Back to Journals » Infection and Drug Resistance » Volume 15
Pyopneumothorax Caused by Trichomonas tenax and Porphyromonas endodontalis Coinfection in a Patient with Previous Cerebral Infarction: A Case Report and Literature Review
Received 20 July 2022
Accepted for publication 3 October 2022
Published 21 October 2022 Volume 2022:15 Pages 6101—6108
DOI https://doi.org/10.2147/IDR.S381859
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
De-Han Cai,1 Xiao-Lin Fang2
1Nephrology Department in Jiangxi Provincial People’s Hospital Affiliated to Nanchang Medical College, Nanchang, People’s Republic of China; 2Department II of Respiratory and Critical Care in Jiangxi Provincial People’s Hospital Affiliated to Nanchang Medical College, Nanchang, People’s Republic of China
Correspondence: Xiao-Lin Fang, Department II of Respiratory and Critical Care in Jiangxi Provincial People’s Hospital Affiliated to Nanchang Medical College, Nanchang, People’s Republic of China, Tel +86 13767049980, Email [email protected]; [email protected]
Background: Even with the advent of NGS and PCR diagnostic tools, cases of chest infections caused by Trichomonas are still very rare. Such pathogens are less likely to be considered by clinicians. These cases frequently involve the pleura and lead to pneumothorax, hydropneumothorax, or pyopneumothorax, making the disease severe.
Case Presentation: A 69-year-old man diagnosed with cerebral infarction a year ago sought medical attention for right-sided pyopneumothorax and respiratory failure. The pathogen found in the pleural fluid was highly suspected to be Trichomonas tenax (T. tenax). Pleural fluid mNGS confirmed T. tenax and Porphyromonas endodontalis coinfection. Metronidazole combined with piperacillin tazobactam was administered to counteract infection. Simultaneously, closed chest drainage and thoracoscopic release of pleural adhesions were performed. The patient was cured, discharged from the hospital, and was in good condition after six months of follow-up.
Conclusion: When chest infections occur in patients with poor oral hygiene and underlying diseases that may lead to aspiration, the identification of Trichomonas infection should be noted. Early confirmation of the diagnosis often requires mNGS and PCR. Metronidazole is essentially effective against Trichomonas, and medical thoracoscopy can be used to manage pleural conditions if necessary.
Keywords: pyopneumothorax, Trichomonas tenax, Porphyromonas endodontalis, aspiration, VATS
Introduction
Trichomonas is a protozoan. Different Trichomonas species can parasitize different parts of the body: Trichomonas vaginalis in the genitourinary tract, Pentatrichomonas hominis in the intestine, and T. tenax in the oral cavity of people with poor oral hygiene.1 The main Trichomonas species associated with chest infections is T. tenax.2 Since cultures are often unsuccessful, diagnosis is primarily determined by the morphology and movement patterns of fresh specimens under the microscope.3 Owing to the availability of PCR and mNGS methods, pulmonary and pleural disorders caused by Trichomonas are being diagnosed more often than before. However, it remains extremely rare. Since Memik reported the first case of chest infection caused by Trichomonas in 1968, surprisingly no more than 20 cases of Trichomonas-associated chest infections have been published in PubMed worldwide.4 Here we present a case of pyopneumothorax caused by T. tenax and Porphyromonas endodontalis coinfection in a patient with previous cerebral infarction. In addition, we reviewed the reported cases of chest infections caused by Trichomonas in the last 20 years.
Case Presentation
The patient was a 69-year-old male farmer who presented to a local hospital with right-sided chest pain, cough, sputum production, and fever for one week. Chest computed tomography (CT) at the local hospital showed multiple right pleural effusions and pneumothorax with right lung atelectasis. This patient had a cerebral infarction a year prior to this visit, with a slight decrease in right-sided muscle strength. He had a history of hypertension for several years and was receiving amlodipine benzoate with poor blood pressure control. The patient denied any history of other chronic diseases such as coronary heart disease, diabetes mellitus and malignancy. He had a 30-year history of smoking, 1 pack/day, had quit smoking for a year, and denied alcoholism. The patient was transferred to our hospital on October 17 after ineffective treatment with anti-infection (details unknown) and oxygen therapy.
