects better QoL, but there are no validated cut-off points to define poor/good QoL.
Treatment satisfaction
Treatment satisfaction was evaluated using the validated, Spanish version of the DTSQ,26 which comprises eight items that can be scored from 0 to 6. Global satisfaction is calculated by adding the scores of six of the items, and a higher score reflects more satisfaction. The other two items assess the perceived frequency of hypo- and hyperglycemia.
Qualitative interviews
An exploratory interview was performed, to identify important aspects in the QoL of people with T1D. A semi-structured design was chosen, in order to guarantee discussion about areas previously identified as relevant. It consisted of eight questions assessing the impact of diabetes, long-term worries, flexibility (diet and dose adjustment), limitations, and self-perception of QoL (Table 1). To design the questions, expert opinion was considered. This was based on the clinical experience of the involved (AC, JN, AMW) and other endocrinologists, as well as that of the interviewer (DA-M), who had worked for 10 years at the local diabetes association, and spontaneous remarks made by the initial 33 patients.
  | Table 1 Questions included in the open interview |
Analysis
Quantitative analysis (clinical information and standardized questionnaires)
DA-M and AMW analyzed the data. Each participant was given a consecutive number as he/she was included in the study and then registered in the database (I1–I102; two excluded due to diagnosis of type 2 diabetes). Analyses were performed using the software package SPSS 16.0 for Windows (SPSS Inc, Chicago, IL, USA). Quantitative variables are described as mean ± standard deviation (SD) or as median (range), according to their distribution (Kolmogorov–Smirnov), and qualitative variables, as percentages. Bivariate correlations were analyzed (Pearson’s r), and comparisons were made between groups (Student’s t-test or analysis of variance [ANOVA], for two or more group comparisons, respectively). In addition, age and diabetes duration were categorized in quartiles and compared using ANOVA and post hoc multiple comparisons (Bonferroni).
In order to find factors associated with QoL (EsDQoL), a step-by-step, multivariate regression analysis was performed. The variables significantly correlated with EsDQoL in the bivariate analysis (except DTSQ results) were included in the model as independent variables, and the model with the best fit (defined by the highest r2) was identified. A two-tailed P<0.05 was considered significant.
Qualitative analysis (interviews)
Qualitative research was based on the performance of semi-structured interviews, based on specifically designed questions. All interviews were literally transcribed by the interviewer during the conversation. Further reading of the transcripts aimed to identify and group the replies. Finally, results were analyzed, summarized questionwise, and described.
Results
Patient characteristics (100 patients) and results of the questionnaires are shown in Tables 2 and 3, respectively.
  | Table 2 Patients’ characteristics |
When patients were stratified according to sex, women showed worse QoL (102.1±18.2 points vs 88.5±24.6 points, P=0.003), less satisfaction (38.6±7.8 points vs 33.0±10.3 points, P=0.004), more impact (37.8±7.9 points vs 32.7±10.2 points, P=0.008), and social concerns (15.1±4.5 points vs 13.1±5.1 points, P=0.051) associated with diabetes than their male counterparts. In addition, patients with longer education showed better QoL (100.6±26.1 points for primary, 93.5±19.8 for secondary, and 86.3±18.4 for university studies, P=0.005), and those with at least one associated cardiovascular risk factor showed reduced QoL (102.5±24.8 points vs 90.1±20.6 points, P=0.009), as did those receiving psychoactive drugs (113.4±27.8 points vs 92.1±21.3 points, P=0.003) and those suffering from more severe, chronic complications (111.2±38.8 points for severe complications, 96.7±21.4 for mild–moderate complications, and 91.8±19.9 for the absence of complications, P=0.05).
Correlations between total QoL and its subscales and other continuous variables are displayed in Table 4. QoL was worse with increasing glycated hemoglobin (HbA1c) and age and improved with treatment satisfaction.
Age
Age was categorized in quartiles, and QoL scores were compared. Age categories and their mean (SD) EsDQoL scores were as follows: 14–20 years: 32.6 (8.4), 21–31 years: 34.3 (7.4), 32–39 years: 35.4 (2.0), and 40–58 years: 39.9 (9.4). A trend toward a difference was found in the satisfaction subscale (P=0.052), whereas no significant differences were found in the other subscales or in total QoL. In multiple post hoc comparisons (Bonferroni), a significant difference for satisfaction was found between Q1 and Q4 (P=0.047).
