Back to Journals » Infection and Drug Resistance » Volume 15
Quality of Antimalarial Drugs in East Africa: A Systematic Review
Authors Girma M, Umeta B , Hasen G , Suleman S
Received 1 May 2022
Accepted for publication 28 September 2022
Published 21 October 2022 Volume 2022:15 Pages 6085—6092
DOI https://doi.org/10.2147/IDR.S373059
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Million Girma,1 Belachew Umeta,2 Gemmechu Hasen,2 Sultan Suleman2,3
1School of Pharmacy, College of Medicine and Health Sciences, Wolaita Sodo University, Wolaita Sodo, Ethiopia; 2School of Pharmacy, Institute of Health, Jimma University, Jimma, Ethiopia; 3Jimma University Laboratory of Drug Quality (JuLaDQ), Jimma University, Jimma, Ethiopia
Correspondence: Belachew Umeta, School of Pharmacy, Institute of Health, Jimma University, Po. Box: 378, Jimma, Oromia, Ethiopia, Email [email protected]
Background: The use of poor quality drugs will have multiple consequences with an extended hazard of growing drug-resistant strains.
Purpose: The review aimed to provide the quality status of antimalarial drugs in East Africa.
Data Source: PubMed, Scopus, Web of Science, and Google Scholar were searched from September 5 to September 12, 2021.
Study Selection: The review included articles available as original research targeted at evaluating the quality of antimalarial drugs. For inclusion, data on at least one of the following quality control parameters were required: packaging and labeling, hardness, friability, weight variation/uniformity of weight, disintegration, dissolution, and assay/percentage purity. Mendeley citation manager version 1.19.4 was used to avoid duplication and organize references, and titles and abstracts were primarily used for screening.
Data Extraction: The sample collection site, drug name, and the quality control parameters tested were retrieved from the selected studies.
Data synthesis: Totally, 300 antimalarial drug samples from Ethiopia, Kenya and Tanzania were included in this review. No antimalarial drug tested failed the identification and disintegration test. However, 15.93% (36/226), 5.00% (15/300), and 1.90% (3/158) of antimalarial samples failed the dissolution, assay and mass uniformity test, respectively. Moreover, amodiaquine and sulfadoxine/pyrimethamine samples failed dissolution and assay tests. In addition, amodiaquine samples failed the mass uniformity test. However, artemether/lumefantrine and quinine passed all quality control parameters tested. Overall, 19.67% (59/300) of antimalarial drug samples did not meet at least one quality control parameter. And the higher faller rate was reported for sulfadoxine/pyrimethamine accounting for 52.86% (37/70).
Conclusions: An unneglected amount of antimalarial drug failed to meet at least one quality control parameter. Strengthening pharmaceutical management systems, including post-marketing surveillance, and providing the resources required for medication quality assurance, are recommended.
Keywords: antimalarial drugs, quality control, coartem, artemether/lumefantrine, assay, dissolution, East Africa
Introduction
Malaria is a life-threatening mosquito-borne disease caused by Plasmodium species and spread by the bites of infected female Anopheles mosquitoes. Fever, headache, chills, and vomiting are symptoms of the disease, and if left untreated, can lead to morbidity and death.1 According to the WHO 2020 report, there were approximately 229 million malaria cases worldwide. The majority of cases were from Africa and Asia.2
In the 1990s, malaria became more prevalent in Sub-Saharan Africa, owing to growing resistance to chloroquine and sulfadoxine-pyrimethamine.3 This tendency was likely worsened by the use of poor quality antimalarial medications.4
There are two management principles for malaria: prevention (vector control, drug prophylaxis, and potential use of a vaccine) and treatment in which drugs play a role.5 Currently, Artemisinin-based combination therapies (ACTs) are becoming the center for managing malaria.6 Successful malaria management is achieved through early diagnosis and immediate treatment with quality ensured medicines.3