The patient’s vital signs on admission were as follows: temperature, 37.4°C; heart rate, 81 beats/min; breathing rate, 24 beats/min; and blood pressure, 165/85 mmHg. His consciousness was clear, and oxygen saturation was 85% (air). Reduced respiratory motility, decreased breathing sounds, and wet rales were observed on the right side. Both the lower extremities were moderately edematous. His right limb muscle strength was of grade V. Routine blood test showed leukocytes 13.5×109/L (normal range 3.5–9.5×109/L), neutrophils 12.4×109/L (normal range 1.4–7.12×109/L), hemoglobin 82 g/L (normal range 130–175 g/L) and normal eosinophils. Blood biochemistry showed only 18.1 g/L of albumin (normal range 40–55 g/L) and mildly elevated liver and kidney function parameters. PCT 4.88 ng/mL (normal range 0–0.05 ng/mL) and CRP 456 mg/l (normal range 0–8 mg/L) were observed. Serum GM and BDG levels were normal. T-SPOT. TB and ANA profiles were negative. T cell subsets and serum immunoglobulins were within normal limits. The chest CT on admission is shown in Figure 1.
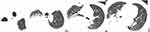 |
Figure 1 Chest CT on admission showing right pleural effusion, and pneumothorax. |
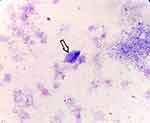
On day 2 of hospitalization, 700 mL of celiac fluid was extracted via closed chest drainage. Pleural fluid tests showed 19.6 g/L total protein, 4.9 g/L albumin, LDH 4700 IU/L (serum normal range 109–245 U/L), and ADA 183.4 U/L (serum normal range 2–17 U/L). The pleural fluid celiac characterization test results were negative. Pleural fluid CEA was within normal limit. Levantine tests were positive, with a nucleated cell count of 273.6×109/L (pleural fluid normal range <500×106/L) and 92% neutrophilic. A protozoan slightly larger in size than leukocytes, oval in shape, with rotational movement, was microscopically observed on a wet smear of pleural fluid. Suspected T. tenax was observed (Figure 2).
On day 3 of hospitalization, intravenous infusion of metronidazole (0.5 g, q8h) combined with piperacillin tazobactam (4.5 g, q8h) was started. Thoracoscopy was performed on day 4 of hospitalization. Yellow flocculent pus was seen and aspirated. Adhesions on the pleural surface and granulation tissue were also visible. The adhesions were partly released and a biopsy of the mural pleura was performed. Pathological examination suggested fibrous connective tissue and many inflammatory fibrin-like exudates. After the operation, the patient felt relieved with chest pain and cough, and continued with closed thoracic drainage. Bronchoscopy was performed on day 5 of hospitalization, and mucous secretions were seen in the right lower lobe, where they were suctioned and lavaged.
On day 6 of hospitalization, the patient agreed to complete mNGS, and pleural fluid was sent for mNGS. The following day, the results were reported as 387 sequences of T. tenax, and 2,139,183 sequences of Porphyromonas endodontalis with a relative abundance of 70.73%. At this time, the diagnosis was clear of right-sided encapsulated pyopneumothorax, T. tenax and Porphyromonas endodontalis coinfection, type I respiratory failure, hypertension, and sequelae of cerebral infarction.
On day 8 of hospitalization, the patient experienced nausea and vomiting during the intravenous infusion of metronidazole, which was considered an adverse drug reaction. Symptomatic treatment was administered, and metronidazole was replaced to levonidazole intravenous infusion (0.5g, q12h). No further gastrointestinal reactions occurred after medication adjustment. The daily chest drainage fluid gradually became clearer and the volume decreased. Ultrasound examination of the pleural fluid showed that the right pleural fluid was separated. Multiple intrapleural injections of urokinase were administered.
Subsequently, the pleural fluid was rechecked several times and no more Trichomonas was detected. The blood culture results were negative. On day 24 of hospitalization, the patient was discharged after significant resolution of the chest lesion (Figure 3) and improvement of the inflammatory indicators. The patient was followed-up for 6 months without any chest infections.
