Back to Journals » Clinical and Experimental Gastroenterology » Volume 10
Quality of care in inflammatory bowel disease: results of a prospective controlled cohort study in Germany (NETIBD)
Authors Langbrandtner J, Hüppe A, Jessen P, Büning J, Nikolaus S, Raspe H, Bokemeyer B
Received 22 February 2017
Accepted for publication 25 June 2017
Published 4 September 2017 Volume 2017:10 Pages 215—227
DOI https://doi.org/10.2147/CEG.S135346
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Andreas M. Kaiser
Jana Langbrandtner,1,* Angelika Hüppe,1,* Petra Jessen,2 Jürgen Büning,3 Susanna Nikolaus,4 Heiner Raspe,5 Bernd Bokemeyer6
1Institute of Social Medicine and Epidemiology, University of Luebeck, Ratzeburger Allee, Luebeck, 2Gastroenterology Practice Altenholz-Kiel, Erdbeerfeld, Altenholz, 3Department of Internal Medicine I, University Hospital Schleswig-Holstein, Campus Luebeck, Ratzeburger Allee, Luebeck, 4Department of Internal Medicine I, University Hospital Schleswig-Holstein, Campus Kiel, Schittenhelmstraße, Kiel, 5Center for Population Medicine and Health Services Research, University of Luebeck, Ratzeburger Allee, Luebeck, 6Gastroenterology Practice Minden, Uferstraße, Minden, Germany
*These authors contributed equally to this work
Background/aims: Patients with inflammatory bowel disease (IBD) need comprehensive, interdisciplinary and cross-sectoral health care. In Germany, evidence-based care pathways have been developed to improve the quality of care of IBD patients. We aimed to evaluate the effects of the implementation of some of these recommendations on patient-related outcomes.
Methods: In a region of North Germany, outpatients with IBD were recruited by gastroenterologists (intervention group). Three activities based on the recommendations of the IBD pathways were implemented, namely, 1) patient participation in a questionnaire-based assessment of 22 somatic and psychosocial problems combined with individualized care recommendations (patient activation procedure); 2) patient invitation to participate in a 2-day patient education program and 3) invitation to their gastroenterologists to participate in periodic interdisciplinary case conferences. For the control group, IBD patients receiving standard care at gastroenterology practices outside the specified region were recruited by their doctors. At baseline, 6- and 12-month follow-up, study patients were invited to complete questionnaires. Generic health-related quality of life, social participation and self-management skills were the main outcomes.
Results: At baseline, 349 patients were included in the study (intervention group: 189; control group: 160); 142 patients from the former and 140 from the latter group returned completed questionnaires at the 12-month follow-up. Over time, improvement in health-related quality of life and social participation was similar in both groups. Participants of the intervention group demonstrated improved self-management skills and more often followed steroid-free medication regimens.
Conclusion: In a real-world clinical context, patient activation procedure combined with patient education and case conferences was less effective than expected. The observed beneficial effects, however, encourage the evaluation of more intensive and addressee-centered activities.
Keywords: Crohn’s disease, ulcerative colitis, IBD care pathways, quality of care, implementation, evaluation, health services research
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC), the most common forms of inflammatory bowel disease (IBD), are characterized by early onset and often associated with a remitting–relapsing course.1–3 Due to flare-ups and both intestinal and extraintestinal complications, IBD patients experience various somatic and psychosocial impairments that affect their quality of life, including employability, work productivity and social participation.4–8
IBD guidelines recommend comprehensive and problem-oriented care to address the wide spectrum of somatic and psychosocial problems.9,10 Health care professionals struggle with this demanding and time-consuming task.11 Numerous criteria for and approaches to optimizing quality of care have been discussed on the basis of both clinical experience and evidence from clinical and health care research. The European Crohn’s Colitis Organization outlined IBD patients’ need for high-quality health care in seven statements,12 emphasizing provision of patient-centered information and individual education. Health care professionals have pointed out that ideal IBD services should be provided in specialized multidisciplinary institutions.13 Such IBD units are considered to offer the best care for IBD,14,15 especially when they follow current guidelines and standards.
In Germany, most people with health problems and first clinical symptoms of IBD consult a general practitioner (GP) in the first instance (primary care). If IBD is suspected, the GP refers them to a specialist, usually a gastroenterologist in a gastroenterology practice (secondary care). Patients with IBD requiring highly specialized care with more complex treatment regimens including immunosuppressants and/or biologics are treated in tertiary care centers (often affiliated to hospitals or specialized gastroenterology practices).16
The German health care system is quite complex and poorly integrated. For IBD patients, this represents a particularly difficult area to negotiate and get access to appropriate information and treatment. In 2009, based on the results of a multiregional survey,7 clinical considerations, patient and expert interviews and systematic review of available data, evidence-based IBD pathways17 were developed by our group in order to improve the quality of IBD health care in Germany.