Medicines are crucial parts of healthcare systems and patient care. Patients, physicians, caregivers, and communities must have confidence in the pharmaceuticals they are using, and the pharmaceuticals needs to be genuine and satisfy the appropriate quality standards.7 Safety, quality, and efficacy of medicines are the criteria utilized to control pharmaceuticals.8 The quality of drugs is particularly vital,9 and almost 15% of drugs circulated on the market are counterfeit.10 The problem of counterfeiting is well recognized within the African region, and in other parts of the developing world.11 Nowadays, among 12 antimalarial drugs utilized in the world, eight have been counterfeited.12 The use of poor quality antimalarial will have multiple consequences with an extended hazard of growing drug-resistant strains of malaria.13
A study conducted in 2008 on six malarious countries reported that 35% of antimalarial samples were substandard and failed to comply quality control parameter of either assay or dissolution.14 A survey conducted by WHO in six African countries in 2011 showed that from 267 tested samples, 28.5% of them failed to meet specifications for different quality control parameters of identification test, assay, dissolution and mass uniformity test.15 Similarly, a study conducted in Kenya detailed antimalarial drugs had higher failure rate (26.8%) than antibiotics (5.3%).16 Another study conducted in Kenya in 2000 reported 3.9% and 69.7% of sulfadoxine/pyrimethamine products failed assay and dissolution tests, respectively.17 Although poor quality anti-malarial drugs have been reported so far in various parts of Africa, there is no comprehensive estimate of the quality status of anti-malarial drugs in the East African region. Therefore, the current review aimed to provide the quality status of antimalarial drugs in selected East African countries through a systematic review of existing evidence.
Methods
Search Method
The review followed the steps recommended in the Preferred Reporting Items for Systematic reviews and Meta-Analyses18 (Figure 1). The studies were identified from PubMed, Scopus, Web of Science, Google Scholar and other data bases. A manual search from Google was done for grey literature screening. The search was conducted from September 5 to September 12, 2021.
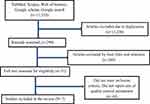 |
Figure 1 PRISMA of included studies. |
The search terms used were quality evaluation, quality control, antimalarial drugs, chloroquine sulphate, coartem, artemether/lumefantrine, artemisinin combination therapy, sulfadoxine/pyrimethamine, fansidar, quinine, amodiaquine, artesunate and amodiaquine co-packed tablet, hardness, friability, weight variation, uniformity of weight, disintegration, dissolution, assay, purity, East Africa. The search terms were constructed in line with the Medical Subject Headings (MeSH) using important connector words. A detail of search strategy for some databases is available in Supplementary File 1. The software Mendeley citation manager 1.19.4 version was used to avoid duplication and organize references.
Selection of Studies, Methodological Quality of Included Studies and Data Extraction
Primarily, titles and abstracts were used for screening, and the remaining articles were subjected to full review to extract essential components.
The data were extracted in Microsoft Excel. Data extraction was handled by MG and GH by using the data extraction form in excel. The data retrieved from the selected studies include the name of the primary author, year of publication, journal, volume, sample collection site, drug name, number of brands included in the study, laboratory test performed (packaging and labeling, identity test, assay, dissolution, uniformity of mass, friability, hardness, content uniformity and disintegration).
After removing irrelevant articles, MG, GH and BU independently reviewed for data quality and methodological validity using a standardized methodological quality evaluation instrument obtained from MEDQUARG guideline19 (Supplementary File 2). Any disagreement was handled by consulting SS.
Inclusion and Exclusion Criteria
Studies available as original research aimed to evaluate the quality of the antimalarial drug, whether it is published or unpublished, studies written in English, and studies conducted in East Africa were included in this review. Data on at least one of the following quality control parameters had to be available for inclusion: packaging and labeling of pharmaceuticals, hardness, friability weight variation/uniformity of weight, disintegration, dissolution and assay/ percentage purity. Editorial and case reports were excluded.
Quality Assessment Parameters for Antimalarial Drugs
The parameters used for quality assessment were packaging and labeling of antimalarial drugs, hardness, friability, weight variation/uniformity of weight, disintegration time, dissolution and assay or percentage purity.
