Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Quality of Life and Its Association with Androgenetic Alopecia Patients in Shanghai: A Cross-Sectional Study
Authors Moorthy S, Yu L, Peng L, Shen L, Han Y, Zhang Z, Li Y, Huang X
Received 16 October 2022
Accepted for publication 22 December 2022
Published 28 December 2022 Volume 2022:15 Pages 2883—2893
DOI https://doi.org/10.2147/CCID.S393633
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
SathishKumar Moorthy,1,* Linli Yu,1,* Lin Peng,1 Liangliang Shen,1 Yu Han,1 Zikai Zhang,2 Yanqiao Li,3 Xin Huang1
1Department of Dermatology, Tongji Hospital, School of Medicine, Tongji University, Shanghai, 200065, People’s Republic of China; 2Department of Science, Tongji Hospital, School of Medicine, Tongji University, Shanghai, 200065, People’s Republic of China; 3Department of Dermatology, Institute of Dermatology and Venereology, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, Chengdu, Sichuan, 610072, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xin Huang, Department of Dermatology, Tongji Hospital, School of Medicine, Tongji University, Shanghai, 200065, People’s Republic of China, Email [email protected]
Background: Health-related quality of life (HRQoL) of androgenetic alopecia patients has become increasingly important, but the influencing factors associated with the different domains are poorly understood.
Objective: This study aimed to investigate the influencing factors in HRQoL of androgenetic alopecia patients and identify its strongly associated domains.
Patients and Methods: We enrolled 170 androgenetic alopecia patients. HRQoL was measured using the World Health Organization Quality of Life Brief Version (WHO-BREF), and Hairdex.
Results: HRQoL was significantly impaired in patients < 30 years, (WHO-BREF: P=0.022, Hairdex: P=0.004), less educated (WHO-BREF: P=0.021, Hairdex: P=0.003), single patients (Hairdex: P=0.023), and urban residence (Hairdex: P=0.043). By domains, those < 30 years were impaired by physical health (P=0.038) and psychological (P=0.030) by WHO-BREF, and symptoms (P=0.002) and emotions (P=0.002) by Hairdex. Singles were impaired by symptoms (P=0.020), and emotions (P=0.009) by Hairdex. Less-educated individuals had impaired all domains in the WHO-BREF and Hairdex, except for physical health. Women had impaired symptoms (P=0.013) and stigmatization (P=0.041) in Hairdex.
Conclusion: Androgenetic alopecia is associated with significantly reduced HRQoL in young, less educated, and single patients. Dermatologists should inquire about Quality of Life and appropriately support androgenetic alopecia patients.
Keywords: quality of life, androgenetic alopecia, WHO-BREF, Hairdex
Introduction
In clinical practice, alopecia is considered a relatively mild skin disease by physicians.1 However, many patients regard hair loss as a crucial problem leading to distress in their daily lives, as well as depression, anxiety, and social phobia. Hairs play an important role in an individual’s self-image, self-confidence, and social behavior.2
Androgenetic alopecia (AGA) is a well-known cause of hair loss, affecting both men and women. In AGA excessive activation of androgen receptors leads to follicular miniaturization through a progressively shorter anagen phase which ultimately results into thinner and shorter hair follicles that may not even penetrate through the epidermis.3 The analysis of pathological specimens had showed a decreased 5:0 ratio of anagen to telogen hair from the normal 12:1 ratio.4,5 In men, AGA is accompanied by a bi-temporal recession of the frontal hairline, followed by diffuse thinning at the vertex. AGA causes diffuse thinning in the crown region in women; however, the frontal hairline is often retained.6 The incidence and prevalence of AGA vary with age and ethnicity. Approximately 30% of men experience AGA by age 30 and 50% by 50 years.7 Among women, 12% experience AGA by age 30, and up to 41%, by 70 years.8
Physicians and patients use various criteria to measure the severity of alopecia. Dermatologists use the different pathological markers and symptoms of the disease to assess androgenetic alopecia, while patients focus on their quality of life (QoL) impact. The effect of lifestyle factors has a great influence on the behavioral patterns which contribute to the occurrence and severity of AGA. Thus, there is an inevitable requirement to understand how AGA impacts patients’ quality of life while determining its severity which can be helpful for AGA patients.9,10
Previous studies have reported that AGA can cause psychosocial difficulties, including low self-esteem, altered self-image, and fewer social engagements. In patients with AGA due to continuous disease progression, quality of life (QOL) gets impaired. Therefore, in such circumstances along with traditional treatment, psychosocial management is very important.11–13 However, previous studies have focused on evaluating QOL in only male AGA patients. In order to have a better understanding of the quality of life (QoL) in AGA patients we assessed using the World Health Organization Quality of Life Brief Version (WHOQOL-BREF)14,15 which is a generic instrument and the Hairdex16 Questionnaire which is a Hair and Scalp specific instrument. In this cross-sectional survey, the goal was to evaluate the sociodemographic variants affecting health-related QOL (HRQoL) in AGA patients in Shanghai and investigate the domain factors that influence their HRQoL.
