Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Pyrroloquinoline Quinone Disodium (PQQ2Na) Has an NLRP Inflammasome-Induced Caspase-1 Release Influence in UVB-Irradiated but Not ATP-Treated Human Keratinocytes but Has No Influence in Increasing Skin Cell Mitochondrial Biogenesis in Either Human Keratinocytes or Fibroblasts
Received 7 October 2021
Accepted for publication 7 January 2022
Published 21 January 2022 Volume 2022:15 Pages 107—115
DOI https://doi.org/10.2147/CCID.S343123
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
James V Gruber,1 Robert Holtz2
1JVG Innovative Consulting, Washington, NJ, USA; 2BioInnovation Laboratories, Inc., Denver, CO, USA
Correspondence: James V Gruber, Email [email protected]
Introduction: Pyrroloquinoline quinone is a bacterial-derived redox factor that has been shown to have numerous benefits in humans. Recently, a model for examining the ability of normal human epidermal keratinocytes (NHEKs) to demonstrate anti-inflammatory benefits via nod-like receptor protein (NLRP)-activated caspase-1 release was reported. The question of whether PQQ2Na might have anti-inflammatory benefits that function through NLRP-activated release of active caspase-1 has not been explored. In addition, it has been reported that PQQ2Na will induce mitochondrial biogenesis in humans when taken orally. Whether or not this effect occurs in skin cells is presently unknown.
Methods: The inflammation studies followed previously published methods that demonstrated both UVB and ATP were able to upregulate the NLRP-activated release of caspase-1 in NHEKs. In addition, NHEK and normal dermal human fibroblasts (NHDF) were treated with PQQ2Na to see if the molecule might stimulate mitochondrial biogenesis measured by increased expression of cyclooxygenase-1 (COX-1) and succinate dehydrogenase complex, subunit A (SDHA).
Results: At non-cytotoxic concentrations between 5 μg/mL and 100 μg/mL in NHEKs and between 0.1 μg/mL and 5 μg/mL in fibroblasts, the PQQ2Na had no influence on cellular mitochondrial biogenesis. In ATP-activated NHEKs at concentrations of PQQ2Na between 0.05 μg/mL and 50 μg/mL, there was no influence of PQQ2Na on release of active caspase-1. In NHEKs irradiated with 60mJ/cm2 of UVB radiation as previously described and treated with 0.05 μg/mL to 50 μg/mL of PQQ2Na, the molecule showed a dose-dependent benefit at reducing the expression of active caspase-1 in the irradiated cells.
Discussion: Benefits of PQQ2Na on various skin cell types which had not been investigated previously were addressed. Surprisingly, the PPQ2Na had no apparent influence on skin cell mitochondrial biogenesis. However, the molecule has a strong suppressing influence on UVB-induced active caspase-1 release in UVB-irradiated NHEKs.