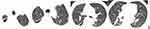 |
Figure 3 Chest CT at discharge showing that the right pleural effusion and pneumothorax were markedly absorbed. |
Discussion and Conclusion
Several species of Trichomonas can be responsible for chest infections, including Trichomonas vaginalis, T. tenax, Pentatrichomonas hominis, and Tetratrichomonas spp. Among these, T. tenax is the most common.5 T. tenax, a difficult-to-culture anaerobic protozoan, is highly prevalent in periodontal diseases.6,7 It is now generally accepted that the organism is thought to enter the respiratory tract through inhalation from the oropharynx.1
In this case, the patient had poor oral hygiene, and the fact that he had a cerebral infarction a year earlier suggests the possibility of aspiration. The pleural fluid mNGS results detected a large amount of oral anaerobic flora in addition to T. tenax, and multiple blood cultures were negative. Therefore, we believe that the T. tenax infection in this patient was caused by oropharyngeal aspiration.
Most articles on Trichomonas causing chest disease are case reports. Although the number of Trichomonas cases has increased over the years with the application of PCR and mNGS techniques, we searched PUBMED and found only 14 cases of chest infections caused by Trichomonas in the last 20 years, which shows that the disease is rare (Table 1). The geographical distribution of the disease worldwide, in descending order, is France, China, Mexico, the United States, and Japan.1,2,8–17
 |
Table 1 Clinical Characteristics of the 15 Cases of Chest Infections Caused by Trichomonas |
Trichomonas species were identified in 14 of 15 cases including our case. One case was Trichomonas vaginalis, five were Tetratrichomonas, and the rest were T. tenax. Trichomonas was co-infected with bacteria in most cases and with fungi in only one case. Among the combined bacterial infections, oral anaerobic flora predominates, and others are mostly parthenogenic anaerobes, such as various streptococci. All these bacteria reside in the oral cavity or upper respiratory tract. Of the 15 cases, two cases did not describe whether the pleura was involved, two cases involved the pleura but did not describe the location, and the remaining 11 cases involved the pleura and described the location. Among them, the right pleura alone was the most involved (8/11), followed by the bilateral pleural involvement (2/11), and only one case involved the left pleura alone.1,2,8–17 Aspiration pneumonia is predominant on the right side due to the steep right main bronchus. These observations further corroborate that the route of Trichomonas chest infection is from the oropharyngeal aspiration to the lower respiratory tract.
In the reviewed cases of Trichomonas infection, we found that sometimes the patients did not actually have severe pneumonia, while the pleural manifestations of the disease were more prominent. In our case, the degree of airway mucosal congestion as well as the amount of secretions observed on bronchoscopy was much less than that in conventional pneumonia. Why does Trichomonas pass through the lower respiratory tract but end up in the pleura-causing lesions? We speculate that this is because the pleural space is more conducive to the growth and reproduction of anaerobic bacteria than the oxygen-rich environment of the lungs, and protozoa including T. tenax feeds on bacteria.1,10,18
Because T. tenax feeds on bacteria, T. tenax alone can hardly cause chest infections. Do these bacteria exist only as food for Trichomonas in pleural diseases? It is shown that anaerobic bacteria are involved in 60%, 80% and 45% of lung abscesses, aspiration pneumonia and pyothorax respectively.19 Furthermore, the success rate of culturing such pathogens is variable.20 It is stated that whether the pleural infection is community-acquired or hospital-acquired, antibiotic selection needs to cover anaerobes unless there is culture confirmation of pneumococci or other specific pathogens.21 In the 2017 the American Association for Thoracic Surgery consensus guidelines for the management of empyema, the need for antibiotic therapy to cover anaerobes remains emphasized. Even if anaerobic cultures return negative results, anti-anaerobic therapy must be continued.20 Data from numerous studies also support the role of oropharyngeal flora, consisting of specialized or parthenogenic anaerobic bacteria, as pathogens causing pleural infections and in the possible pathogenic mechanisms of pleural infections.22 Coupled with the fact that our pleural fluid mNGS test showed high sequence number and relative abundance values of Porphyromonas endodontalis, we concluded that this case was a coinfection of T. tenax and Porphyromonas endodontalis resulting in pyopneumothorax.