The German IBD pathways call for early diagnosis, guideline-based diagnostic and therapeutic procedures, as well as communication and collaboration between physicians and other allied health care professionals. They recommend periodic comprehensive screening for identifying IBD-related physical and psychosocial problem areas, intensified involvement of patients in their own care, patient-tailored education programs in small groups and interdisciplinary IBD case conferences. These recommendations are in agreement with statements from current qualitative initiatives for IBD care in other countries.18–20
In 2010, we conducted a pilot study to gain some experience in implementing one of the central recommendations of the German IBD pathways. A regional network of medical and nonmedical health care providers was set up in North Germany.21 It allowed us to test the practicability and acceptability of a self-administered screening questionnaire on IBD-related physical and psychosocial problem areas combined with a computer-assisted data analysis and written individualized care recommendations (designated “patient activation procedure”).6 Subsequently, a nationwide randomized controlled trial of patients with IBD covered by one specific German statutory health insurance was carried out to test the efficacy of the patient activation procedure. Beneficial effects were seen in patient-reported health-related quality of life, social participation and self-management skills.22
The effects of the patient activation procedure applied in a routine care context have not been evaluated so far. In this study (NETIBD-Study), we investigated the results of implementing this procedure, together with two additional recommendations of the IBD pathways, in IBD outpatients under the care of gastroenterologists.
Materials and methods
We conducted a two-arm prospective cohort study to compare the impact on patient-reported outcomes (PROs) of our activation procedure together with two additional activities recommended by the IBD pathways, on the one hand, with the standard gastroenterological care, on the other. The study included three measurement points (baseline, 6- and 12-month follow-up).
Patient recruitment
Patient enrollment started in May 2013 and lasted about 9 months. IBD patients for the intervention group (IG) were recruited by IBD networks of gastroenterologists working at their own practices or at the university outpatient clinics of tertiary care centers (NETIBD) in a region of North Germany (including the cities of Luebeck and Kiel and the districts Ostholstein, Ploen and Segeberg). Outside this region, gastroenterologists from all over Germany working at the same care level recruited patients for the control group (CG). Outpatients with CD, UC or indeterminate colitis aged at least 18 years and who provided written informed consent to participate in the study were consecutively included.
During initial consultation, eligible patients were given a short study flyer to raise interest in study participation. Patients expressing interest were given informed consent documents with detailed study information and the baseline questionnaire. The attending doctors were requested to fill in a two-page patient record form (PRF; documenting clinical data and current as well as previous medication of patients). Only patients with completed PRF and who had returned completed questionnaires were included in the final analysis.
Identical postal questionnaires were sent to IG and CG participants 6 and 12 months later. Patients who did not return follow-up questionnaires within 4 weeks were sent a single reminder. Gastroenterologists were requested to fill in PRFs at follow-up visits.
No financial compensation was paid to the recruiting physicians of either group.
IG
For improving the quality of IBD care, gastroenterologists of the NETIBD-study agreed upon the implementation of recommendations of the IBD pathways shown in Table 1.
  | Table 1 Activities of the NETIBD to implement recommendations of the IBD pathways Abbreviations: IBD, inflammatory bowel disease; IG, intervention group. |
In implementing the IBD recommendation pathways, our focus was on the “patient activation procedure”. Beside the outcome measures, the study questionnaires comprised questions on 6 somatic and 16 psychosocial IBD-related problem areas. Based on their responses at baseline, all IG patients received written feedback on their personal problem profile, together with individualized recommendations for appropriate health care options.24
IG patients were explicitly encouraged to discuss the written feedback with their gastroenterologists. A detailed description of this questionnaire-based problem assessment has been given previously.22
During the 12-month study period, IG participants were invited to register for a 2-day patient education program developed and offered by the patient organization DCCV e.V. (German Crohn’s Disease/Ulcerative Colitis Association). The aim of this program was to improve patient knowledge about the disease, promote health literacy and enhance self-management skills.25 Sessions for 15–20 patients were offered on five different weekends either at Kiel or Luebeck. Additionally, the NETIBD-gastroenterologists were invited to discuss challenging cases (not only study patients) within an interdisciplinary group led by senior gastroenterologists. At Kiel and Luebeck, regular IBD case conferences take place – weekly at Kiel and once every 4 weeks at Luebeck. Surgeons are always present, whereas radiologists, pathologists, dermatologists, ophthalmologists, rheumatologists and microbiologists are present as needed. As a spin-off of the professional interaction, we expected an increase of guideline adherence and quality of care in the intervention areas.
CG
In contrast to IG, the CG participants were sent written feedback on their personal problem profile together with individually tailored recommendations for health care options (“patient activation procedure”) after completion of the 12-month follow-up assessment. Apart from this, the CG patients received standard care from their doctors.
Outcome measures
As the main outcome indicators, we assessed changes in patient-reported health-related quality of life, restrictions on social participation and self-management skills.