Keywords: pyrroloquinoline quinone disodium, inflammasomes, mitochondrial biogenesis, caspase-1, inflammation
Introduction
In 1979, Salisbury et al structurally identified a novel coenzyme found in methylotrophic bacteria associated with bacterial alcohol dehydrogenase.1 They initially called the enzyme methoxatin and it was found to be a novel quinone structure which was termed pyrroloquinoline quinone (PQQ), Figure 1. This discovery was later expanded to include an ability to isolate the novel quinone derivative via fermentation from various microorganisms.2 The abundance of the molecule in numerous bacterial and plant sources raised a question, still not entirely answered, of whether PQQ might be another human vitamin as it was discovered to occupy various body tissues and sites.3–5 Since these early discoveries, it has now been found that PQQ is highly conserved in numerous bacteria and plants.6 One particularly important aspect of the role of PQQ in mammals is as a possible redox cofactor.7 It has been suggested that in this redox capacity, PQQ may have an ability to oxidatively and reductively recycle approximately 20,000 times which makes it more stable in this capacity than vitamin C by a factor of about 5000. In addition, the molecular stability to oxidative and reductive conditions makes it much more stable than some very popular nutritional and skin antioxidants including quercetin (25-fold), EGCG (30-fold) and DOPA (1000-fold).7
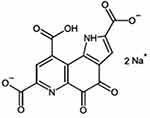 |
Figure 1 Chemical structure of pyrroloquinoline quinone disodium (PQQ2Na). |
It has been suggested that in humans, PQQ may act to increase mitochondrial biogenesis which is the growth of new cellular mitochondria.8–10 This is important because it suggests that PQQ may interact closely with cellular mitochondria in several ways.11,12 Of particular interest is the ability of PQQ to control cellular mitochondrial ROS which have been suggested to be directly related to cellular inflammation pathways.13,14 The studies by Yang et al are particularly interesting as they suggest that the PQQ may influence inflammation via the NF-ƙβ nuclear pathways.14 This raises the possibility that PQQ may influence the formation of ROS and control of the skin’s innate immune response driven through the formation of the NLRP inflammasomes and expression of active Caspase-1.15–17 The relationship between the NF-ƙβ nuclear pathways, NLRP3 inflammasome activation and mitochondria wellbeing is important as it appears to influence direct aspects of the extracellular matrix protein expression and mitochondrial health.18,19
This paper will discuss recent studies that directly examine the influence of PQQ as the water-soluble disodium salt (called PQQ2Na) on Normal Human Epidermal Keratinocytes (NHEK) and Normal Human Dermal Fibroblasts (NHDF) for the ability of the molecule to increase mitochondrial biogenesis in these important skin cells. More specifically, the studies examine the influence of PQQ2Na on the expression of two established markers for mitochondrial biogenesis, Cyclooxygenase-1 (COX-1) and Succinate dehydrogenase complex, subunit A (SDHA). Both mitochondrial biomarkers have been shown previously to demonstrate mitochondrial biogenesis in PQQ-treated human-induced Pluripotent Stem Cells.20
In addition, the studies examine the impact of PQQ2Na on the control of NLRP-induced active Caspase-1 release in UVB and ATP-activated NHEKs using previously reported testing procedures.17,21,22 Like mitochondrial biogenesis, the NLRP-induced inflammasome activation of active Caspase-1 is intimately related to the mitochondrial ROS activity.23
Materials and Methods
Pyrroloquinoline Quinone
The Pyrroloquinoline quinone disodium (PQQ2Na) employed in these studies was obtained from a commercial source selling the purified ingredient as a nutritional supplement. The purity of the molecule was shown to be greater than 98%.
Cell Culture
Adult human epidermal keratinocytes and dermal fibroblasts were obtained from Thermofisher. The keratinocytes were grown using EpiLife Media (60 µM calcium) supplemented with 0.2% v/v bovine pituitary extract, 1 µg/mL recombinant human insulin-like growth factor-I, 0.18 µg/mL hydrocortisone, 5 µg/mL bovine transferrin, 0.2 ng/mL human epidermal growth factor. The fibroblasts were grown in DMEM media supplemented with 10 ug/mL insulin, 1 ng/mL bFGF and 2% fetal bovine serum.
Toxicity Prescreen
Keratinocytes and fibroblasts were seeded into the wells of a 96-well plate and grown until confluent. Once confluent, the cells were treated for 48 hours with the test materials. At the end of the treatment period, the culture media was replaced with media supplements with 0.5 mg/mL MTT. The cells were incubated at 37°C for thirty minutes and then washed with PBS. Two hundred microliters of isopropanol were added to each well to extract the reduced MTT and the plate was read with a plate reader at 540 nm using isopropanol as the blank. The results for the toxicity prescreen are presented as percent viability using an untreated group to represent 100% viability.