Diabetes duration
Diabetes duration was also categorized in quartiles (0–7 years, 8–13 years, 14–20 years, and 21–41 years). No differences in EsDQoL scores were found using ANOVA or post hoc multiple comparisons. Total QoL scores (SD) were as follows: 87 (17.6), 95.6 (24.5), 92.3 (28.5), and 103.6 (22.2) (P=0.078 for ANOVA and P=0.067 for Q1 vs Q4 in post hoc comparisons). No significant differences were found for any of the subscales (data not shown).
No differences were found between men and women regarding treatment satisfaction. Longer education tended to be associated with lower HbA1c (9.0±1.9 vs 8.6±2.0 ± vs 7.7±1.3 for primary, secondary, and university studies, respectively, P=0.053), and patients receiving psychoactive drugs tended to have worse control (9.5±2.9 vs 8.4±1.6, P=0.067).
Multiple regression analysis showed that higher HbA1c, female sex, and severity of complications explain 25.2% of the variance in QoL (Table 5). If level of education was also included in the model, this variance increased to 28.3%. Age, psychoactive drug treatment, and cardiovascular risk factors did not reach statistical significance in multivariate analysis.
Semi-structured interview (67 patients)
Question 1
Having diabetes had changed the lives of 68.5% of the participants. When replying to how it had changed, patients gave replies like the following.
I have a lot of restrictions [Patient code I36, 20 years of diabetes, male]
More worries and limitations [I33, 37 years of diabetes, female]
Having to inject, control food, etc [I38, 6 years of diabetes, female]
Having to program everything in my life, changing my habits [I44, 2 years of diabetes, female]
They are more observant of me [I70, 2 years of diabetes, female]
Question 2
A total of 83.5% of the participants named chronic complications as their main long-term concern. Some of their replies were the following.
Blindness, disability [I42, 23 years of diabetes, female]
That my organs are damaged [I56, 4 years of diabetes, male]
Complications, not being constant with treatment [I64, 10 years of diabetes, female]
That it affects my organs and that my children inherit it [I91, 8 years of diabetes, female]
It worries me to dependent as I am [I37, 41 years of diabetes, male]
Question 3
Regarding treatment, 37.3% identified monitoring and registering glycemia as the most difficult part, and 16.4% said they hated injecting insulin. Some of their statements included the following.
Being observant of [glucose] controls [I42, 23 years of diabetes, female]
Glycemia, having to check so many and writing them down [I44, 2 years of diabetes, female]
I hate injecting; I prefer not to eat, to avoid injections [I48, 20 years of diabetes, female]
I feel ashamed of injecting insulin in a public place [I49, 11 years of diabetes, male]
I hate pricking my finger [I62, 34 years of diabetes, male]
Not having time to dedicate to diabetes [I64, 10 years of diabetes, female]
Diet and, earlier, I was afraid of injecting [I70, 2 years of diabetes, female]
Having to carry a glucose meter, food and insulin with me [I81, 16 years of diabetes, female]
Having to diet, take insulin and do [glucose] controls [I85, 8 years of diabetes, male]
Dieting, not being able to eat everything, although I do it unseen [I91, 8 years of diabetes, female]
Question 4
35.8% felt limited by their diet, and 31.3% reported avoiding insulin doses above those recommended by their endocrinologist. Some replies included the following.
I am almost always on a strict diet [I35, 27 years of diabetes, female]
I feel guilty when I eat things I shouldn’t [I41, 11 years of diabetes, female]
Question 5
Most (64.1%) expressed that they felt happy and satisfied with their diabetes management. Some patients’ replied as follows.
Yes, because the pump has helped me improve [I34, 17 years of diabetes, male]
I had not been to the hospital for 5 years [I98, 20 years of diabetes, male]
I am not satisfied; I should get more involved, but I don’t know how [I96, 13 years of diabetes, female]
Question 6
Regarding the patients’ personal definition of QoL, 26.8% defined it as not having complications, 14.9% as not having diabetes, and 23.8% as being well controlled or having an acceptable HbA1c. Some of the replies were as follows.