Materials and Methods
Participants
This cross-sectional study was conducted from January 2021 to January 2022 at the Shanghai Tongji Hospital affiliated with Tongji University. This study was conducted as a face-to-face survey. Patients aged 18–60 years and diagnosed with AGA through clinical examination by a dermatologist were included in the study. The stages of hair loss were measured by using the Hamilton-Norwood scale17 (H-N scale) for males and the Sinclair scale18 for females by dermatologists. The severity of the disease was classified as mild (H-N scale Type I–Type II and Sinclair Type I), moderate (H-N scale Type IIa–Type III vertex, Sinclair Type II), or severe (H-N scale Type IV–VII, Sinclair Type III–V). HRQoL was assessed using the World Health Organization Quality of Life Brief Version (WHOQOL-BREF)14,15 and the Hairdex Questionnaire.16 All participants provided informed consent before participation and gave instructions on how to fill in the study questionnaires. Patients with known mental disorders were excluded from this study. The study was approved by the ethics committee of Shanghai Tongji Hospital (ID: K-2022-001).
QoL Instruments
World Health Organization Quality of Life Brief Version (WHOQOL-BREF).
The WHOQOL-BREF (Supplementary 1) is the most popular generic instrument for assessing QoL. The questions were grouped into four domains: psychological, social, environmental, and physical. Individual questions were rated on a five-point Likert scale. Subsequently, the scores were converted to a 0–100 scale. Domain scores are scaled in a positive direction (ie, higher scores denote a higher quality of life).14,15
Hairdex
The Hairdex (Supplementary 2) is a specific health-related QoL measurement tool designed to determine the specific impact of hair loss on patients’ QoL. It consists of 48 items and is divided into five categories: symptoms, functioning, emotion, self-confidence, and stigmatization. Each question is scored on a scale of 0 to 4, with a higher score associated with poorer QOL.16
Analysis
All data analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, U.S.A). The normality of the distribution was verified using the Shapiro–Wilk test. Continuous variables that satisfied normality were presented as the mean ± standard deviation (SD), and those that were not satisfied were presented as medians (IQR). Categorical variables were presented as frequencies (percentages). The box plots are used to describe the distribution of different domains of the Hairdex score and WHO-BREF score among AGA patients.
The t-test and Mann–Whitney U-test were used to compare differences in WHO-BREF, and Hairdex scores according to sex, age group, relationship status, place of birth, and family history. ANOVA and Kruskal–Wallis tests were used to investigate the association between polytomous variables (education level, duration of AGA, and severity of disease) and WHO-BREF, and Hairdex scores. We analyzed their associations with potential risk factors in an identical manner.
Multiple linear regression analysis was performed using WHO-BREF, or Hairdex scores and their sub-domains as the dependent variable, whereas independent variables included sex, age group, relationship status, place of birth, family history, education level, AGA duration, and disease severity. P<0.05 was considered statistically significant, and all P-values were two-tailed.