Mitochondrial Biogenesis Assay
Keratinocytes and fibroblasts were seeded into the wells of a 96-well plate and grown until confluent. Once confluent, the cells were treated for 48 hours with the test materials. At the end of the treatment period mitochondrial biogenesis was assessed using a MitoBiogenesis In-Cell ELISA Kit (Abcam) per the manufacturer’s instructions. Briefly, the cells were fixed in 4% paraformaldehyde prepared in PBS for 10 minutes at room temperature. At the end of the fixation period, the cells were washed three times with PBS and then incubated overnight at 4°C with PBS supplemented with 0.02% sodium azide to decrease any residual peroxidase activity in the cells. On the following day the sodium azide solution was replaced with a Quenching solution to decrease any residual phosphatase activity and the well plate was incubated for 10 minutes at room temperature. After washing the plate three times with PBS, the fixed cells were permeabilized by incubating them for 30 minutes in a 0.1% Triton X-100 solution prepared in PBS. After the cells were permeabilized, they were incubated for two hours at room temperature in a 2x blocking solution. At the end of the blocking incubation, the cells were probed with an antibody cocktail containing both an anti-Cox-1 and an anti-SDHA antibody in 1x blocking buffer. After incubating the plate for two hours at room temperature and then washing it three times with PBS, the cells were probed with a second antibody cocktail containing an HRP-conjugated secondary antibody to detect anti-Cox-1 and an AP-conjugated secondary antibody to detect anti-SDHA. The plate was then incubated for 2 hours at room temperature, washed twice with PBS, and then incubated with a development solution with fluorescence substrates for HRP and AP. The plate was then read after 25 minutes of incubation using a fluorometer set to an ex/em of 355/485 for SDHA and 530/590 for COX1. After reading the plate, the development solution was replaced with a Janus Green stain and incubated for 5 minutes at room temperature. At the end of the incubation, the wells were washed 5 times with distilled water to remove any unbound dye. The cellular bound Janus Green stain was then extracted using 0.5 N HCl and the plate was read on a plate reader at 620 nm. The fluorescence-based measurements for SDHA and COX1 were then normalized to cell number using the Janus Green measurements. The results for this assay are expressed as a percent using untreated cells to represent 100% expression of SDHA or COX1.
Inhibition of Caspase-1 Activation Assay
The ability of the test material to inhibit ATP or UVB-induced caspase-1 activation was assessed using previously established methods.17 Briefly, keratinocytes were either exposed to 60 mJ/cm2 of UVB light or cultured in the presence of 5 mM ATP to induce the activation of caspase-1. For the UVB treatment, the cell culture media was replaced with PBS for the UVB exposure and then immediately after the UVB irradiation the PBS was replaced with media containing the test materials. For the ATP treatment, the cells were treated with ATP and the test materials simultaneously over the treatment period. For both stresses, caspase-1 activation was measured 20 hours after the initiation of the stress using the luminescence-based Caspase-Glo 1 Inflammasome Assay kit (Promega). The results of the caspase-1 activation assay are expressed in luminescence intensity, where an increase in intensity represents an increase in caspase-1 activity.
Statistics
All the assays were performed in triplicate with the treatment means compared using an ANOVA and a p < 0.05 as a cutoff for statistical significance.
Results and Discussion
MTT Assay Results
Both Normal Human Epidermal Keratinocytes and Normal Human Dermal Fibroblasts were examined for their ability to respond to PQQ2Na at various doses to examine the cell’s abilities to resist toxicity to PQQ2Na. The results of these assays are shown in Figure 2. It was found that the Normal Human Epidermal Keratinocytes were more stable to the PQQ2Na, responding positively to treatments as high as 100 µg/mL (100 ppm) before the cellular survival dropped below 80%, the cutoff at which in vitro studies are considered to become problematic due to excessive cellular toxicity. Interestingly, the fibroblasts were shown to not be as robustly resistant to the PQQ2Na as the keratinocytes demonstrating pronounced cytotoxic response at 10 µg/mL. The reason for this cytotoxicity difference is unknown but may be an interesting finding that demonstrates a difference between skin cells known to move through an exfoliation and cellular differentiation process (keratinocytes) and skin cells known to enter apoptosis and cellular death (fibroblasts) as they age. For this reason, further mitochondrial biogenesis studies on keratinocytes were run at concentrations between 5 µg/mL and 100 µg/mL while similar studies on fibroblasts were run at PQQ2Na concentrations between 0.1 µg/mL and 5 µg/mL.