Having more flexibility in my life and not having complications [I33, 37 years of diabetes, female]
Living like someone without diabetes [I36, 20 years of diabetes, male]
That diabetes does not interfere with my everyday life [I42, 23 years of diabetes, female]
Having a good HbA1c [I53, 12 years of diabetes, male]
Having diabetes is not having quality of life [I54, 2 years of diabetes, male]
Having a less aggressive treatment [I58, 6 months of diabetes, male]
There is no quality of life with diabetes [I66, 14 years of diabetes, female]
Accepting and managing the disease [I73, 15 years of diabetes, male]
Not having complications [I86, 28 years of diabetes, male]
Being more relaxed, because I feel stressed [I101, 10 years of diabetes, male]
Question 7
More than half (59.7%) of the patients regarded that they needed more training on diet, carbohydrate counting, and insulin dose adjustments.
Yes, on eating and dose adjustment [I33, 37 years of diabetes, female]
Yes, I need to develop some habits [I50, 15 years of diabetes, female]
Yes, adjusting food and insulin [I54, 2 years of diabetes, male]
Working on my attitude is what I need [I91, 8 years of diabetes, female]
Question 8
41.7% said that they had had some limitations during their lives, especially with work.
Yes, I didn’t risk having more children [I35, 27 years of diabetes, female]
Professionally [I36, 20 years of diabetes, male]
Accepting the disease has been very difficult. I have set my own limits [I42, 23 years of diabetes, female]
Yes, professional diving [I45, 5 years of diabetes, male]
Yes, with my driving license [I60, 6 years of diabetes, male]
I was not accepted at a school camp for being different [I74, 5 years of diabetes, female]
The first year at work I hid my diabetes [I49, 11 years of diabetes, male]
Nobody knows about it at work, I don’t tell anyone [I55, 11 years of diabetes, male]
None of my friends knows about it; I hide it [I71, 12 years of diabetes, female]
Discussion
The results of the present study suggest that, in our patients with T1D, HbA1c, severe chronic complications, female sex, and having a shorter education were associated with higher EsDQoL scores, that is, with worse QoL. The open interviews yielded additional, very relevant information and showed a higher degree of concern for glycemic control, eating, and chronic complications.
Our study aimed to identify patients’ worries and needs before the implementation of an education program. The Diabetes, Attitudes, Wishes and Needs 2 study is one of the largest and most ambitious designed to date. It included not only patients but also family members and health care professionals from several countries. Attention was drawn to patient implication, self-management, and psychological support. In Spain, a need for patient and health care professional education was identified.27 In the present study, QoL was assessed using the EsDQoL, one of the two validated tools in Spain and one of the most frequently used tools worldwide. This questionnaire has no validated cut-off points and is interpreted based on mean scores. It is sometimes difficult to compare studies using DQoL, since some authors use an inverse scoring system (higher scores reflecting better QoL). In the present study, we used the original scoring method and found results (total QoL score 94.6±22.9) that were similar to a previous study performed in Spain (92.5±16.15).28 DTSQ scores were also similar to what has been reported.29,30
Previous studies where DQoL was also used showed worse QoL in patients with chronic complications,7,12–14,16,31,32 especially with increasing number and severity.7,12,32 Some have also shown an association between glycemic control and QoL in patients with T1D,13,16,17,32–35 whereas others have not28,36,37 Indeed, HbA1c on its own was not a very strong predictor of QoL in the present study. In agreement with our results, previous work also shows that women have less satisfaction and more impact of diabetes on their lives,23 as well as more worries about diabetes38 and worse well-being,39 and younger people show better QoL.23 Finally, contrary to our findings, other studies using DQoL have shown an effect of duration of the disease on QoL.19,23 The semi-structured interviews in the present study revealed dietary constraints as a relevant concern. In agreement with this, other studies have identified them as one of the main factors determining QoL,40,41 and indeed, a flexible diet is associated with improved QoL.42,43
The present study combines a quantitative and a qualitative approach, which was found to be complementary. The semi-structured interview revealed more concerns for glycemic control, food, insulin injections, and complications than what could be concluded from the EsDQoL. Although EsDQoL is a validated questionnaire with good internal consistence, the patients’ role in the management of diabetes has changed, since it was developed for the DCCT. No cure has been found for the disease, but many aspects of diabetes management have evolved: glucose meters have improved, insulin treatment is more flexible, patients have easier access to information, and therapeutic education is more patient-centered. All of these factors should be taken into consideration when measuring QoL: living with T1D now is very different from what it was 20 years ago.