Results
Demographic and Clinical Characteristics
The study included 170 clinically diagnosed participants with AGA where the response rate was 100%. The majority of participants were men (63.53%), less than 30 years old (66.47%), single (70.59%). The mean age was 28.99 and the standard deviation (SD) was 6.79 with a male: female ratio of 1.7:1. (Table 1)
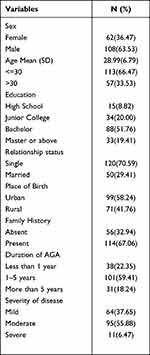 |
Table 1 Patient Social and Demographic Characteristics |
Quality of Life Impairment in AGA
Participants aged < 30 years showed significantly worse HRQoL in the WHO-BREF and Hairdex (P=0.022, P=0.004), and high school qualifications had significantly worse HRQoL impairment in the WHO-BREF and Hairdex (P=0.021, P=0.003). Single individuals showed significantly worse HRQoL in the Hairdex (P=0.023) than married individuals. Furthermore, Hairdex showed that participants born in rural regions had significantly worse HRQoL (P =0.043). (Table 2)
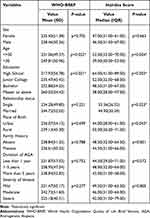 |
Table 2 Variables Associated with Worse Quality of Life in Androgenetic Alopecia Patients |
Among domains, the most impaired domain in WHO-BREF was physical health with a Mean of 53.029, SD 11.99 whereas the least impaired domain was the environment with a Mean of 62.818, SD 15.284. On the Hairdex scale, the most impaired domain was Emotions with a Mean of 13.529 and SD 9.402 and the least impaired domain was Symptoms with a Mean of 5.835 and SD 4.074. (Figure 1)
 |
Figure 1 Domains impact on androgenetic alopecia patients. |
Multivariate Linear Regression Analysis of Factors Influencing Domains in AGA Patients
In the WHO-BREF domain, patients older than 30 years had better psychological QoL (P=0.004). The participant’s masters and above were associated with better QoL in psychological (P=0.000), social relationship (P=0.002), and environment (P=0.000) than that associated with high school. In terms of disease severity, moderate QoL was associated with better psychological (P=0.027) and social relationships (P=0.043) than mild QoL (Table 3).
 |
Table 3 Variables Associated with Domains of World Health Organization Quality of Life Brief Version (WHOQOL-BREF) in Androgenetic Alopecia Patients |
The Hairdex domains showed that men had better QoL in terms of symptoms (P=0.028), worsen QoL in function (P=0.027), and stigmatization (P=0.039) in comparison to women. Patients aged ≥ 30 years had better self-confidence (p = 0.049). In education, masters and above was associated with better QoL in all domains (symptoms: P=0.018, function: P=0.001, emotions: P=0.003, self-confidence: P=0.006, stigmatization: P=0.018) compared to that associated with high school, and patients with a positive family history had better QoL in self-confidence (P=0.002) than patients with a negative family history. (Table 4)
 |
Table 4 Variables Associated with Domains of Hairdex Scale in Androgenetic Alopecia Patients |
Correlation Between Hairdex Score and WHO-BREF Score Among AGA Patients
The observation of correlation between each of Hairdex domains and each of the WHO-BREF domains represented positive correlations between the symptoms and Physical Health (r = −0.3278; P < 0.001); Psychological (r = −0.2664, P = 0.0004); Social relationship (r = −0.4399; P < 0.001); Environment (r = −0.4040; P < 0.001). Functions and Physical Health (r = −0.1712; P =0.0256); Psychological (r = −0.2270, P =0.0029); Social relationship (r = −0.3367; P < 0.001); Environment (r = −0.3105; P < 0.001). Emotions and Physical Health (r = −0.2784; P =0.0002); Psychological (r = −0.3204, P < 0.001); Social relationship (r = −0.4211; P < 0.001); Environment (r = −0.4143; P < 0.001). Self-confidence and Physical Health (r = 0.2366; P =0.0019); Psychological (r = 0.1996, P =0.0091); Social relationship (r = 0.1765; P = 0.0213). Stigmatization and Physical Health (r = −0.2241; P = 0.0033); Psychological (r = −0.2170, P = 0.0045); Social relationship (r = −0.3439; P < 0.001); Environment (r = −0.3138; P < 0.001). However, no correlation was observed between self-confidence and Environment (r = 0.1376; P = 0.0736). (Table 5)
 |
Table 5 Correlation Between Hairdex Score and WHO-BREF Score Among AGA Patients |
Discussion
