Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
QT/QTc safety and efficacy evaluation of teneligliptin in Indian type 2 diabetes mellitus patients: the “thorough QT/QTc” study (Q-SET study)
Authors Erande S , Sarwardekar S, Desai B
Received 24 January 2019
Accepted for publication 20 March 2019
Published 21 June 2019 Volume 2019:12 Pages 961—967
DOI https://doi.org/10.2147/DMSO.S202458
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
S Erande,1 S Sarwardekar,2 B Desai3
1Department of Medicine, Akshay Hospital, Pune 411004, India; 2Department of Medicine, Sawardekar Clinic, Mumbai 400055, India; 3Department of Cardiology, Desai Heart Care Clinic, Mumbai 400092, India
Background: Newer therapies, such as dipeptidyl peptidase-IV inhibitors, are increasingly being used in the treatment of type 2 diabetes mellitus (T2DM). Teneligliptin, a DPP4 inhibitor, currently commonly used as monotherapy or as add-on therapy, was generally well tolerated in patients with T2DM during clinical trials. No AEs related to QT prolongation were detected with 40 mg/day of teneligliptin, but were seen at a supratherapeutic dose of 160 mg/day.
Aims and objective: To evaluate the safety of teneligliptin in type 2 diabetes patients with respect to QTc prolongation.
Methodology: This was an open-label, prospective, multi-centric trial conducted in patients with T2DM aged ≥18 to ≤65 years with a hemoglobin A1c (HbA1c) ≥7.0% and gliptin naïve. Teneligliptin 20 mg once a day was added to the standard treatment. The dose of teneligliptin was increased to 40 mg once a day if required, on the basis of glycemic parameters. Twelve-lead ECG was recorded at baseline and follow-up visits. The QTc was calculated by using the Bazett’s formula (QTc=QT/√RR).
Results: The mean QT interval at screening (Visit 1, Day 0, baseline ECG) was 0.33±0.07 seconds, while at visit 2 (Day 1, post 2 hours of Teneligliptin dosing) it was 0.32±0.04 seconds, at visit 3 (Day 15) it was 0.32±0.04 seconds, and at visit 4 (Day 90) it was 0.32±0.03 seconds. The mean QTc interval at baseline was 0.37±0.04 seconds, while at visit 2 it was 0.37±0.04 seconds, at visit 3 it was 0.37±0.03 seconds, and at visit 4 it was 0.37±0.03 seconds. There was a significant reduction in fasting blood glucose (P=0.002), postprandial blood glucose (P<0.001), and HbA1c (P<0.001) at the end of the 3 months as compared to baseline.
Conclusion: Teneligliptin at a therapeutic dose of 20 mg/day or 40 mg/day improved glycemic parameters significantly and did not cause QT/QTc interval prolongation.
Keywords: diabetes, teneligliptin, QT prolongation
A Letter to the Editor has been published for this article
Introduction
Diabetes mellitus (DM) is a complex disease characterized by chronic hyperglycemia, metabolic abnormalities, and long-term macro- and microvascular complications. It is one of the leading global health issues of the 21st century.1 India is one of the countries with the largest number of people having DM. In 2017, ~73 million people were diagnosed with DM in India. The rapid shift in the Indian economy is one of the factors behind the increase in the prevalence of DM. If this trend continues, by 2045, almost 134 million people will have diabetes.2
Standard therapies for the treatment of type 2 diabetes mellitus (T2DM) include metformin, sulfonylureas, meglitinides, thiazolidinediones, and insulin. Newer therapies, such as dipeptidyl peptidase-IV inhibitors, are increasingly being used in the treatment of type 2 diabetes. But, a major question that arises is whether these newer therapies can also be used safely and effectively across the spectrum of T2DM patients. The latest ADA-EASD joint statement released in October 2018 recommends usage of the newer cardio-friendly drugs for treatment of T2DM. Usage of DPP-4 inhibitors has consistently increased in India since the introduction of teneligliptin.3
Teneligliptin, a DPP4 inhibitor, was approved for the management of type 2 diabetes mellitus in Japan (2012), in South Korea (2014), and in India (2015).4 In addition to effective glycemic control, results of various clinical trials also suggested that teneligliptin, as monotherapy or add-on therapy, was generally well tolerated in patients with T2DM.4 Teneligliptin is orally administered at a dosage of 20 mg once daily, which can be increased up to 40 mg per day. According to a strict QT/QTc evaluation study and clinical studies for type 2 diabetes conducted in Japan and other countries, no adverse events (AEs) related to QT prolongation were detected with 20 mg/day or with 40 mg/day of teneligliptin, which is the maximal dosage used in clinical practice.5 But, teneligliptin at 160 mg/day dose, during initial safety studies, was associated with changes in QT interval.6 The importance of establishing the cardiac safety of any antidiabetic drug is particularly important since diabetic patients have an increased risk for cardiovascular disease, and this makes these patients more susceptible to the effects which any drug may have on heart function. There is no published data regarding the safety of teneligliptin, with therapeutic doses, in Indian type 2 diabetic patients with respect to QTc prolongation.