During the performance of the study, a discordance between what patients spontaneously said and their results according to the standardized questionnaires was detected. Therefore, the open, structured interview was only performed in 67 participants, after the first 33 had been assessed already. Although this represents only two-thirds of the total sample, previous studies including open interviews are smaller and assess between four and 30 participants. Despite the difficulty in quantifying and summarizing the results obtained from this kind of interview, previous, smaller studies identify similar concerns to those described in the present paper.44–48 We recognized everyday needs and worries not detected through the standardized questionnaires.
One of the strengths of the study is sample size, which is relatively large (67 patients) for a study involving qualitative, semi-structured interviews. It provides a precedent for the creation of a new instrument to measure QoL in patients with T1D and to design a therapeutic education program tailored to patients’ needs.49
However, we do acknowledge some limitations of this study. The population need not be representative of all patients with T1D. The endocrinology department at our center treats patients aged 14 and older. Thus, young children and their parents are not represented. Furthermore, people with insufficient knowledge of written and spoken Spanish were not included, either. In addition, although most did, not all patients who were invited to participate accepted, and we did not register the percentage of acceptance. We cannot rule out these facts as potential sources of bias. Finally, the interview was not audio-recorded but literally transcribed. Although the transcriptions allow for independent review of the data, we are aware that certain nuances could have been missed.
Conclusion
Poor glycemic control, lower education, complications, and female sex are associated with worse QoL in our population. Open, semi-structured interviews identified aspects not included in the standardized questionnaires. The results of the present study show the need to investigate further in the QoL of patients with T1D. A new, updated questionnaire should be designed and validated, to include aspects of everyday life with diabetes, and not only negative consequences such as poor glycemic control and complications. In fact, a new instrument has been developed by our group and is now being validated. It includes aspects such as QoL perception, social and family aspects, leisure time, employment limitations, self-management, sexual life, physical activity, complications, physical and psychological well-being, sleep, and disease acceptance, among others. This instrument could potentially be used in the future to assess intervention programs in T1D.
Acknowledgments
DA-M is funded by a predoctoral fellowship (Agencia Canaria de Investigación, Innovación y Sociedad de la Información TESIS20120050). During the performance of the study, the authors have received grants from the European Foundation for the Study of Diabetes (EFSD/JDRF/Novo Nordisk 2008 Programme for type 1 Diabetes) (AMW, JN) and Instituto de Salud Carlos III (PI08/01113 – JN, AMW; PI10/02310 – DA-M, RV, AC, and AMW; PI11/02441 – AMW).
Author contributions
DA-M and AMW conceived and designed the study. RV, AC, FJN, and AMW participated in patient recruitment. DA-M performed the interviews. DA-M, RMS-H, and AMW analyzed the data. DA-M and AMW wrote the paper. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they do not have any competing interests related to the contents of this study.