Health-related quality of life was measured by EuroQol (EQ visual analog scale [EQ-VAS]),26 a generic, valid and reliable instrument.26,27 Respondents reported on their current health status on a visual analog scale (VAS) ranging from 0 (the worst possible) to 100 (the best possible) health status. Social participation restrictions were measured by the “Index for Measuring Participation Restriction” (IMET), a German generic and International Classification of Functioning, Disability and Health (ICF)-oriented measurement tool.28–30 Restrictions in nine different areas of life within the past 3 months (self-care, daily duties and responsibilities at and outside home, recreation, social activities, personal relations, sex, nutrition) were assessed by numerical rating scales from 0 (not disabled) to 10 (highly disabled). The scores of the nine items are added to give a total score. In addition, participants were asked to estimate the number of days their disease kept them from carrying out their usual activities (work, education, household) within the past 3 months (0–90 “disability days”).
Self-management skills were assessed with the Health Education Impact Questionnaire (heiQ).31–33 We chose the two scales “Constructive Attitudes” (“When I have health problems, I have a clear understanding of what I need to do to control them”) and “Self-monitoring” (“I know what things can trigger my health problem”). The scales consist of five and six items, respectively, and measure independent constructs on a four-point Likert scale (strongly disagree to strongly agree). Higher values indicate better outcome.
A wide range of secondary outcome variables was assessed by means of the questionnaires filled by the study patients. Disease activity was measured by the symptom-based German Inflammatory Bowel Disease Activity Index (GIBDI). GIBDICD follows the Crohn’s Disease Activity Index (CDAI),34 and GIBDIUC follows the Clinical Activity Index (CAI).35 Values for GIBDIUC and GIBDICD range from 0 to 21. The index is described in detail elsewhere.36
As a measure of “disease complexity”, the number of active IBD-related somatic and psychosocial problems (x out of 22) was assessed.
We asked participants if they contacted and if so, how often, any of 16 different medical specialists (e.g., ophthalmologist, dermatologist) involved in IBD outpatient care. Additionally, participants were asked if they had used the services of 13 specified nonmedical therapists and health care providers (e.g., nutritionist, physiotherapist) at least once in the previous 12 months.
Patient satisfaction with IBD health care was assessed by a numerical rating scale (0=not at all satisfied to 10=very satisfied).
Using PRFs, gastroenterologists documented patients’ medication history and current medications and assessed disease activity using the Harvey Bradshaw Index (HBI) in CD37 and the partial Mayo Score in UC.38
At the 12-month follow-up, we assessed (among IG patients) acceptance of our written feedback recommendations, 2) interest in the education program and 3) participation of gastroenterologists in the case conferences.
Sample size
We calculated the sample size on the ability to detect a small difference of at least six points between treatment groups in health-related quality of life measured by EQ-VAS (based on IBD-data from the study by Stark et al27). We estimated that 136 patients per arm are required to detect a statistically significant difference at the 5% level with at least 80% power. Calculating a 15% dropout rate between baseline and 6- and 12-month follow-up, we aimed at enrolling 188 patients per arm.
Statistical analysis
Dropout analyses were conducted to assess attrition bias. Patient characteristics are presented as numbers with percentages, and mean values with standard deviation (SD). Missing data were not replaced. Differences between groups were tested with independent t-test or chi-square test. For continuous outcomes, we used two-factorial analysis of covariance (group × time) with repeated measurements on the factor time to uncover the main and interaction effects of intervention. We used the parameters showing baseline differences as covariates. To describe differences between IG and CG, adjusted mean values and 95% confidence intervals (CIs) were reported. Effect sizes (ES) were calculated according to Morris,39 based on the mean pre–post change in the IG minus the mean pre–post change in the CG, divided by the pooled pretest standard deviation (ES=dppc2). Significance tests were performed without alpha adjustment; therefore, the results are considered exploratory.40 All analyses were performed using IBM SPSS Statistics 20.
Ethical considerations
The study was approved by the ethics committee of the University at Luebeck (AZ 13–028).
Results
Participation
According to the recruiting gastroenterologists, 611 patients (IG: 339, CG: 272) expressed interest in participating in the study (recruiting period: 05/2013–12/2013) and were given detailed study documents. Out of these, 387 (63.3%) returned the baseline questionnaire to the study center. There were 189 outpatients in the IG and 160 in the CG. Thirty-eight patients (IG: 14, CG: 24) were excluded because they did not fulfill the inclusion criteria (Figure 1); 282 study patients who returned the completed 6- and 12-month follow-up postal questionnaires (IG: 142, CG: 140) were included in the final analyses.
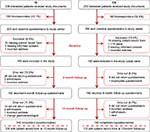  | Figure 1 Participant flow diagram. Abbreviations: CG, control group; IG, intervention group. |
These 282 study patients (IG=142, CG=140) were recruited by 28 gastroenterologists: patients of the IG were recruited by 11 gastroenterologists and 2 tertiary care centers, each enrolling between 1 and 36 patients; of the 140 patients in the CG, 15 gastroenterology treatment centers enrolled between 3 and 35 patients.