In the active Caspase-1 release studies because the cells are placed into an NLRP-induced active Caspase-1 release profile which already places cellular inflammatory stress on the cells, the keratinocytes were treated with 0.05 µg/mL to 50 µg/mL of PQQ2Na following the procedures previously outlined by Gruber et al.17 Briefly, the keratinocytes were irradiated using 60 mj/cm2 of UVB radiation to activate the NLRP-induced release of Caspase-1 followed by replacement of the cell culture media with media containing the various treatments with the PQQ2Na. The measurements were made at the 20-hour timeframe after treatment with the PQQ2Na as previously described. Likewise, for the ATP-treated cells, 5 mM of ATP was employed to activate the keratinocytes to release Caspase-1 followed by a 20-hour timeframe to measure active Caspase-1 expression again as previously described.
Keratinocyte COX-1 and SDHA Mitochondrial Biogenesis Results
Results from the treatment of NHEKs with PQQ2Na looking at COX-1 releases are shown in Figure 3.
Figure 4.Fibroblast COX-1 and SDHA Mitochondrial Biogenesis Results
Results from the treatment of NHDFs with PQQ2Na looking at COX-1 expression are shown in Figure 5.
Figure 6.From the results of both the keratinocyte and fibroblast treatments, considering the adjustments made to the measurements by the Janus Green staining to account for final cell density, it is apparent that neither cell type responded within the 48 hours of treatment by increasing cellular mitochondria density. This result was somewhat surprising given that previous work by Augustyniak et al, with PQQ had demonstrated a positive influence on mitochondrial biogenesis in induced human Pluripotent Stem Cells.20 In addition, PQQ has been demonstrated in human oral studies to increase mitochondrial biogenesis.10 However, in both important cell types that make up the epidermis and dermis of human skin, topical treatment with PQQ2Na does not influence mitochondrial biogenesis.
UVB-Induced Active Caspase-1 Expression Results
In earlier work previously reported by Gruber et al, NHEKs were activated to express Caspase-1 using 60 mJ/cm2 of UVB radiation.17 In these earlier studies, fibroblasts were not investigated and so had not been shown previously to respond to UVB and ATP to express active Caspase-1 like NHEKs do. For this reason, only NHEKs were examined for their ability to respond to PQQ in this inflammasome model. Results from these studies are shown in Figure 7.
 |
Figure 7 Caspase-1 assay results expressed in luminescence on UVB-treated NHEKs treated with various levels of PQQ2Na. Asterisks indicate statistical significance (p ≤ 0.05) versus untreated control. |
ATP-Induced Active Caspase-1 Expression Results
Again, following the previously reported methods, NHEKs were treated with 5 mM of ATP to induce expression of active Caspase-1. Results from these studies are shown in Figure 8.
From the results, as found previously, both UVB and ATP induced a robust increase in the expression of active Caspase-1 as measured using the Promega Caspase-1 Glo assay.17 For the cells expressing active Caspase-1 induced by previous treatment with ATP, the PQQ2Na showed no influence on the expression of active Caspase-1. However, in the UVB-treated keratinocytes, the PQQ2Na showed a statistically significant suppression of active Caspase-1 release at 50 µg/mL of treatment and what appears to be a dose-dependent decrease in active Caspase-1 from 0.05 µg/mL to 50 µg/mL although the treatments less than 50 µg/mL were not shown to be statistically significant to the untreated controls.