Hence, the current prospective study was intended to evaluate the safety of teneligliptin in type 2 diabetes patients with respect to QTc prolongation.
The primary objective of the study was to evaluate change in HbA1c and the percentage of patients with ECG changes of QT/QTc prolongation. The secondary objectives were to evaluate the safety and tolerability of teneligliptin in patients with type 2 diabetes. The effect of teneligliptin on fasting blood sugar (FBS) and post prandial blood sugar (PPBS) was also evaluated.
Methodology
The study was an open-label, prospective, multi-centric study. A total of 66 patients were enrolled in the study. The study was conducted in accordance with the principles of International Conference on Harmonization (ICH) guidelines for Good Clinical Practice (GCP). Independent Ethics Committee (Suraksha Ethics committee. Reg No: ECR/644/Inst/MH/2014) approval was obtained prior to commencement of the study. After the patients gave written informed consent they underwent screening procedures, which included complete general and systemic examination. The comprehensive physical examination included the respiratory system and cardiovascular system, per abdomen, and neurological examination.
The patients included in the study were male or female patients with uncontrolled Type 2 diabetes mellitus, aged ≥18 to ≤65 years, with a hemoglobin A1c (HbA1c) ≥7.0%, and were gliptin naïve. The exclusion criteria were: patients with type 1 diabetes, severe diabetic complications such as ketoacidosis, marked baseline prolongation of QT/QTc interval (eg, repeated demonstration of a QTc interval >450 milliseconds (ms), history of additional risk factors for Torsade de pointes (TdP), eg, heart failure, hypokalemia, family history of Long QT Syndrome, the use of concomitant medications that have the potential to prolong the QT/QTc interval, liver dysfunction, pregnant or nursing women and those who might be pregnant, patients with a history of seizures, history of stroke, and cardiovascular events, and any patient whom the investigator judged to be inappropriate for this study.
Sample size calculation
A literature survey was done prior to sample size calculation to study the reported reduction in HbA1c in earlier clinical trials of teneligliptin and other gliptins. Gliptins at a therapeutic dose are reported to have no effect on QT interval. We considered papers for similar molecules and similar end point. We checked the reduction in the HbA1c from baseline to end point. While published papers have spoken of HbA1c reduction of >1%, we have considered an HbA1c reduction of 1%. Therefore, with a 95% confidence level, 90% power, and 10% drop out rate, a sample size of 66 (~70) subjects would be sufficient to determine the teneligliptin safety trial.
Calculation is based on the formula:
n=f(α/2, β)×2×σ2/(μ1−μ2)2
where μ1 and μ2 are the mean outcome in the control and experimental group, respectively, and σ is the standard deviation.
Study procedure
Patients who satisfied the inclusion and exclusion criteria were enrolled into the study. Teneligliptin 20 mg once a day was added to the standard treatment. The dose of teneligliptin was increased to 40 mg once a day if required, on the basis of glycemic parameters. At each visit the patients underwent comprehensive systemic examination where vitals and 12-lead ECG were recorded. The normal ECG at screening was considered as baseline ECG. The 12-lead ECG recording on each follow-up visit was performed 2 hours after the dosing of teneligliptin. Fasting blood glucose and postprandial blood glucose were estimated at each visit.
Safety assessments consisted of monitoring and recording all adverse reactions reported by the patients. The changes in vitals, ECG, and hematology and biochemistry at the end of treatment were compared with the findings at baseline.
Calculation of QT and QTc
The QT interval represents the time of ventricular activity, including both depolarization and repolarization. It is measured from the beginning of the QRS complex to the end of the T wave. Normally, the QT interval is 0.36–0.44 seconds (9–11 boxes). The QT interval will vary with patient gender, age, and heart rate.
The QT interval should be corrected for heart rate to enable comparison with reference values. Because heart rate is the principal determinant of repolarization length, many correction formulae have been developed to calculate a corrected QT interval (QTc) value corresponding to a QT value normalized at a heart rate of 60 beats/minute. This allows comparison of QT values over time at different heart rates, and improves detection of patients at increased risk of arrhythmias.