Figure 1 presents the detailed recruitment flow chart.
Nonresponder and dropout analysis
Demographic and clinical data were not available of all of the patients who expressed an interest in participation and had been given study documents (n=611). Dropout analyses revealed some small differences between the study completers and dropouts at 6- and 12-month follow-up. In each case, completers had higher levels of education and lower disease activity than dropouts; there were no significant differences in age, gender, employment and disease duration, disease course or diagnosis at baseline (data not shown).
Study participants
Baseline characteristics of the 282 IBD patients (mean age: 43 years; average duration of disease: 13 years; females: 61%; CD: 50%) who returned all three completed questionnaires are summarized in Table 2. We found some significant differences between the IG and CG. Compared to IG patients, CG patients were older (40.8±12.5 vs. 44.8±12.3 years; p=0.006), had a lower level of education (p=0.004) and were treated less frequently with biologics at baseline (p<0.001). The differences in the other characteristics were not statistically significant.
Main outcomes
The most important PROs at the 6- and 12-month follow-up are summarized in Table 3.
No significant interaction effects (group × time) were seen with respect to EQ-VAS, IMET-score and disability days. There was no improvement in health-related quality of life or social participation restrictions in members of the IG. Both IG and CG seemed to improve slightly over time, but without reaching significance. Across all the parameters measured, the CG showed less favorable values than the IG, resulting in significant group effects.
There were significant interaction effects in both the heiQ-scales Self-monitoring and Constructive Attitudes (p=0.013 and p=0.029, respectively). Scores improved over time only in IG members, who reported more constructive attitudes toward their disease and better self-care capabilities, whereas the scores of CG members in these two areas remained nearly unchanged (Figure 2).
The differences between IG and CG in changes from baseline to 12-month follow-up correspond to ES of dppc2=0.28 (Self-monitoring) and 0.29 (Constructive Attitudes), respectively.
Secondary outcomes
The results of the patient-reported secondary outcomes are summarized in Table 4.
Disease burden
During the course of the study, both groups reported a slight reduction in disease activity and mean number of active somatic and psychosocial problems. No beneficial effects of intervention were seen.
Health care utilization
At the 12-month follow-up, there was no difference between the study groups in the self-reported total number of contacts with the specified 16 medical specialists or in the utilization of the services of allied health professionals (e.g., physiotherapy, nutritional consultations), as shown in Table 4. In both study groups, satisfaction with IBD health care remained unchanged at a high level of eight points (numerical rating scale [NRS]: 0–10; 10=very satisfied).
Table 5 summarizes the physician-reported secondary outcomes, namely, disease activity and medication. Completed PRFs were not available for all of the study participants at the 12-month follow-up. Only those participants with completed PRFs and who had returned the completed questionnaires at the 12-month follow-up were included in the following analyses (IG: 128; CG: 112). Over 12 months, no significant changes in physician-reported disease activity could be observed in either study group (Table 5).
At baseline as well as 12 months later, immunosuppressants were more frequently used by the CG than the IG, whereas biologics were more frequently used by the IG than the CG. The latter difference was more pronounced at the 12-month follow-up (Table 5).
At baseline, the prevalence of steroid use was nearly identical in both groups. One year later, gastroenterologists reported a significantly lower frequency of patients under steroids in the IG (8%) than in the CG (20%; p=0.009), with a reduction of about 9% in IG and about 2% in CG. Figure 3 shows the frequency of steroid use at baseline and 12-month follow-up, together with the changes between the two measurements (Figure 3).
Acceptance among patients and physicians
Patient activation procedure
Of the 142 IG patients, 16% reported the procedure as being excellent, 50% as good, 20% as satisfactory, 2% as fair and 2% as poor (10% missing values). On average, the helpfulness of feedback recommendations was rated with 6/10 points (0: not helpful at all, 10: very helpful). Treatment recommendations were given to 104 of 142 IG patients for at least one active problem; 48% (50/104) reported that they followed at least one recommendation in the past 12 months and 22% (23/104) reported that they discussed the results of the assessment questionnaire with their physicians.
Patient education program
Of the 189 invited IG patients, 33 expressed interest in the weekend education program. Adequate number of patients signed up (required minimum number of participants was 15) for only one of the five weekend programs offered, with 20 IG patients participating. These were satisfied with the program and assigned it four points (on a scale from 1=no benefit to 5=high benefit).
IBD case conferences
At the 12-month follow-up, 9 of the 13 IG gastroenterologists reported participating in at least one IBD case conference in the last 6 months. On a five-point Likert scale ranging from 1 (strongly agree) to 5 (strongly disagree). They strongly agree with the statement that IBD case conferences are helpful for their work. All of them expressed their intention to attend future IBD case conferences and found them useful enough that they would recommend these to their colleagues.