The purpose of this study was to assess the various factors that have an impact on HRQoL in androgenetic alopecia patients. AGA is considered to be a benign condition with mainly cosmetic effects.1 However, hair loss’s psychological and sociological effects can be considerable. Lower physical attractiveness, low self-esteem, fear of aging, and negative effects on social life are all caused by hair loss.19 The ability to perform everyday tasks according to one’s age and primary social role is referred to as one’s quality of life (QoL).14 Previous studies have reported that in patients with AGA due to continuous disease progression, quality of life (QOL) gets impaired. Therefore, psychosocial management is very important in such circumstances along with traditional treatment.11–13
In AGA QoL assessment has become more and more important, and to assess how the disease has affected people’s quality of life, many different indices that are often measured by self-report questionnaires are used.20 In order to evaluate the impact of androgenetic alopecia on QOL we used a twenty six-item World Health Organization Quality of Life Brief Version (WHOQOL-BREF)14,15 which is a generic instrument and a forty eight item Hairdex16 Questionnaire which is a disease-specific instrument. The advantage of using both generic and disease-specific instruments is that they are sensitive enough to distinguish specific aspects of a disease’s impact on the QOL. Our study showed that androgenetic alopecia is associated with reduced HRQoL in young, less educated, and single patients. The most impacted domain in WHO-BREF was physical health with a mean of 53.01 and in the Hairdex emotion domain with a mean of 13.52 followed by self-confidence with a mean of 12.21. Similarly, a study conducted by Abolfotouh et al also reported that hair follicle disorders significantly impact emotions rather than other domains.21
In modern times appearances are used as a measurement of attractiveness and sexuality for many people, and visible hair loss can have a major negative impact, particularly in some women, on self-perceptions of feminine characteristics and attractiveness.22 Our findings indicated that there was no significant difference between the sexes in QoL. However, Russo et al reported that females with AGA had more impaired QoL than men.23 This might be due to female dominance in their study. At the same time, the multivariate linear regression model proved that women have worsened QoL in terms of symptoms (P=0.013) and men have worsened QoL in stigmatization (P=0.041) of the Hairdex domain.
Our analyses revealed that AGA patients aged < 30 years were more impaired with lower QoL in WHO-BREF with a Mean of 231.06 and Hairdex with a Median of 52.00, specifically with the physical health (P=0.038) and psychological domain (P=0.030) in WHO-BREF, and symptoms (P=0.002) and emotion (P=0.002) domains in Hairdex. This indicates hair loss symptoms impact physical health and create a psychological and emotional negative impact on age <30 years. Additionally, the results confirm with those of recent studies examining QoL in AGA patients, which found that young AGA patients had lower QoL.1,10,11,24,26–28 These findings suggest that older AGA patients might have an improved coping mechanism compared with younger patients with AGA. However, Gonal et al demonstrated that QoL is not affected by patient age. They concluded that cultural and traditional beliefs might influence the perception of hair loss in different communities.25
Education was found to have a positive effect on Quality of life and well-being through income and health.29 Similarly, our analyses determined that less education was associated with a lower QoL in WHO-BREF with a Mean of 217.93 and Hairdex with a Median of 64.00. In the WHO-BREF, three domains (psychological, social relationship, environment) showed statistically impaired QoL in the less-educated patients, and the most impaired domain was the environment (P=0.001). In the Hairdex, all domains (symptoms, function, emotions, self-confidence, stigmatization) showed impaired QoL, and the most impaired domain was function (P=0.001). This indicates that QoL in AGA patients were more influenced by education level and a higher level of education provides better QoL.
The analyses of results revealed that single patients had a highly impaired QoL than married patients on the Hairdex scale with a Median of 53.56. Symptoms and emotions were highly impaired domains in the Hairdex scale. Similar to our results, Elsaie et al reported that single patients with AGA had poor QoL compared to that of married individuals.12 Hence, this proves that being single has a greater influence on QoL by AGA. The reason might be that single patients have more need to socialize and make friends, so their quality of life is more affected than that of married people.