VATS is considered to be the treatment of choice in both the early and late stages of empyema.20 Because our patient had an encapsulated pyopneumothorax with more compartments, it had not yet reached stage III of pyothorax, forming a cortex that compressed the surface of the visceral pleura. Considering the poor effect of chest drainage alone, we released the pleural adhesions thoracoscopically.
It has been suggested that Trichomonas infection is often complicated by pulmonary diseases or immunosuppression.8 While in the 15 patients, 2 had no underlying disease, 4 had brain disorders or surgery, 3 cases had malignancy, 1 case had heart transplantation, 1 case had postoperative reflux esophagitis, and 4 cases were not described. There appeared to be no significant relationship between the presence of the disease and the immune status of the patient. Conversely, diseases that predispose to patients’ aspiration, such as brain disorders, are more susceptible to Trichomonas infections. The prognosis of this disease is generally good. All patients had a clinically improved outcome, except for three patients with glioma, advanced adenocarcinoma, and postoperative reflux esophagitis, respectively, who were fatal outcomes.
Unfortunately, in this case, we did not complete NGS testing of BALF and pleural biopsies. No specific Trichomonas antibodies were available. These uncompleted tests may contribute to further understanding the mechanism of Trichomonas pathogenesis.
This case reminds us that in the management of pleural disease with or without pulmonary lesions, care should be taken to identify Trichomonas infection if common antibiotics are not effective and the patient has poor oral hygiene and underlying disease with the potential for aspiration. As this pathogen is not easy to culture, the use of mNGS or PCR can rapidly target pathogens and clarify the specific species of Trichomonas. Trichomonas is basically sensitive to metronidazole. While treating Trichomonas, the use of antibiotics needs to take into account its possible coinfection with anaerobic bacteria. The need for thoracoscopic local intervention is determined by the patient’s pleural lesions. The main preventive measures are good personal oral hygiene and immediate medical attention in cases of oral diseases.
Abbreviations
T. tenax, Trichomonas tenax; NGS, next-generation sequencing; PCR, polymerase chain reaction; CT, computed tomography; GM, galactomannan; BDG, 1-3-β-D-glucan; TB, tuberculosis; ANA, anti-nuclear antibody; BALF, bronchoalveolar lavage fluid; VATS, video-assisted thoracic surgery.
Data Sharing Statement
The data are available from the corresponding author on reasonable request.
Consent for Publication
Written informed consent was obtained from the patient and his family for the publication of this manuscript. Institutional approval is not required to release case details.
Acknowledgment
The authors are grateful to Dr Yun-wei Zheng for providing photos of the microorganism.
Funding
There is no funding to report.
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
1. Lewis KL, Doherty DE, Ribes J, Seabolt JP, Bensadoun ES. Empyema caused by trichomonas. Chest. 2003;123(1):291–292. PMID: 12527635. doi:10.1378/chest.123.1.291
2. Chiche L, Donati S, Corno G, et al. Pleuro-pneumopathie à Trichomonas tenax [Trichomonas tenax in pulmonary and pleural diseases]. Presse Med. 2005. 34(19 Pt 1):1371–1372. French. PMID: 16292189. doi:10.1016/s0755-4982(05)84193-4
3. Yao C, Ketzis JK. Aberrant and accidental trichomonad flagellate infections: rare or underdiagnosed? Trans R Soc Trop Med Hyg. 2018;112(2):64–72. PMID: 29608771. doi:10.1093/trstmh/try027
4. Memik F. Trichomonads in pleural effusion. JAMA. 1968;204(12):1145–1146. PMID: 5694759. doi:10.1001/jama.1968.03140250125023
5. Lin C, Ying F, Lai Y, et al. Use of nested PCR for the detection of trichomonas in bronchoalveolar lavage fluid. BMC Infect Dis. 2019;19(1):512. PMID: 31182037; PMC6558733. doi:10.1186/s12879-019-4118-9
6. Benabdelkader S, Andreani J, Gillet A, et al. Specific clones of Trichomonas tenax are associated with periodontitis. PLoS One. 2019;14(3):e0213338. PMID: 30856220; PMC6411126. doi:10.1371/journal.pone.0213338
7. Bracamonte-Wolf C, Orrego PR, Muñoz C, et al. Observational cross-sectional study of Trichomonas tenax in patients with periodontal disease attending a Chilean university dental clinic. BMC Oral Health. 2019;19(1):207. PMID: 31484557; PMC6727549. doi:10.1186/s12903-019-0885-3
8. Porcheret H, Maisonneuve L, Estève V, Jagot JL, Le Pennec MP. Trichomonase pleurale à Trichomonas tenax [Pleural trichomoniasis due to trichomonas tenax]. Rev Mal Respir. 2002;19(1):97–99. French. PMID: 17546821.