Discussion
We have previously carried out a randomized controlled trial (RCT) on the effectiveness of the patient activation procedure on IBD patients recruited via a statutory health insurance. The study showed the procedure to be effective and beneficial in terms of improving self-reported health-related quality of life, social participation and self-management skills. All observed ES were small, with values between 0.2 and 0.3.22 The aim of this two-arm, parallel, observational, cohort study was to explore its effects (combined with two additional elements and compared to usual IBD care) within the context of routine gastroenterological care. We see this study as a “pragmatic” doable substitute for a much more time- and resource-intensive (cluster) randomized study and is thus not redundant.
The core of our complex intervention was twofold: 1) a screening questionnaire on a range of patients’ physical and psychosocial problems, to be filled in by the patients and 2) based on the completed questionnaire, written tailored feedback to patients comprising specific health care recommendations. The aim of this procedure was to enhance patients’ self-responsibility and action in planning and accessing appropriate health services, especially for psychosocial problems.
In this study, we were not able to replicate our previous findings on quality of life and social participation. Both (by and large comparable) cohorts showed similar, but small improvements in these variables after adjusting for the markedly different medication regimens and other variables (age and level of education). Whereas the positive changes in the IG are in the range of what we observed in the earlier study (which was an RCT22), the improvements in the CG in this study were much larger than what we expected to find.
This may be due to a particularly active, competent and/or competitive group of gastroenterologists attending to CG patients. Gastroenterologists recruiting patients for the CG might have felt a need to compete with those recruiting for the IG. However, we have no evidence to support this suggestion.
In contrast to the PROs on quality of life and social participation, we were able to replicate our previous findings on self-management skills. IG participants reported more constructive attitudes toward their chronic disease and a greater ability to monitor it. These observed positive effects were statistically significant, but again rather small with an ES of just below 0.3.
This seems to be at least a promising result in the proclaimed “age of self-management”,41,42 where numerous self-management interventions have been developed, with heterogeneous and, where positive, generally weak effects.42–45 The newly developed and recently evaluated “German psychoeducational self-management program” for IBD may serve as an example.23,25 As far as hospitalized rehabilitation patients are concerned, no significant intergroup differences were seen in the primary and secondary outcomes including the heiQ-scale “Constructive Attitudes and Approaches”.25 In outpatients, the same self-management program proved superior compared to an untreated CG at 3-month follow-up with an ES of day=0.17 for the same scale.23
At the 12-month follow-up, we did not see significant beneficial effects in the secondary outcomes, “disease burden” or “health care utilization”. The only exception to the general pattern was the physician-reported steroid use: based on the available PRFs, we observed a reduction of steroid prescriptions to IG patients (from about 18% to 8%) with no relevant changes among CG patients (from about 22% to 20%) as an indirect sign of an optimized medication regimen in keeping with the current guidelines (Figure 3). Whether the bundling of medical expertise in the case conferences resulted in implementation of current best evidence-based recommendations is a matter of speculation. Networking of caregivers for IBD patients has been reported to optimize medical treatment.46
Given the mainly “negative” result in the primary objectives of the study, could it be a “false-negative” result?
We do not believe this to be the case, first because of the consistent pattern in primary and secondary PROs, and second, this result was seen despite the slightly less favorable baseline values in most of the patients of the CG. Furthermore, there is no indication for ceiling effects in IG. Such an effect would suggest that a high proportion of participants have maximum scores in the observed primary outcomes at baseline so that they can hardly score any higher at follow-ups. In this study, health-related quality of life and social participation of IG and CG at baseline were not better than those seen in other German IBD study samples.22,47 In addition, positive changes seen in this study were comparable in the range of what we observed in the RCT.22 Both observations make a ceiling effect unlikely.
In principle, the occurrence of a Type II error (failing to detect an effect that is present) cannot be excluded. In his book Complementary Methodology for Clinical Research,48 Kiene discusses 16 factors potentially facilitating false-negative results. One is “contamination”, and this might be relevant for our study. We cannot exclude that our own work contributed to the quality of care in the CG by our publication of care pathways in 2009, by providing both IG and CG gastroenterologists with the same well-accepted PRF (two out of three gastroenterologists would recommend it to their colleagues) and by distributing patient questionnaires via the gastroenterologists. It may have led to more structured and comprehensive treatment in both groups and, thus, to similar clinical and PROs.
What remains is the effect of our complex intervention on two attitudes relevant for successful coping. Whereas the baseline values of the two main outcomes were practically identical in both groups and directly comparable to what we saw in our RCT22 with self-monitoring (13.4 in IG/13.1 in CG) and constructive attitudes (11.8 in IG/11.5 in CG), again only the IG showed comparable-sized, statistically significant effects as were seen in the RCT.22
Even though not much is known about the association between self-management skills and health-related quality of life or social participation,49 different stakeholder groups (patients, family, health care professionals) have identified self-management skills as an important generic outcome.50
Limitations of the study
In interpreting the findings of this study, some limitations need to be mentioned.