In the present study, the QTc was calculated by using the Bazett’s formula (QTC=QT/√RR).
Statistical analysis
All characteristics were summarized descriptively. For continuous variables, data were represented using Mean±SD. For categorical data, the number and percentage were used in the data summaries. The difference of the means of analysis variables between the two independent groups was tested by unpaired t-test. The difference of the means of analysis variables between two time points in the same group was tested by paired t-test. The difference of the means of analysis variables between more than two time points was tested by ANOVA and F-test of testing of equality of variance.
Results
The present study enrolled 66 patients, which includes 41 (62%) males and 25 (38%) females. The average age of patients enrolled was 50.2±9.1 years (Table 1). The average duration of diabetes was 3–4 years, and average BMI was 26±3.8. (19–1 to 36.9). Fifty-five (83.33%) patients received teneligliptin 20 mg once a day, and 11 (16.66%) patients received teneligliptin 40 mg per day as an adjuvant therapy along with other oral anti-diabetic drugs. Metformin was the most common drug prescribed in 50 (75.75%) patients (Table 1), and combination of Glimepiride and Metformin was the preferred combination in 16 (24.24%) patients, along with teneligliptin (Table 1).
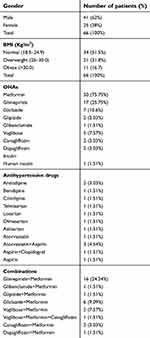 | Table 1 Basic demographic characteristics and OHAs and other drug prescribed |
The mean QT interval at screening visit 1( Day 0, baseline ECG) was 0.33±0.07 seconds, while at visit 2 (Day 1, post 2 hours of Teneligliptin dosing) it was 0.32±0.04 seconds, at visit 3 (Day 15) it was 0.32±0.04 seconds, and at visit 4 (Day 90) it was 0.32±0.03 seconds. The average change in the mean QT interval from baseline ECG, on visit 2 (Day 1, post 2 hour of Teneligliptin dosing) was −0.01±0.03 seconds, on visit 3 (day 15) it was −0.01±0.03 seconds, and on visit 4 (day 90) it was −0.01±0.04 seconds. No significant difference was seen in the QT interval at each visit subsequent to the baseline visit (Table 2).
 | Table 2 QT/QTc in patients treated with teneligliptin 20/40 mg per day |
The mean QTc interval at baseline was 0.37±0.04 seconds, while at visit 2 it was 0.37±0.04 seconds, at visit 3 it was 0.37±0.03 seconds, and at visit 4 it was 0.37±0.03 seconds. The average change in the mean QTc interval from baseline on visit 2 was 0 seconds, on visit 3 (day 15) it was 0±0.01 seconds, and on visit 4 (day 90) it was 0±0.01 seconds. Therefore, the QTc interval did not change from baseline, and there was no statistically significant difference in QTc interval from baseline to any subsequent follow-up visits. There was no significant change in the QT interval (P=0.517) and QTc interval (P=0.998) at baseline and the end of study (Table 2). Among the 11 patients who were treated with teneligliptin 40 mg per day, no significant change in the QT (P=0.880) and QTc (P=0.757) interval was seen at visit 2, visit 3, and visit 4, as compared to baseline (Table 2).
There was a significant reduction in fasting blood glucose (P=0.002), postprandial blood glucose (P<0.001), and HbA1c (P<0.001) at the end of the 3 months, as compared to baseline (Table 3). The average decrease in fasting blood glucose was 15.75±16.67 mg/dL, that of post prandial blood glucose was 45.48±25.96 mg/dL, and that of HbA1C was 0.69±0.31%. There was also no significant change in the vital signs at visit 3 or visit 4 as compared to baseline (Table 4).
 | Table 3 Effect of teneligliptin added to standard treatment on FBG, PPBG, and HbA1c |
 | Table 4 Effects on vital signs |
Hypoglycemia or any other adverse effect was not reported in any of the patients. The drug was well tolerated in all patients.