Conclusions
The results of the active Caspase-1 assays demonstrate that PQQ2Na is influencing the skin cell’s innate immune response driven through UVB-induced active Caspase-1 release. UVB would be considered a Danger Associated Molecular Pattern or DAMP.22,24 This lends further support to the work by Yang et al, that PQQ can influence the NF-ƙβ nuclear pathways in other cells besides nerve cells. This likely occurs through a potent mitochondrial ROS-reducing ability of the PQQ2Na which supports the molecule’s benefits as a redox-stable antioxidant that can impact cellular health. However, it appears that PQQ2Na does not affect the expression of active Caspase-1 when the external influence is ATP. ATP is both a DAMP and a Pathogen Associated Molecular Pattern or PAMP. It functions to create ROS in pathways that are different than direct exposure to UVB which causes damage to mitochondria directly. This difference in performance of the molecule may be important for when the molecule should be applied topically. For instance, the strong UVB influence suggests that the ingredient would be particularly beneficial in topical applications where potential UV or high energy impacts are affecting the skin. Likewise, in oxidative environments such as chronic ozone exposure or chronic small particulate pollution (PM2.5) exposure, both of which have recently been shown to activate the NLRP inflammasome pathways via ROS-induced pathways, the PQQ2Na may also have beneficial effects.25,26
Disclosure
The authors report no conflicts of interest in this work.
References
1. Salisbury SA, Forrest JS, Cruse WBT, Kennard O. A novel coenzyme from bacterial primary alcohol dehydrogenases. Nature. 1979;280:843–844. doi:10.1038/280843a0
2. Ameyama M, Hayashi M, Matsushita K, Shinagawa E, Adachi O. Microbial production of pyrroloquinoline quinone. Agric Biol Chem. 1984;48:561–565.
3. Kumazawa T, Sato K, Seno H, Ishii A, Suzuki O. Levels of pyrroloquinoline quinone in various foods. Biochem J. 1995;307:331–333. doi:10.1042/bj3070331
4. Killgore J, Smidt C, Duich L, et al. Nutritional importance of pyrroloquinoline quinone. Science. 1989;245:850–852. doi:10.1126/science.2549636
5. Bishop A, Gallop PM, Kamovsky MC. Pyrroloquinoline quinone: a novel vitamin? Nut Rev. 1998;56:287–293. doi:10.1111/j.1753-4887.1998.tb01661.x
6. Naveed M, Tariq K, Sadia H, Ahmad H, Mumtaz AS. The life history of pyrroloquinoline quinone (PQQ): a versatile molecule with novel impacts on living systems. Int J Mol Biol. 2016;1:1–20.
7. Ricker R, Chowandadisai W, Nakano M. Potential physiological importance of pyrroloquinoline quinone. Alternat Med Rev. 2009;14:268–277.
8. Chowanadisai W, Bauerly KA, Tchaparian E, Wong A, Cortopassi GA, Rucker RB. Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element binding protein phosphorylation and increased PGC-1α expression. J Biol Chem. 2010;285:142–152. doi:10.1074/jbc.M109.030130
9. Saihara K, Kamikubo R, Ikemoto K, Uchida K, Akagawa M. Pyrroloquinoline quinone, a redox-active o-quinone, stimulates mitochondrial biogenesis by activating the SIRT1/PGC-1α signaling pathway. Biochem. 2017;56:6615–6625. doi:10.1021/acs.biochem.7b01185
10. Hwang PS, Machek SB, Cardaci TD, et al. Effects of pyrroloquinoline quinone on aerobic exercise performance and indices of mitochondrial biogenesis in untrained men. J Am Coll Nutri. 2020;39:547–556. doi:10.1080/07315724.2019.1705203
11. Kong X, Wang R, Xue Y, et al. Sirtuin-3, a new target of PGC-1α, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5:1–13. doi:10.1371/journal.pone.0011707
12. Cherry AD, Piantadosi CA. Regulation of mitochondrial biogenesis and its intersection with inflammatory responses. Antiox Redox Signal. 2015;22:965–976. doi:10.1089/ars.2014.6200
13. Harris CB, Chowanadisai W, Mishchuk DO, Satre MA, Slupsky CM, Rucker RB. Dietary pyrroloquinoline quinone (PQQ) alters indicators of inflammation and mitochondrial-related metabolism in human subject. J Nutr Biochem. 2013;24:2076–2084. doi:10.1016/j.jnutbio.2013.07.008
14. Yang C, Yu L, Kong L, et al. Pyrroloquinoline quinone (PQQ) inhibits lipopolysaccharide-induced inflammation in part via downregulated NF-ƙβ and p38/JNK activation in microglial and attenuates microglia activation in lipopolysaccharide treatment mice. PLoS One. 2014;9:1–11.