Data Synthesis and Analysis
The data extracted from included articles were imported into a Microsoft Excel spreadsheet and presented in the form of tables and figures. Summary results were presented by frequency and percentage using tables and figures.
Results
Search Result
Initially, 11,534 articles were accessed from electronic databases of PubMed, Scopus, Web of Science, Google Scholar, Google search and other data bases. The titles and abstracts of 299 records were screened for eligibility after duplicates were removed. Of 299 studies, 248 were excluded after the title and abstract were reviewed. The remaining articles were assessed for full-text review. And 44 articles were excluded because of not related to quality evaluation of anti-malarial drugs and assess safety and efficacy of antimalarial drugs. Finally, 7 studies were included in the review.20–26
Characteristics of Studies
The articles included in this review were conducted in three countries. Ethiopia,20,21,26 Tanzania,22,24 and Kenya23,25). Around 50% (n=159) of the samples included in this review were collected from Ethiopia and 48 (16.00%) included samples were collected from Kenya (Table 1).
 |
Table 1 Distribution of Samples by Country |
Quality Control Parameters Tested
All studies included in the review were conducted on the quality control parameters of packaging and labeling. Except for one study,26 all conducted the identification test. All of the included studies were done an assay test. Except for one study,23 of the studies evaluated the dissolution test. In this review, 300 samples of different antimalarial drugs were included (Table 1).
Drugs Included in the Review
In this review, different brands of antimalarial drugs were included. Artemether/lumefantrine (artefan, artemine, IPCA, coartem), sulfadoxine/pyrimethamine (sulphadar, tansidar, tansin, malostat, neopyrin, laridox, tansimax, orodar, medodoxin, malareich, fansidar), artesunate +amodiaquine co-packed product, chloroquine (APF, cadila, Epharm), quinine (QSM300, quinil, quinine remedica) and amodiaquine tablets were included (Table 2).
 |
Table 2 Quality Control Parameters Evaluation of Antimalarial Drugs Included in the Review |
Sample Collection Site and Types of Pharmacopeias Used to Test Drug Quality
The samples were collected from hospital pharmacies, health center pharmacies, drug stores, wholesales pharmacies, and retail outlet pharmacies (Table 3).
 |
Table 3 Sample Collection Site for Tested Drugs and Utilized Pharmacopeia Method for Quality Control Test |
Different Pharmacopeias were utilized to evaluate the quality of antimalarial drugs. The commonly used pharmacopeias were USP, Combination of USP and BP, International Pharmacopeia (Table 3).
Types of Analytical Techniques Used and Quality Control Parameters
Four different analytical techniques were utilized to evaluate the quality of antimalarial drugs. High-Performance Liquid Chromatography (HPLC) was used for assay and identification tests. UV-spectroscopy was also used to perform quality control test for assay (Table 4).
 |
Table 4 Analytical Techniques Used for Quality Control of Antimalarial Drugs |
Quality Control Tests of Anti-Malarial Dugs Included in the Review
All the seven studies included in this review evaluated the packaging and labeling of antimalarial drugs. Of the 300-samples of antimalarial drugs tested for packaging and labeling, 3 (1.00%) failed to comply with packaging and labeling of pharmaceuticals. Chloroquine phosphate (3-sample) was the one that failed to comply (Table 2).
All of the antimalarial drugs included in the review had active pharmaceutical ingredients (Table 2).
Of the 300-samples, 15-samples failed to comply with the assay test. And amodiaquine tablets (10-samples) and Sulfadoxine/pyrimethamine (5-samples) were the antimalarial drugs that failed to comply (Table 2).
Three samples, 1.90% (3/158), failed to comply with the mass uniformity test. Amodiaquine tablets (3-samples) were the tablets that failed to comply with the mass uniformity test. Only one sample of Sulfadoxine/pyrimethamine failed to comply with the friability and hardness test (Table 2).
Of 300 samples of antimalarial drugs included in this review, 15.9% (36/226) of the samples failed to comply with the dissolution test. Sulfadoxine/pyrimethamine (30-samples) and amodiaquine tablet (6-samples) was the samples failing to comply with the test (Table 2).