References
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. | ||
Carrillo Dominguez A. Incidence of type 1 diabetes mellitus in the Canary Islands (1995–1996). Epidemiologic Group of the Canary Society of Endocrinology and Nutrition. Rev Clin Esp. 2000;5: 257–260. | ||
Belinchón Sz-Somoza BM, Hernández Bayo JA, Cabrera Rodríguez R. Incidence of childhood type 1 diabetes (0–14 yrs) in La Palma Island: 1993–2007. Diabetologia. 2008;51(S1):158. | ||
Rodríguez C, García-Núñez M, Marrero D, et al. Factores predisponentes y tratamiento de la cetoacidosis diabética en el área sur de Gran Canaria. Av Diabetol. 2009;25(supl 1):90. | ||
Anderson RM, Donnelly MB, Dedrick RF. Measuring the attitudes of patients towards diabetes and its treatment. Patient Educ Couns. 1990;16:231–245. | ||
Colagiuri R, Eigenmann CA. A national consensus on outcomes and indicators for diabetes patient education. Diabet Med. 2009;26:442–446. | ||
Aalto AM, Uutela A, Aro AR. Health related quality of life among insulin-dependent diabetics: disease-related and psychosocial correlates. Patient Educ Couns. 1997;30:215–225. | ||
Delamater AM, Jacobson AM, Anderson B, et al. Psychosocial therapies in diabetes: report of the Psychosocial Therapies Working Group. Diabetes Care. 2001;24:1286–1292. | ||
Glasgow RE, Toobert DJ, Gillette CD. Psychosocial barriers to diabetes self-management and quality of life. Diabet Spectrum. 2001;14:33–41. | ||
Kanbara S, Taniguchi H, Sakaue M, et al. Social support, self-efficacy and psychological stress responses among outpatients with diabetes in Yogyakarta, Indonesia. Diabet Res Clin Prac. 2008;80:56–62. | ||
Senécal C, Nouwen A, White D. Motivation and dietary self-care in adults with diabetes: are self-efficacy and autonomous self-regulation complementary or competing constructs? Health Psychol. 2000;19:452–457. | ||
Hahl J, Hämäläinen H, Sintonen H, Simell T, Arinen S, Simell O. Health-related quality of life in type 1 diabetes without or with symptoms of long-term complications. Qual Life Res. 2002;11:427–436. | ||
Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev. 1999;15:205–218. | ||
The Diabetes Control and Complications Trial Research Group. Influence of intensive diabetes treatment on quality-of-life outcomes in the diabetes control and complications trial. Diabetes Care. 1996;19: 195–203. | ||
Hoey H, Aanstoot HJ, Chiarelli F, et al. Good metabolic control is associated with better quality of life in 2,101 adolescents with type 1 diabetes. Diabetes Care. 2001;24:1923–1928. | ||
Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Educ Couns. 2001;42:123–131. | ||
Tan SMK, Shafiee Z, Wu LL, Rizal AM, Rey JM. Factors associated with control of type I diabetes in Malaysian adolescents and young adults. Int J Psychiatry Med. 2005;35:123–136. | ||
Wikblad K, Leksell J, Wibell L. Health-related quality of life in relation to metabolic control and late complications in patients with insulin dependent diabetes mellitus. Qual Life Res. 1996;5:123–130. | ||
Sparring V, Nyström L, Wahlström R, Jonsson PM, Ostman J, Burström K. Diabetes duration and health-related quality of life in individuals with onset of diabetes in the age group 15–34 years – a Swedish population-based study using EQ-5D. BMC Public Health. 2013;13:377. | ||
Speight J, Reaney MD, Barnard KD. Not all roads lead to Rome-a review of quality of life measurement in adults with diabetes. Diabet Med. 2009;26:315–327. | ||
Gibbons E, Fitzpatrick R. A Structured Review of Patient-Reported Outcome Measures (PROMs) for Diabetes. Patient-Reported Outcome Measurement Group 2009. Oxford: Department of Public Health University of Oxford; 2009. | ||
American Diabetes Association. Standards of medical care in diabetes-2012. Diabetes Care. 2012;35(suppl 1):S11–S63. | ||
Millán MM, Reviriego J, Del Campo J. Revaluación de la versión española del cuestionario diabetes quality of life (EsDQOL) [Reappraisal of the Spanish version of the diabetes quality of life questionnaire]. Endocrinología y Nutrición. 2002;49:322–324. | ||
The DCCT Research Group. Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT). The DCCT Research Group. Diabetes Care. 1998;11:725–732. | ||
Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. | ||
Ramón Gomis JL, Herrera-Pombo A, Calderón C, Rubio-Terrés P. Validación del cuestionario Diabetes Treatment Satisfaction Questionnaire (DTSQ) en la población española. Pharmacoeconomics. 2006;3:7–18. | ||