Design limitations
There was a control condition, but participants were not randomly assigned to the two treatment groups; so, uncontrolled or uncontrollable confusion by the authors may have implications. Differences between IG and CG in age, level of education, medication and several outcome variables were seen at baseline (and were statistically adjusted for). Other unmeasured confounding factors may have been present.
Database
Our main effect parameters are PROs. This may, from a clinical point of view, seem insufficient (though necessary according to the current standards). Further studies may, if possible, include more objective measures such as the results of more or less specific laboratory tests, ultrasound or endoscopy.
Representativeness
IG and CG patient recruitment was only carried out through gastroenterologists practicing in outpatient settings, mostly in gastroenterology practices. In contrast to the CG, the practices recruiting the IG were all in one German region; 40 out of 142 IG patients were recruited via two tertiary outpatient clinics. This may affect the external validity of our results. Our sample with 66% patients in actual remission is, however, well comparable with other German samples of IBD outpatients treated in gastroenterological practices (e.g., Blumenstein et al51 reported 67% and Bokemeyer et al52 reported 61% patients in remission).
Our sampling roster/recruitment basis carries the risk of underestimating the actual role of GPs in the care of IBD patients. Only 6% of our study patients mentioned a GP as their main source of care. In two earlier studies, this proportion was 40%, reflecting the relevant role of primary care in both the initial work-up and long-term care.22,53
Limited acceptance
Not all implemented activities of the NETIBD had the same level of acceptance. The five educational classes offered were found to be less interesting than expected, therefore only one was actually conducted. The 2-day weekend training in small groups, now offered by the patient organization DCCV e.V. all across Germany, is in high demand and has long waiting lists. We suspect that the reason for the low level of interest shown by the IG in the free and local training offered might be that, at the baseline, only 10% of the participants expressed a need for more information. Thus, well-informed patients may find a 2-day training course as being much too long. This assessment was also expressed by the participating physicians in their feedback on the study. Also, the case conferences were attended only by two-thirds of the IG physicians.
Strengths of the study
Evaluation research on approaches and strategies targeting improved quality of IBD care is rare and frequently amounts to nothing more than one pre- and one post-intervention measurement without any CG. More evidence-based studies are needed.12–15 The NETIBD study included a comparison group and condition, as well as parallel measurements of relevant variables at baseline and after 6 and 12 months. It took place in (self)selected, nevertheless ordinary gastroenterological practices. Our study, thus, demonstrates the feasibility of descriptive and analytical health services research embedded in routine care of patients.
Conclusion
The implementation of some of the recommendations of the IBD pathways to improve the quality of care of IBD patients treated in gastroenterology practices faced obstacles from doctors and patients alike. Educational classes (for patients) and case conferences (for doctors) found less acceptance than expected. Nevertheless, there should be continued offer of these classes, one reason being the observed tendency toward optimizing IBD therapy resulting from it.
In contrast, there was greater acceptance among physicians to recruit patients and distribute questionnaires and greater readiness among patients to complete and return the questionnaires; these were analyzed by an automated procedure in our institute, and patients were sent individualized written responses. Thus, this approach to improve IBD care was successful.
Overall, the study results are rather “negative” than “positive”; they do not show a positive effect of our three-pronged intervention on the clinically relevant PROs, health-related quality of life and social participation. Favorable but small effects were seen on two variables characterizing beneficial self-management skills. We consider this to be a result mainly of our well-accepted screening questionnaire combined with written individualized health care recommendations. An electronic version of the screening questionnaire is meanwhile available as open access with a printable automated analysis and feedback.54
Acknowledgments
We thank all patients and recruiting gastroenterologists participating in our research. This study was supported by the “Association of German Outpatient Gastroenterologists (bng)”.
Disclosure
The authors report no conflicts of interest in this work.