Discussion
The “thorough QT/QTc study” is intended to determine whether the drug has a threshold pharmacologic effect on cardiac repolarization.7 QT prolongation is quantitavely associated with the risk of TdP (polymorphic ventricular tachyarrhythmia). The current threshold set by USFDA level of regulatory concern for cardiac safety of any drug that prolongs the mean QT/QTc interval by around 5 ms or less does not appear to cause TdP, while drugs that prolong the mean QT/QTc interval by >20 ms have a substantially increased likelihood of being proarrhythmic.7 The data on drugs that prolong the mean QT/QTc interval by more than around 5 and less than 20 ms are inconclusive, but some of these compounds have been associated with proarrhythmic risk.7
In the present “thorough QT/QTc study”, data from 66 T2DM patients on teneligliptin or combination therapy with other OADs was analyzed. ECG pattern were recorded at baseline and at 2 hours post-teneligliptin dosing, on the 15th and 90th day, to determine changes in the QT/QTc interval. No significant difference was seen in the QT interval (P=0.517) and QTc interval (P=0.998) at baseline and the end of study or any of the follow-up visits. Those on a 40 mg dose of teneligliptin also showed no significant change in the QT (P=0.880) and QTc (P=0.757) interval at baseline and end of study or any of the follow-up visits. Thus, 40 mg and 20 mg doses of teneligliptin were associated with no QT/QTc prolongation. A thorough QT/QTc evaluation study was also conducted by Kishimoto,6 which was a moxifloxacin-controlled, parallel-group comparative study conducted in foreign healthy adult males and females, where placebo, teneligliptin 40 mg, and 160 mg were administered orally once daily for 4 days (placebo group, 40 mg group, and 160 mg group). Regarding the difference in QTc interval from baseline, the difference between the teneligliptin group and the placebo group with its two-sided 90% CI in the 40 mg group and 160 mg group were 3.9 (0.2–7.6) and 9.3 (5.6–13.0) milliseconds, respectively, at the end of 3 hours, with the upper limit of the one-sided 95% CI exceeding 10 milliseconds. Thus, no AEs related to QT prolongation were detected with 40 mg/day of teneligliptin, which is the maximal dosage used in clinical practice. However, because when 160 mg/day of the drug was administered, slight prolongation of the QTc interval was detected temporally at the high concentrations of the drug (around tmax level). Similar results were seen with sitagliptin,8 where the clinical dose of sitagliptin 100 mg was not associated with an increase in QTc interval but the supratherapeutic 800-mg dose of sitagliptin was associated with minimal, clinically insignificant prolongation of the QTc interval at concentrations approximately 11-fold higher than maximal concentrations following the 100-mg clinical dose. The PK/QTc model demonstrated a shallow relationship between the plasma concentration of sitagliptin and the placebo-subtracted QTc change from baseline, with a 0.59-millisecond increase in QTc for every 1000-nM increment in sitagliptin plasma concentration.9
Several factors may increase the Cmax of teneligliptin, including renal impairment, hepatic impairment, and concomitant use with a CYP3A4/P-gp inhibitor. Cmax of teneligliptin increases approximately 1.04–1.12-fold in subjects with renal impairment, 1.25–1.38-fold in subjects in hepatic impairment, and 1.37-fold in subjects administered concomitantly with a CYP3A4/P-gp inhibitor.9 In a thorough QT/QTc prolongation study, the Cmax reached at teneligliptin 160 mg was estimated to be within 4.3–5.2 -times and 2.1–2.6 -times the Cmax reached following the administration of teneligliptin at 20 mg and 40 mg in the presence of multiple factors, respectively, even if the upper limit of the CI is taken into account.9,10 Therefore, at clinically recommended doses (20 mg and 40 mg), teneligliptin does not seem to cause QTc prolongation, as shown in the study document submitted to PMDA (Pharmaceuticals and Medical Devices Agency, Japan) by the originator in Japan.12 In the teneligliptin data submitted to PMDA, Japan, it has been shown that there is no QT/QTc prolongation with a 40 mg dose of teneligliptin based on the thorough QT/QTc study. It is recommended that the coadministration of teneligliptin with drugs known to cause QT prolongation on their own, such as class IA or class III antiarrhythmic drugs, should be performed with caution.6 Moreover, hypoglycemia being one of the strong QTc prolongators, in combination with other hypoglycemic drugs, may need strict pharmacovigilance.11
In the present study, apart from its favorable effect on QT/QTc interval, teneligliptin 20 mg and 40 mg per day showed a significant decrease in the fasting blood glucose (P=0.002), post prandial blood glucose (P<0.001), and HbA1C (P<0.001) by the end of the study. Kutoh et al,13 in a 3-month study of 31 drug-naïve Japanese T2DM patients, evaluated teneligliptin daily 20 mg as a monotherapy. This study found a significant reduction in HbA1c (from 10.34±2.06 to 8.38±2.23%, P<0.00001) and fasting blood glucose (from 211.3±68.4 to 167.3±70.2 mg/dL, P<0.0002) from the baseline. Similar results were reported in the TREAT INDIA study,14 where mean HbA1c, FPG, and PPG were significantly reduced by 1.37%±1.15%, 51.29±35.41 mg/dL, and 80.89±54.27 mg/dL, respectively, at the end of 3 months of teneligliptin therapy. The long-term efficacy of teneligliptin has also been studied in two 52-week, open-label, multicenter, interventional studies.15 The changes in HbA1c from baseline to week 52 were −0.63±0.65% in the teneligliptin monotherapy group, −0.76±0.70% in the glinide combination therapy group, −0.78±0.75% in the biguanide combination therapy group, −0.89±0.64% in the alpha-glucosidase inhibitor combination therapy group, and −0.81±0.76% in the SU combination therapy group.