Peyrot M, Burns KK, Davies M, et al. Diabetes attitudes wishes and needs 2 (DAWN2): a multinational, multi-stakeholder study of psychosocial issues in diabetes and person-centred diabetes care. Diabetes Res Clin Pract. 2013;99(2):174–184. | ||
Machado A, Anarte MT, Ruiz de Adana MS. Predictores de Calidad de Vida en Pacientes con Diabetes Mellitus Tipo 1. Clínica Y Salud. 2010;21:35–47. | ||
Ashwell SG, Bradley C, Stephens JW, Witthaus E, Home PD. Treatment satisfaction and quality of life with insulin glargine plus insulin lispro compared with NPH insulin plus unmodified human insulin in individuals with type 1 diabetes. Diabetes Care. 2008;31(6):1112–1117. | ||
Witthaus E, Stewart J, Bradley C. Treatment satisfaction and psychological well-being with insulin glargine compared with NPH in patients with Type 1 diabetes. Diabet Med. 2001;18:619–625. | ||
Huang ES, Brown SES, Ewigman BG, Foley EC, Meltzer DO. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care. 2007;30(10):2478–2483. | ||
Jacobson AM, Braffett BH, Cleary PA, Gubitosi-Klug RA, Larkin ME. The long-term effects of type 1 diabetes treatment and complications on health-related quality of life: a 23-year follow-up of the diabetes control and complications/epidemiology of diabetes interventions and complications cohort. Diabetes Care. 2013;36:3131–3138. | ||
Wikby A, Hörnquist JO, Stenström U, Andersson PO. Background factors, long-term complications, quality of life and metabolic control in insulin dependent diabetes. Qual Life Res. 1993;2:281–286. | ||
Guttmann-Bauman I, Flaherty BP, Strugger M, McEvoy RC. Metabolic control and quality-of-life self-assessment in adolescents with IDDM. Diabetes Care. 1998;21:915–918. | ||
Ambler GR, Fairchild J, Crai ME, Cameron FJ. Contemporary Australian outcomes in childhood and adolescence type 1 diabetes: 10 years post the diabetes control and complications trial. J Paediatr Child Health. 2007;43:403–410. | ||
Ingersoll G, Marrero DG. A modified quality of life measure for youths: psychometric properties. Diabetes Educ. 1991;17:114–118. | ||
Grey M, Boland E, Yu C, Sullivan-Bolyai S, Tamborlane WV. Personal and family factors associated with quality of life in adolescents with diabetes. Diabetes Care. 1998;21:909–914. | ||
Trento M, Panero F, Porta M, et al; Piedmont Study Group for Diabetes Epidemiology. Diabetes-specific variables associated with quality of life changes in young diabetic people: the type 1 diabetes Registry of Turin (Italy). Nutr Metab Cardiovasc Dis. 2013;23:1031–1036. | ||
Eiser C, Flynn M, Green E, et al. Quality of life in young adults with type 1 diabetes in relation to demographic and disease variables. Diabet Med. 1992;9:375–378. | ||
Singh H, Bradley C. Quality of life in diabetes. Int J Diabet Develop Countries. 2006;26:7–10. | ||
Bradley C. Measuring quality of life in diabetes. Diabet Annu. 1996;10:207–224. | ||
Study Group DAFNE. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. Br Med J. 2002;325(7367):746. | ||
Rapley P, Axon S, Babel G, et al. Dose adjustment for normal eating: longer term perspectives of adults with type 1 diabetes. J Diabet Mellitus. 2014;04:179–188. | ||
Watts S, O’Hara L, Trigg R. Living with type 1 diabetes: a by-person qualitative exploration. Psychol Health. 2010;25(4):491–506. | ||
Escudero-Carretero MJ, Prieto-Rodríguez M, Fernandez-Fernandez I, March-Cerda JC. Expectations held by type 1 and 2 diabetes mellitus patients and their relatives: the importance of facilitating the health-care process. Health Expect. 2007;10(4):337–349. | ||
Millan MM, del Campo J, Millan MD, Anton S, Reviriego J. Analysis of the experience of diabetes mellitus through case study: an approach to patient’s quality of life. Med Clin (Barc). 2002;114(suppl 3):90–92. | ||
Isla P. Living with diabetes: quality of care and quality of life. Patient Prefer Adherence. 2011;5:65–72. | ||
Schäfer I, Pawels M, Küver C, et al. Strategies for improving participation in diabetes education. A qualitative study. PLoS ONE. 2014;9(4):e95035. | ||
Sánchez RM, López Plasencia Y, et al. Preliminary evaluation of the ANAIS education programme for type 1 diabetes (T1D): a randomised controlled trial. Diabetes. 2014;63(suppl 1):A17. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.