References
Ott C, Obermeier F, Thieler S, et al. The incidence of inflammatory bowel disease in a rural region of Southern Germany: a prospective population-based study. Eur J Gastroenterol Hepatol. 2008;20(9):917–923. | ||
Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380(9853):1590–1605. | ||
Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380(9853):1606–1619. | ||
Burisch J, Jess T, Martinato M, Lakatos PL; ECCO-Epicom. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7(4):322–337. | ||
Kemp K, Griffiths J, Lovell K. Understanding the health and social care needs of people living with IBD: a meta-synthesis of the evidence. World J Gastroenterol. 2012;18(43):6240–6249. | ||
Hüppe A, Langbrandtner J, Raspe H. Komplexe psychosoziale problemlagen bei morbus crohn und colitis ulcerosa – fragebogengestütztes assessment als erster schritt zur aktivierung von patientinnen und patienten. Z Gastroenterol. 2013;51(3):257–270. | ||
Hardt J, Muche-Borowski C, Conrad S, Balzer K, Bokemeyer B, Raspe H. Chronisch entzündliche darmerkrankungen als multifokale erkrankungen: körperliche und psychosoziale probleme von patienten mit CED. Ergebnisse eines Fragebogen-Surveys. Z Gastroenterol. 2010;48(3):381–391. | ||
Lönnfors S, Vermeire S, Greco M, Hommes D, Bell C, Avedano L. IBD and health-related quality of life – discovering the true impact. J Crohns Colitis. 2014;8(10):1281–1286. | ||
European Crohn’s and Colitis Organisation [webpage on the Internet]. ECCO Consensus guidelines for IBD. Crohn’s Disease (CD) Guidelines. Vienna: ECCO, 2010. Available from: https://www.ecco-ibd.eu/index.php/publications/ecco-guidelines-science/published-ecco-guidelines.html. Accessed January 24, 2017. | ||
Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6(10):965–990. | ||
Ostbye T, Yarnall KS, Krause KM, Pollak KI, Gradison M, Michener JL. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3(3):209–214. | ||
Elkjaer M, Moser G, Reinisch W, et al. IBD patients need in health quality of care ECCO consensus. J Crohns Colitis. 2008;2(2):181–188. | ||
Mikocka-Walus A, Andrews JM, Rampton D, Goodhand J, van der Woude J, Bernstein CN. How can we improve models of care in inflammatory bowel disease? An international survey of IBD health professionals. J Crohns Colitis. 2014;8(12):1668–1674. | ||
Panes J, O’Connor M, Peyrin-Biroulet L, Irving P, Petersson J, Colombel JF. Improving quality of care in inflammatory bowel disease: what changes can be made today? J Crohns Colitis. 2014;8(9):919–926. | ||
Louis E, Dotan I, Ghosh S, Mlynarsky L, Reenaers C, Schreiber S. Optimising the inflammatory bowel disease unit to improve quality of care: expert recommendations. J Crohns Colitis. 2015;9(8):685–691. | ||
Bokemeyer B. CED-behandlung in deutschland. Betrachtung zur sinnvollen vernetzung. Der Gastroenterologe. 2007;2(6):447–455. | ||
Raspe H, Conrad S, Muche-Borowski C. Evidenzbasierte und interdisziplinär konsentierte versorgungspfade für patientinnen und patienten mit morbus crohn oder colitis ulcerosa. Z Gastroenterol. 2009;47(6):541–562. | ||
Melmed GY, Siegel CA. Quality improvement in inflammatory bowel disease. Gastroenterol Hepatol (NY). 2013;9(5):286–292. | ||
IBD Standards Group. Standards for the Healthcare of People who have Inflammatory Bowel Disease (IBD). IBD Standards 2013. St Albans: Crohn’s and Colitis UK, 2013. Available from: http://www.ibdstandards.org.uk/uploaded_files/ibdstandards.pdf. Accessed January 24, 2017. | ||
Crohn’s & Colitis Australia. Interim Australian IBD Standards: Standards of healthcare for people with inflammatory bowel disease in Australia. Camberwell: Crohn’s & Colitis Australia, 2015. Available from: https://www.crohnsandcolitis.com.au/site/wp-content/uploads/IBD-Standards.pdf. Accessed August 18, 2017. | ||
Langbrandtner J. Wegweisend. Netzwerk für Patienten mit chronisch entzündlichen Darmerkrankungen [Network for patients with chronic inflammatory diseases]. Gesundheitsland Schleswig-Holstein Jahrbuch 2011/2012; 2011:72. German. | ||
Hueppe A, Langbrandtner J, Raspe H. Inviting patients with inflammatory bowel disease to active involvement in their own care: a randomized controlled trial. Inflamm Bowel Dis. 2014;20(6):1057–1069. | ||
Berding A, Witte C, Gottschald M, et al. Beneficial effects of education on emotional distress, self-management, and coping in patients with inflammatory bowel disease: a prospective randomized controlled study. Infamm Intest Dis. 2016;1:182–190. | ||
Hüppe A, Langbrandtner J, Raspe H. Operationalisation of an active problem. Luebeck: University Luebeck, 2013. Available from: http://www.forschung-patientenorientierung.de/files/operationalisation.pdf. Accessed August 15, 2017. | ||
Reusch A, Weiland R, Gerlich C, et al. Self-management education for rehabilitation inpatients suffering from inflammatory bowel disease: a cluster-randomized controlled trial. Health Educ Res. 2016;31(6):782–791. | ||
EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. | ||