Specific Antimalarial Drug Quality and Quality Control Tests
Seventy samples of sulfadoxine/ pyrimethamine were tested for different quality control parameters, and 52.86% (37/70) samples failed to comply with at least one quality control parameter tested. 42.86% (30/70) of them failed the dissolution test, and 7.14% (5/70) of them failed the assay test (Table 2). Of 44 tablets of amodiaquine tested for quality, 43.18% (19/44) of them failed to comply with at least one quality control parameter. From these parameters, 22.73% (10/44) of them failed the assay test, and 13.64% (6/44) of them failed the dissolution test.
Forty-four samples of chloroquine were included in this review, and 6.82% (3/44) of them failed to comply with at least one parameter of the quality control test. Three of the tablets failed the packaging and labeling inspection (Table 2).
From different quality control parameters tested for the antimalarial drugs included in this review, the highest faller rate was reported for the dissolution test (15.93%), followed by the assay test accounting for 5.00% (Table 2).
As per this review, 19.67% (59/300) of antimalarial drugs failed to comply with at least one quality control parameter. Of the different quality control parameters tested, the dissolution test encountered the higher faller rate (15.93%), followed by the assay test accounting for 5.00% (Table 2).
Discussion
When determining whether medicine is substandard, damaged, or counterfeit, visual inspection of packaging and labeling is essential. Some products contain genuine active pharmaceutical ingredients, but packaged in fake packaging.27 The WHO checklist for physical characteristics, packaging and labeling information, or the USP tool for visual inspection of medicine were used in all studies included in the review to inspect packaging and labeling.28,29 All tablets passed the packaging and labeling of pharmaceuticals, except three samples of chloroquine phosphate tablets. The study conducted on the malarious world reported that out of 9348 anti-malarial samples, 30.1% (2813) failed chemical/packaging quality tests.32 To identify the products, HPLC was utilized to compare the retention times of the analyzed drug peak to those of a reference standard (RS). Product peak retention time and RS chromatogram peak retention times were compared and determined to be similar.20 All drugs under investigation passed the identification tests.
The assay test is used to determine how much active pharmaceutical ingredients are present in a dosage form and its critical quality control parameter. Of the 300-antimalarial drug samples tested for percentage purity, 15-samples failed the test. The tablets that failed the assay test were amodiaquine 22.73% (10/44) and sulfadoxine/pyrimethamine 7.14% (5/70). A study conducted in South-East Nigeria on the quality of anti-malarial reported that 60 (37%) of the anti-malarial drugs tested did not meet the USP specifications for the amount of active ingredients.31 The ability of a drug to dissolve is a crucial part of its evaluation, and it is one of the quality control tests for ensuring product uniformity and batch-to-batch equivalence.30 According to the current review, 226-samples of antimalarial drugs were examined for dissolution. In contrast, 36 (15.93%) antimalarial drugs failed the dissolution test. The samples that failed the dissolution test were sulfadoxine/pyrimethamine (30/70) and amodiaquine (6/44). The finding is comparable with the study conducted in six malarious countries in 2008 reported that, in total, 35% (73/210) of the samples were substandard and failed either the TLC or the dissolving tests. Failure by TLC, dissolution, or both of the individual pharmaceutical kinds, happened in 38% of SP and 48% of amodiaquine.14 Uniformity of weight or content uniformity is used to ensure the consistency of dosage units. And each unit in a batch should have a drug substance within a range around the label claim.30 Of 158 samples tested for mass uniformity, 1.93% (4/158) of them failed to comply with the test.
Disintegration, friability and hardness tests are the quality control parameter for tablets. Tablets must maintain their integrity from the time they are manufactured till they reach the patients, and they must not break apart during long shipments on boats and in trucks.9 The disintegration, friability, and hardness tests were performed in three studies. And the majority of the samples met the requirements. The friability and hardness tests for the sulfadoxine/pyrimethamine tablet were failed to comply. Overall, 19.67% of samples (59/300) failed at least one quality control parameter. The faller is higher for sulfadoxine/ pyrimethamine and amodiaquine. However, the quality control tests for artemether/lumefantrine and quinine were in line with the specification. The majority of antimalarial drugs failed the dissolution and assay tests, which is a critical quality control parameter.