In our study results on the place of birth, there was an adversely affected impact on QoL in rural populations by the Hairdex scale. However, Elsaie et al reported no statistically significant association between urban and rural patients with AGA.12 This may be due to the urban living environment and economic development being better compared to rural regions in China. Furthermore, awareness and knowledge regarding AGA is lacking in rural regions.
The current study found that 67.07% of patients had a positive family history of hair loss, and the results showed that the presence or absence of family history in AGA had no significant difference in the total score of the questionnaires. Similar to our results, Elsaie et al found no significant association between the presence or absence of family history.12 However, our analysis in the linear regression of the Hairdex domain revealed that patients with a positive family history had better self-confidence. This suggests that people with a positive family history of AGA have an early awareness of the disease, so their quality of life is less affected.
Our analysis of the disease duration revealed no statistically significant differences found among groups. Similar to our results, previous studies have shown that the longer the condition of AGA, the greater the impact on QoL. However, no statistically significant difference was found among the groups in any of the studies.1,10,12,25,27 This suggests that duration of AGA has no effect on the QoL.
In our analysis of the disease severity, no statistically significant difference was found in QoL among groups by the total score. Regarding the commonest type/grade of presentation in male patients by Hamilton-Norwood scale17 Type III Vertex is the most common type with 51.85% and in female by Sinclair Sclae18 Type I is the most common type with 66.12%. Similarly, Xu et al study showed in shanghai Type III Vertex as the commonest type of presentation in men and Type 1 as the commonest type of presentation in women.30 However, some studies have shown different results. A study in an Indian population by Sehgal VN et al showed type II and III as the commonest presentation.31 A Chinese study by Wang et al32 which included multiple cities in China showed type IV as the commonest type, while the Korean study by Paik et al33 showed type III as the commonest type. A study of a Turkish population by Salman KE et al showed Type III was found to be the more prevalent type.34
Although this study drew several important conclusions, it had several limitations. First, it was limited by its small sample size. Second, our study was conducted in a single tertiary hospital-based design, which limits the generalizability of our results. Third, all the questionnaires were self-reported. In these cases, reporting biases might have acted as confounding factors.
Conclusion
Our findings demonstrate that there was no significant QoL difference between the gender in AGA. Patients aged less than 30 years, single, and less educated have negatively impacted QoL. As per domains, women have worsened QoL in terms of symptoms and men have worsened QoL in stigmatization. Emotions, symptoms, physical health, and psychological domains were the most impaired domains in age less than 30 years and single patients with AGA. Physical health and self-confidence domains are most significantly affected in those aged above 30 years. Less-educated patients suffer in all domains in Hairdex and WHO-BREF, except for the physical health domain. Dermatologists should inquire about QoL in patients with AGA, identify the factors, provide appropriate support to raise awareness and understanding of AGA in patients, and consider ways to improve patients QoL when discussing treatment options.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with Ethics Guidelines
The present study was conducted in accordance with the World Medical Association Declaration of Helsinki. This study was approved by the Ethics Committee of Tongji Hospital affiliated with Tongji University (ID K-2022-001). Copyright permission is not required to reproduce the Hairdex Questionnaire. All registry participants provided written informed consent and authorization prior to participating.
Acknowledgment
The authors would like to thank the patients who participated in the survey. The authors are grateful to Professor Yongfu Yu and Jiahuan Peng, School of Public Health, Fudan University; Professor Jue Li, School of Medicine, Tongji University, Professor Huamei Yan, Clinical Research Center of Tongji Hospital Affiliated to Tongji University, and Professor Xiuqiang Ma of Naval Military Medical University for their guidance and help in statistical work. SathishKumar Moorthy and Linli Yu are co-first authors for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors acknowledge the financial support received from the National Natural Science Foundation of China (No. 82073452 and No.81772161), Natural Foundation Project of Shanghai Science and Technology Commission (17ZR1426300), Shanghai Tongji Hospital Clinical Research and Cultivation Key Project (ITJ(ZD)1903), Shanghai General Hospital Integrated Traditional Chinese and Western Medicine Special Project (ZHYY-ZXYJHZX-202002), and Shanghai Outstanding Young Medical Talent Training Funding Program.
Disclosure
The authors declare no conflict of interest in this work.