Stark RG, Reitmeir P, Leidl R, Konig HH. Validity, reliability, and responsiveness of the EQ-5D in inflammatory bowel disease in Germany. Inflamm Bowel Dis. 2010;16(1):42–51. | ||
Deck R, Mittag O, Hüppe A, Muche-Borowski C, Raspe H. Index zur Messung von Einschränkungen der Teilhabe (IMET) - Erste Ergebnisse eines ICF-orientierten assessmentinstruments. Praxis Klinische Verhaltensmedizin und Rehabilitation. 2007;76:113–120. | ||
Deck R. IMET. Index zur Messung von Einschränkungen der Teilhabe. In: Begel J, Wirtz M, Zwingmann C, eds. Diagnostische Verfahren in der Rehabilitation. Göttingen: Hogrefe; 2008:372–374. | ||
Deck R, Walther A, Staupendahl A, Katalinic A. Einschränkung der Teilhabe in der Bevölkerung – Normdaten für den IMET auf der Basis eines Bevölkerungssurveys in Norddeutschland. Rehabilitation. 2015;54(6):402–408. | ||
Schuler M, Musekamp G, Faller H, et al. Assessment of proximal outcomes of self-management programs: translation and psychometric evaluation of a German version of the Health Education Impact Questionnaire (heiQ). Qual Life Res. 2013;22(6): 1391–1403. | ||
Osborne RH, Elsworth GR, Whitfield K. The health education impact questionnaire (heiQ): an outcomes and evaluation measure for patient education and self-management interventions for people with chronic conditions. Patient Educ Couns. 2007;66(2):192–201. | ||
Nolte S, Elsworth GR, Sinclair AJ, Osborne RH. The extent and breadth of benefits from participating in chronic disease self-management courses: a national patient-reported outcomes survey. Patient Educ Couns. 2007;65(3):351–60. | ||
Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70(3):439–444. | ||
Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298(6666):82–86. | ||
Janke KH, Raible A, Bauer M, et al. Questions on life satisfaction (FLZM) in inflammatory bowel disease. Int J Colorectal Dis. 2004;19(4): 343–353. | ||
Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1(8167):514. | ||
Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14(12):1660–1666. | ||
Morris S. Estimating effect sizes from pretest-posttest-control group designs. Organ Res Meth. 2008;11(2):364–386. | ||
Abt K. Descriptive data analysis: a concept between confirmatory and exploratory data analysis. Methods Inf Med. 1987;26(2):77–88. | ||
Saibil F, Lai E, Hayward A, Yip J, Gilbert C. Self-management for people with inflammatory bowel disease. Can J Gastroenterol. 2008;22(3):281–287. | ||
Department of Health. The Expert Patient: A New Approach To Chronic Disease Management For The 21st Century. London: Department of Health; 2001. | ||
Barlow C, Cooke D, Mulligan K, Beck E, Newman S. A critical review of self-management and educational interventions in inflammatory bowel disease. Gastroenterol Nurs. 2010;33(1):11–18. | ||
Rakshit RC, Mayberry JF. What is the role of patient education in the care of IBD? Inflamm Bowel Dis. 2008;14(Suppl 2):S66–S67. | ||
Timmer A, Preiss JC, Motschall E, Rucker G, Jantschek G, Moser G. Psychological interventions for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2011;2:CD006913. | ||
Blumenstein I, Tacke W, Filmann N, et al. Integrierte Versorgung von Patienten mit chronisch-entzundlichen Darmerkrankungen im Rhein-Main-Gebiet: Ergebnisse der ersten integrierten Versorgung CED in Deutschland. Z Gastroenterol. 2013;51(7):613–618. | ||
Stark RG, Reitmeir P, Leidl R, König HH. Validity, reliability, and responsiveness of the EQ-5D in inflammatory bowel disease in Germany. Inflamm Bowel Dis. 2010;16(1):42–51. | ||
Kiene H. Komplementäre Methodenlehre der klinischen Forschung – Cognition-Based Medicine. Limitierung randomisierter Studien: Die prinzipielle Tendenz zu falsch negativen Ergebnissen. Berlin: Springer-Verlag; 2001. | ||
Conley S, Redeker N. A systematic review of self-management interventions for inflammatory bowel disease. J Nurs Scholarsh. 2016;48(2):118–127. | ||
Boger E, Ellis J, Latter S, et al. Self-management and self-management support outcomes: a systematic review and mixed research synthesis of stakeholder views. PLoS One. 2015;10(7):e0130990. | ||
Blumenstein I, Herrmann E, Filmann N, et al. Female patients suffering from inflammatory bowel diseases are treated less frequently with immunosuppressive medication and have a higher disease activity: a subgroup analysis of a large multi-centre, prospective, internet-based study. J Crohns Colitis. 2011;5(3):203–210. | ||
Bokemeyer B, Hardt J, Hüppe D, et al. Clinical status, psychosocial impairments, medical treatment and health care costs for patients with inflammatory bowel disease (IBD) in Germany: an online IBD registry. J Crohns Colitis. 2013;7(5):355–368. | ||
Hüppe A, Steimann G, Janotta M, et al. Auf dem Prüfstand: stationäre medizinische Rehabilitation bei chronisch entzündlichen Darmerkrankungen. Die Rehabilitation. 2016;55(4):248–255. | ||
Raspe H, Hüppe A, Langbrandtner J. Online-Fragebogen für Betroffene mit chronisch entzündlicher Darmerkrankung. Luebeck: University Luebeck, 2013. Available from: http://www.ced-aktiv-werden.de. Accessed August 15, 2017. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