Strength and Limitations
The review tried to assess the quality of antimalarial drugs circulating in East Africa from existing evidence. However, the availability of limited data results in difficulty in including more articles. In addition, quantitative analysis and heterogeneity of the articles were not performed.
Conclusions
Overall, 19.67% of the sample failed to meet at least one of the quality control parameters. Amodiaquine and sulfadoxine/pyrimethamine samples had a higher faller rate than others. However, artemether/lumefantrine and quinine samples passed all quality control tests. The dissolution test had the highest faller rate among the quality control parameters, followed by the assay.
Data Sharing Statement
The data set used for analysis is available from the corresponding author upon request.
Disclosure
The authors disclose no conflicts of interest in this work.
References
1. World Health Organization. Malaria Fact sheet - World malaria report 2015; 2011 [September 5, 2012]: 6–8. Available from: http://www.who.int/malaria/world_malaria_report_2011/WMR2011_factsheet.pdf.
2. World malaria report 2020: 20 years of global progress and challenges. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO.
3. Gomes M, Wayling S, Pang L. Interventions to improve the use of antimalarials in South-East Asia: an overview. Bull World Health Organ. 1998;76(1):9–19.
4. Basco LK. Molecular epidemiology of malaria in Cameroon. XIX. Quality of antimalarial drugs used for self-medication. Am J Trop Med Hyg. 2004;70(3):245–250. doi:10.4269/ajtmh.2004.70.245
5. Enayati A, Hemingway J. Malaria management: past, present, and future. Annu Rev Entomol. 2010;55(1):569–591. doi:10.1146/annurev-ento-112408-085423
6. World Health Organization. Guidelines for the treatment of malaria. World Health Organization; 2015.
7. Tschida SD. A Systematic Review on Antibiotic Quality [Master’s thesis], University of Oslo/Norway, The Faculty of Medicine, Institute of Health and Society, Department of Community Medicine; 2016.
8. World Health Organization. Effective drug regulation: what can countries do? Theme paper for discussion, Geneva 16–19 March 1999. World Health Organization; 1999.
9. MCA. Towards safe medicines. A guide to the control of safety, quality and efficacy of human medicines in the United Kingdom. Revised edition; 1997: 1–93.
10. Cockburn R, Newton PN, Agyarko EK, Akunyili D, White NJ. The global threat of counterfeit drugs: why industry and governments must communicate the dangers. PLoS Med. 2005;2(4):e100. doi:10.1371/journal.pmed.0020100
11. World Health Organization. The quality of antimalarials: a study in selected African countries. World Health Organization; 2003.
12. Hall KA, Newton PN, Green MD, et al. Characterization of counterfeit artesunate antimalarial tablets from Southeast Asia. Am J Trop Med Hyg. 2006;75(5):804–811. doi:10.4269/ajtmh.2006.75.804
13. Walker EJ, Peterson GM, Grech J, Paragalli E, Thomas J. Are we doing enough to prevent poor-quality antimalarial medicines in the developing world? BMC Public Health. 2018;18(1):1–6. doi:10.1186/s12889-018-5521-7
14. Bate R, Coticelli P, Tren R, Attaran A. Antimalarial drug quality in the most severely malarious parts of Africa–a six country study. PLoS One. 2008;3(5):e2132. doi:10.1371/journal.pone.0002132
15. World Health Organization. Survey of the quality of selected antimalarial medicines circulating in six countries of sub-Saharan Africa. WHO; 2011.
16. Thoithi GN, Abuga KO, Nguyo JM. Drug quality control in Kenya: observation in the drug analysis and research unit during the period 2001–2005. East Cent Afr J Pharmaceut Sci. 2008;11:3.
17. Kibwage IO, Ngugi JK. Sulphadoxine/pyrimethamine tablet products on the Kenyan market: quality concerns. East Cent Afr J Pharmaceut Sci. 2000;3(1):14–19.