Teneligliptin as an add-on therapy to other agents such as glimepiride, metformin, and pioglitazone, was generally well tolerated in our study. No hypoglycemia or any other adverse effect was reported by patients involved in the trial.
Our study was limited by its small sample size and open label nature, which warrants further need of a large scale randomized double blinded trial. In addition, taking into account that there are diabetic patients who have concurrent diseases such as arrhythmia and ischemia, and that teneligliptin may be administered to such patients for a long period of time, a real world evidence study will help in understanding the drug–drug interaction and factors which increase the QT/QTc interval, if any.
Conclusion
Teneligliptin at a therapeutic dose of 20 mg/day or 40 mg/day does not cause QT/QTc interval prolongation. In the present study it was very well tolerated, with significant reduction in FBS, PPBG, and HbA1C. The results of the current study corroborate the findings published in the literature that teneligliptin at therapeutic doses does not cause QT/QTc interval prolongation in patients.
Disclosure
The abstract of this paper was presented at the APICON (Association of Physicians of India Conference) 2019 conference as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Journal of the Association of Physicians of India (JAPI): http://www.apiconkochi2019.com/userfiles/TK-Raman-Poster-Area-12-3(1).pdf: http://www.japi.org/february_2019/poster/diabetes.html. The authors report no conflicts of interest in this work.
References
1. Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res ClinPract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023
2.
3. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Available from: http://care.diabetesjournals.org/content/diacare/early/2018/09/27/dci18-0033.full.pdf.
4. Patel DK, Sharma RT, Patel HA, Barkate HV. Teneligliptin: a review on cardio-renal safety. Int J Basic Clin Pharmacol. 2016;5:229–234. doi:10.18203/2319-2003.
5. Fisman EZ, Tenenbaum A. Antidiabetic treatment with gliptins: focus on cardiovascular effects and outcomes. Cardiovasc Diabetol. 2015;14:129. doi:10.1186/s12933-015-0294-0
6. Kishimoto M. Teneligliptin: a DPP-4 inhibitor for the treatment of type 2 diabetes. Diabetes Metab Syndrome Obesity. 2013;6:187–195. doi:10.2147/DMSO
7. Guidance for industry E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. Available from: file:///C:/Users/admin/Downloads/USFDA%20Guidance%20document-E14%20Clinical%20Evaluation%20of%20QTc.pdf.
8. Bloomfield DM, Krishna R, Hreniuk D, et al. A thorough QTc study to assess the effect of sitagliptin, a DPP4 inhibitor, on ventricular repolarization in healthy subjects. J Clin Pharmacol. 2009;49(8):937–946. doi:10.1177/0091270009337511
9.
10. Halabi A, Maatouk H, Siegler KE, Faisst N, Hinrichsen H. Pharmacokinetics and safety of teneligliptin in subjects with hepatic impairment. Clin Pharmacol Drug Dev. 2014;3(4):290–296. doi:10.1002/cpdd.89
11. Singh AK. Efficacy and safety of teneligliptin. Indian J Endocrinol Metab. 2017;21(1):11–17. doi:10.4103/2230-8210.193163
12.
13. Kutoh E, Hirate M, Ikeno Y. Teneligliptin as an initial therapy for newly diagnosed, drug naïve subjects with type 2 diabetes. J Clin Med Res. 2014;6:287–294. doi:10.14740/jocmr1809w
14. Ghosh S, Trivedi S, Sanyal D, Modi KD, Kharb S. Teneligliptin real-world efficacy assessment of type 2 diabetes mellitus patients in India (TREAT-INDIA study). Diabetes Metab Syndrome Obesity. 2016;9:347–353. doi:10.2147/DMSO.S121770
15. Kadowaki T, Marubayashi F, Yokota S, Katoh M, Iijima H. Safety and efficacy of teneligliptin in Japanese patients with type 2 diabetes mellitus: A pooled analysis of two phase III clinical studies. Expert Opin Pharmacother. 2015;16:971–978. doi:10.1517/14656566.2015.1032249
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