References
1. Zhang M, Zhang N. Quality of life assessment in patients with alopecia areata and androgenetic alopecia in the People’s Republic of China. Patient Prefer Adherence. 2017;11:151. doi:10.2147/PPA.S121218
2. Reid EE, Haley AC, Borovicka JH, et al. Clinical severity does not reliably predict quality of life in women with alopecia areata, telogen effluvium, or androgenic alopecia. J Am Acad Dermatol. 2012;66(3):e97–e102. doi:10.1016/j.jaad.2010.11.042
3. Ho CH, Sood T, Zito PM. Androgenetic Alopecia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022.
4. Bienenfeld A, Azarchi S, Lo Sicco K, Marchbein S, Shapiro J, Nagler AR. Androgens in women: androgen-mediated skin disease and patient evaluation. J Am Acad Dermatol. 2019;80(6):1497–1506. doi:10.1016/j.jaad.2018.08.062
5. Sadick NS, Callender VD, Kircik LH, Kogan S. New insight into the pathophysiology of hair loss trigger a paradigm shift in the treatment approach. J Drugs Dermatol. 2017;16(11):s135–s140.
6. Kanti V, Messenger A, Dobos G, et al. (S3) guideline for the treatment of androgenetic alopecia in women and in men-short version. J Eur Acad Dermatol Venereol. 2018;32(1):11–22. doi:10.1111/jdv.14624
7. Severi G, Sinclair R, Hopper JL, et al. Androgenetic alopecia in men aged 40–69 years: prevalence and risk factors. Br J Dermatol. 2003;149(6):1207–1213. doi:10.1111/j.1365-2133.2003.05565.x
8. Yip L, Zaloumis S, Irwin D, et al. Gene‐wide association study between the aromatase gene (CYP19A1) and female pattern hair loss. Br J Dermatol. 2009;161(2):289–294. doi:10.1111/j.1365-2133.2009.09186.x
9. Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male‐pattern and female‐pattern hair loss, 2017 version. J Dermatol. 2018;45:1031–1043. doi:10.1111/1346-8138.14470
10. Tanaka Y, Aso T, Ono J, Hosoi R, Kaneko T. Androgenetic alopecia treatment in Asian men. J Clin Aesthet Dermatol. 2018;11(7):32.
11. Han SH, Byun JW, Lee WS, et al. Quality of life assessment in male patients with androgenetic alopecia: result of a prospective, multicenter study. Ann Dermatol. 2012;24(3):311–318. doi:10.5021/ad.2012.24.3.311
12. Elsaie LT, Elshahid AR, Hasan HM, Soultan FA, Jafferany M, Elsaie ML. Cross sectional quality of life assessment in patients with androgenetic alopecia. Dermatol Ther. 2020;33(4):e13799. doi:10.1111/dth.13799
13. Sawant N, Chikhalkar S, Mehta V, Ravi M, Madke B, Khopkar U. Androgenetic alopecia: quality-of-life and associated lifestyle patterns. Int J Trichology. 2010;2(2):81. doi:10.4103/0974-7753.77510
14. World Health Organization. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998;28(3):551–558. doi:10.1017/S0033291798006667
15. Bonomi AE, Patrick DL, Bushnell DM, Martin M. Validation of the United States’ version of the world health organization quality of life (WHOQOL) instrument. J Clin Epidemiol. 2000;53(1):1–2. doi:10.1016/s0895-4356(99)00123-7
16. Fischer TW, Schmidt S, Strauss B, et al. Hairdex: a tool for evaluation of disease-specific quality of life in patients with hair diseases [in German]. Hautarzt. 2001;52(3):219–227. doi:10.1007/s001050051293
17. Hamilton JB. Patterned loss of hair in man: types and incidence. Ann N Y Acad Sci. 1951;53(3):708–728. doi:10.1111/j.1749-6632.1951.tb31971.x
18. Sinclair R, Jolley D, Mallari R, Magee J. The reliability of horizontally sectioned scalp biopsies in the diagnosis of chronic diffuse telogen hair loss in women. J Am Acad Dermatol. 2004;51(2):189–199. doi:10.1016/s0190-9622(03)00045-8
19. Katoulis AC, Christodoulou C, Liakou AI, et al. Quality of life and psychosocial impact of scarring and non-scarring alopecia in women. J Dtsch Dermatol Ges. 2015;13:137–142. doi:10.1111/ddg.12548
20. Sampogna F, Tabolli S, Abeni D. Impact of different skin conditions on quality of life. G Ital Dermatol Venereol. 2013;148:255–261.