15. Zhong Z, Umemura A, Sanchez-Lopez E, et al. NF-ƙβ restricts inflammasome activation via elimination of damaged mitochondria. Cell. 2016;164:896–910. doi:10.1016/j.cell.2015.12.057
16. Zhao W, Ma L, Cai C, Gong X. Caffeine inhibits NLRP3 inflammasome activation by suppressing MAPK/NF-ƙβ and A2aR signaling in LPS-induced THP-1 macrophages. Int J Biol Sci. 2019;15:1571–1581. doi:10.7150/ijbs.34211
17. Gruber JV, Holtz R. In vitro expression of NLRP inflammasome-induced active Caspase-1 expression in normal human epidermal keratinocytes (NHEK) by various exogenous threats and subsequent inhibition by naturally derived ingredient blends. J Inflamm Res. 2019;12:219–230. doi:10.2147/JIR.S215776
18. Kuang J, Xie M, Xiaolin W. The NALP3 inflammasome is required for collagen synthesis via the NF-ƙβ pathway. Int J Mol Med. 2018;41:2279–2287. doi:10.3892/ijmm.2018.3404
19. Holley CL, Schroder K. The rOX-stars of inflammation: links between the inflammasome and mitochondrial meltdown. Clin Transl Immunol. 2020;9:e01109. doi:10.1002/cti2.1109
20. Augustyniak J, Lenart J, Zychowicz M, et al. Sensitivity of hiPSC-derived neural stem cells (NSC) to pyrroloquinoline quinone depends on their developmental stage. Toxicol in Vitro. 2017;45:434–444. doi:10.1016/j.tiv.2017.05.017
21. Gruber JV, Stojkoska V. NLRP inflammasomes and induced skin inflammation, barrier recovery and extended skin hydration. Int J Cosmet Sci. 2020;42:68–78. doi:10.1111/ics.12588
22. Gruber JV. Do normal human epidermal keratinocytes require a priming step to activate the NLRP3 Inflammasomes to exogenous threats? JOJ Dermatol Cosmet. 2021;3:555621. doi:10.19080/JOJDC.2021.03.555621
23. Sorbara MT, Girardin SE. Mitochondrial ROS fuel the inflammasome. Cell Res. 2011;21:558–560. doi:10.1038/cr.2011.20
24. Sanchez-Rodriguez R, Munan F, Angioni R, et al. Targeting monoamine oxidase to dampen NLRP3 inflammasome activation in inflammation. Cell Mol Immunol. 2020;18:1311–1313. doi:10.1038/s41423-020-0441-8
25. Ferrara F, Pambianchi E, Pecorelli A, et al. Redox regulation of cutaneous inflammasome by ozone exposure. Free Rad Biol Med. 2020;152:561–570. doi:10.1016/j.freeradbiomed.2019.11.031
26. Dong L, Hu R, Yang D, et al. Fine particulate matter (PM2.5) upregulates expression of inflammasome NLRP1 via ROS/NF-ƙβ signaling in HaCaT cells. Int J Med Sci. 2020;17:2200–2206. doi:10.7150/ijms.46962
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