9. Mallat H, Podglajen I, Lavarde V, Mainardi JL, Frappier J, Cornet M. Molecular characterization of Trichomonas tenax causing pulmonary infection. J Clin Microbiol. 2004;42(8):3886–3887. PMID: 15297557; PMC497589. doi:10.1128/JCM.42.8.3886-3887.2004
10. Wang HK, Jerng JS, Su KE, Chang SC, Yang PC. Trichomonas empyema with respiratory failure. Am J Trop Med Hyg. 2006;75(6):1234–1236. PMID: 17172399. doi:10.4269/ajtmh.2006.75.1234
11. Bellanger AP, Cabaret O, Costa JM, Foulet F, Bretagne S, Botterel F. Two unusual occurrences of trichomoniasis: rapid species identification by PCR. J Clin Microbiol. 2008;46(9):3159–3161. PMID: 18632901; PMC2546736. doi:10.1128/JCM.00322-08
12. Mantini C, Souppart L, Noël C, et al. Molecular characterization of a new Tetratrichomonas species in a patient with empyema. J Clin Microbiol. 2009;47(7):2336–2339. PMID: 19420167; PMC2708534. doi:10.1128/JCM.00353-09
13. Leterrier M, Morio F, Renard BT, Poirier AS, Miegeville M, Chambreuil G. Trichomonas in pleural effusion: case report, literature review and utility of PCR for species identification. New Microbiol. 2012;35(1):83–87. PMID: 22378558.
14. Lopez-Escamilla E, Sanchez-Aguillon F, Alatorre-Fernandez CP, et al. New tetratrichomonas species in two patients with pleural empyema. J Clin Microbiol. 2013;51(9):3143–3146. PMID: 23784131; PMC3754616. doi:10.1128/JCM.01056-13
15. Arase M, Yamashita M, Mimasu M, Mizuno S, Yamada K, Ohkusu K. [A first case report of empyema caused by Tetratrichomonas species in Japan]. Rinsho Byori. 2014;62(12):1197–1202. Japanese. PMID: 25823234.
16. Dong N, Jin X, Huang J, et al. Tetratrichomonas in pyopneumothorax. Am J Emerg Med. 2019;37(6):1215.e1–1215.e4. PMID: 31023584. doi:10.1016/j.ajem.2019.03.029
17. Wu Y, Ye Y, Yang Y, Yang W, Lin J, Cao K. Pyopneumothorax from coinfection by Trichomonas tenax and Geotrichum capitatum in a child from China: a case report. BMC Infect Dis. 2021;21(1):842. PMID: 34416850; PMC8377835. doi:10.1186/s12879-021-06539-0
18. Bedawi EO, Hassan M, Rahman NM. Recent developments in the management of pleural infection: a comprehensive review. Clin Respir J. 2018;12(8):2309–2320. PMID: 30005142. doi:10.1111/crj.12941
19. Germaud P. Rôle des bactéries anaérobies strictes au cours des suppurations pleuropulmonaires Obligate anaerobic bacteria in pleuropulmonary suppurations. Médecine et Maladies Infectieuses. 2000;30(Supplement 2):132s–136s. doi:10.1016/S0399-077X(00)89116-7
20. Shen KR, Bribriesco A, Crabtree T, et al. The American Association for thoracic surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg. 2017;153(6):e129–e146. PMID: 28274565. doi:10.1016/j.jtcvs.2017.01.030
21. Davies HE, Davies RJ, Davies CW; BTS Pleural Disease Guideline Group. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii41–53. PMID: 20696693. doi:10.1136/thx.2010.137000
22. Hassan M, Cargill T, Harriss E, et al. The microbiology of pleural infection in adults: a systematic review. Eur Respir J. 2019;54(3):1900542. PMID: 31248959. doi:10.1183/13993003.00542-2019
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.