21. Abolfotouh MA, Al-Khowailed MS, Suliman WE, Al-Turaif DA, Al-Bluwi E, Al-Kahtani HS. Quality of life in patients with skin diseases in central Saudi Arabia. Int J Gen Med. 2012;5:633–642. doi:10.2147/IJGM.S33276
22. Helms RL, O’Hea EL, Corso M. Body image issues in women with breast cancer. Psychol Health Med. 2008;13(3):313–325. doi:10.1080/13548500701405509
23. Russo PM, Fino E, Mancini C, Mazzetti M, Starace M, Piraccini BM. HrQoL in hair loss‐affected patients with alopecia areata, androgenetic alopecia and telogen effluvium: the role of personality traits and psychosocial anxiety. J Eur Acad Dermatol Venereol. 2019;33(3):608–611. doi:10.1111/jdv.15327
24. Gupta S, Goyal I, Mahendra A. Quality of life assessment in patients with androgenetic alopecia. Int J Trichology. 2019;11(4):147. doi:10.4103/ijt.ijt_6_19
25. Gonul M, Cemil BC, Ayvaz HH, Cankurtaran E, Ergin C, Gurel MS. Comparison of quality of life in patients with androgenetic alopecia and alopecia areata. An Bras Dermatol. 2018;93(5):651–658. doi:10.1590/abd1806-4841.20186131
26. Bade R, Bhosle D, Bhagat A, Shaikh H, Sayyed A, Shaikh A. Impact of androgenic alopecia on the quality of life in male subjects: results of an observational study from tertiary care hospital. J Med Sci Clin Res. 2016;4(10):12900–12907.
27. Mubki TF, Dayel SA, AlHargan AH, AlGhamdi KM, AlKhalifah AI. Quality of life and willingness-to-pay in patients with androgenetic alopecia. Egypt J Dermatol Venerol. 2019;39(1):31. doi:10.4103/ejdv.ejdv_33_18
28. Jun M, Keum DI, Lee S, Kim BJ, Lee WS. Quality of life with alopecia areata versus androgenetic alopecia assessed using Hair Specific Skindex-29. Ann Dermatol. 2018;30(3):388–391. doi:10.5021/ad.2018.30.3.388
29. Powdthavee N, Lekfuangfu WN, Wooden M. What’s the good of education on our overall quality of life? A simultaneous equation model of education and life satisfaction for Australia. J Behav Exper Econom. 2015;54:10–21. doi:10.1016/j.socec.2014.11.002
30. Xu F, Sheng -Y-Y, Mu Z-L, et al. Prevalence and types of androgenetic alopecia in Shanghai, China: a community-based study. Br J Dermatol. 2009;160(3):629–632. doi:10.1111/j.1365-2133.2008.08909.x
31. Sehgal VN, Kak R, Aggarwal A, Srivastava G, Rajput P. Malepattern androgenetic alopecia in an Indian context: a perspective study. J Eur Acad Dermatol Venereol. 2007;21:473–479. doi:10.1111/j.1468-3083.2006.01920.x
32. Wang TL, Zhou C, Shen YW, et al. Prevalence of androgenetic alopecia in China: a community-based study in six cities. Br J Dermatol. 2010;162(4):843–847. doi:10.1111/j.1365-2133.2010.09640.x
33. Paik JH, Yoon JB, Sim WY, Kim BS, Kim NI. The prevalence and types of androgenetic alopecia in Korean men and women. Br J Dermatol. 2001;145:95–99. doi:10.1046/j.1365-2133.2001.04289.x
34. Salman KE, Altunay IK, Kucukunal NA, Cerman AA. Frequency, severity and related factors of androgenetic alopecia in dermatology outpatient clinic: hospital-based cross-sectional study in Turkey. An Bras Dermatol. 2017;92(1):35–40. doi:10.1590/abd1806-4841.2017524
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