18. Yepes-Nuñez JJ, Urrútia G, Romero-García M, The A-FS. PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol. 2021;74(9):790–799. doi:10.1016/j.rec.2021.07.010
19. Newton PN, Lee SJ, Goodman C, et al. Guidelines for field surveys of the quality of medicines: a proposal. PLoS Med. 2009;6(3):e1000052. doi:10.1371/journal.pmed.1000052
20. Abuye H, Abraham W, Kebede S, Tatiparthi R, Suleman S. Physicochemical quality assessment of antimalarial medicines: chloroquine phosphate and quinine sulfate tablets from drug retail outlets of South-West Ethiopia. Infect Drug Resist. 2020;13:691. doi:10.2147/IDR.S234684
21. Belew S, Suleman S, Mohammed T, et al. Quality of fixed dose artemether/lumefantrine products in Jimma Zone, Ethiopia. Malar J. 2019;18(1):1. doi:10.1186/s12936-019-2872-1
22. Hebron Y, Tettey JN, Pournamdari M, Watson DG. The chemical and pharmaceutical equivalence of sulphadoxine/pyrimethamine tablets sold on the Tanzanian market. J Clin Pharm Ther. 2005;30(6):575–581. doi:10.1111/j.1365-2710.2005.00687.x
23. Amin AA, Snow RW, Kokwaro GO. The quality of sulphadoxine‐pyrimethamine and amodiaquine products in the Kenyan retail sector. J Clin Pharm Ther. 2005;30(6):559–565. doi:10.1111/j.1365-2710.2005.00685.x
24. Minzi OM, Moshi MJ, Hipolite D, et al. Evaluation of the quality of amodiaquine and sulphadoxine/pyrimethamine tablets sold by private wholesale pharmacies in Dar es Salaam Tanzania. J Clin Pharm Ther. 2003;28(2):117–122. doi:10.1046/j.1365-2710.2003.00470.x
25. Ndwigah S, Stergachis A, Abuga K, Mugo H, Kibwage I. The quality of anti-malarial medicines in Embu County, Kenya. Malar J. 2018;17(1):1–5. doi:10.1186/s12936-018-2482-3
26. Hussein B. Post-market in vitro quality evaluation of artemether-lumefantrine tablets marketed in Addis Ababa and selected areas of Oromia Region in Ethiopia [Doctoral dissertation]; Addis Ababa University; 2015.
27. Newton PN, McGready R, Fernandez F, et al. Manslaughter by fake artesunate in Asia—will Africa be next? PLoS Med. 2006;3(6):e197. doi:10.1371/journal.pmed.0030197
28. World Health Organization. Tool for visual inspection. Geneva, Switzerland. 2005; Available from: https://www.globalforumljd.org/sites/default/files/documents/virtualLibrary/USP%20VisualInspectionTool%20%282005%29.pdf.
29. World Health Organization. Tool for visual inspection, A checklist for visual inspection of medicines in order to identify suspicious products for further examination, Geneva: World Health Organization. Geneva World Heal Organ [Internet]. 2015; Available from: http://www.whpa.org/Toolkit_BeAware_Inspection.pdf.
30. Umeta B, Bekele A, Mohammed T, Duguma M, Teshome H, Mekonnen Y. Dissolution profile evaluation of eight brands of metformin hydrochloride tablets available in Jimma, Southwest Ethiopia. Diabetes Metab Syndr Obes. 2021;14:3499. doi:10.2147/DMSO.S316187
31. Onwujekwe O, Kaur H, Dike N, et al. Quality of anti-malarial drugs provided by public and private healthcare providers in south-east Nigeria. Malar J. 2009;8(1):1–9. doi:10.1186/1475-2875-8-22
32. Tabernero P, Fernández FM, Green M, Guerin PJ, Newton PN. Mind the gaps - The epidemiology of poor-quality anti-malarials in the malarious world - Analysis of the WorldWide antimalarial resistance network database. Malar J. 2014;13(1):1–14. doi:10.1186/1475-2875-13-139
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
