Back to Journals » Cancer Management and Research » Volume 12
PVT1 Mediates Cell Proliferation, Apoptosis and Radioresistance in Nasopharyngeal Carcinoma Through Regulating miR-515-5p/PIK3CA Axis
Authors Han Y, Li F, Xie J, Wang Y, Zhang H
Received 9 April 2020
Accepted for publication 12 August 2020
Published 13 October 2020 Volume 2020:12 Pages 10077—10090
DOI https://doi.org/10.2147/CMAR.S257583
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Yanyan Han,1 Fang Li,2 Jun Xie,2 Yi Wang,2 Hua Zhang1
1Department of Otolaryngology, The First Affiliate Hospital of Xinjiang Medical University, Urumqi, Xinjiang, People’s Republic of China; 2Department of Otolaryngology, Urumqi Eye and ENT Specialist Hospital, Urumqi, Xinjiang, People’s Republic of China
Correspondence: Hua Zhang Tel +86-991-4366067
Email [email protected]
Background: Radioresistance greatly hinders the treatment of nasopharyngeal carcinoma (NPC). Long noncoding RNA (lncRNA) plasmacytoma variant translocation 1 (PVT1) has been corroborated to participate in diverse cancers, including NPC. Our aim was to investigate the underlying molecular mechanism of PVT1 in NPC radioresistance.
Methods: Quantitative real-time polymerase chain reaction (qRT-PCR) was utilized to measure the expression levels of PVT1, microRNA (miR)-515-5p and phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) in NPC tissues and cells. Cell counting kit-8 (CCK8) assay, colony formation assay and flow cytometry assay were employed to detect cell proliferation, radiosensitivity and apoptosis, respectively. The protein levels of Cyclin D1, B-cell lymphoma 2 associated X (Bax), Cleaved-caspase-3, PIK3CA, protein kinase B (AKT) and phosphorylated AKT (p-AKT) in samples were measured by Western blot. The starBase was used to predict the binding sites between miR-515-5p and PVT1 or PIK3CA. Dual-luciferase reporter assay and RNA immunoprecipitation (RIP) assay were performed to verify the interaction. Xenograft tumor model was established to investigate the biological role of PVT1 in vivo.
Results: The levels of PVT1 and PIK3CA were upregulated in NPC tissues and cells, opposite to the expression of miR-515-5p. Knockdown of PVT1 inhibited cell proliferation, radioresistance and promoted cell apoptosis in NPC cells. Meanwhile, PVT1 silencing downregulated Cyclin D1, and upregulated Bax and Cleaved-casp-3 in NPC cells after radiotherapy. Besides, miR-515-5p interacted with PVT1 and targeted PIK3CA in NPC cells. Further studies indicated that PVT1 regulated radioresistance via miR-515-5p/PIK3CA axis and modulated the AKT pathway by interacting with miR-515-5p. Moreover, knockdown of PVT1 suppressed tumor growth in vivo.
Conclusion: Downregulation of PVT1 inhibited proliferation, radioresistance and promoted apoptosis by downregulating PIK3CA via sponging miR-515-5p in NPC cells.
Keywords: NPC, PVT1, miR-515-5p, PIK3CA, radioresistance
Introduction
Nasopharyngeal carcinoma (NPC) is a cancer arising from the nasopharynx epithelium, and radiotherapy is the primary and only curative treatment for NPC.1 However, radioresistance is a huge obstacle for the treatment of NPC.2,3 Hence, it is compulsory to figure out the hidden molecular mechanism of radioresistance in NPC.
Long noncoding RNAs (LncRNAs) are a kind of RNA molecules with the length exceeding 200 nucleotides and they cannot encode proteins.4 Growing studies showed that lncRNAs were associated with radioresistance in diverse human cancers,5,6 including NPC.7,8 Recently, lncRNA plasmacytoma variant translocation 1 (PVT1) was reported to induce radioresistance in NPC.9 However, the function of PVT1 in mediating radioresistance in NPC is still poorly understood.
MicroRNAs (miRNAs) are small noncoding RNAs (nearly 22 nucleotides in length), which regulate gene expression by targeting the 3ʹ-untranslated region (3ʹUTR) at the post-transcriptional level.10 Emerging reports have intensely deepened our comprehension of the crucial role of miRNAs in radioresistance.11–13 MiR-515-5p was reported to be correlated with breast cancer,14 gastric cancer15 and prostate cancer.16 Nevertheless, the role of miR-515-5p in radioresistance in NPC is little known and worth investigating.
Phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) was involved in diverse human cancers. A previous study reported that PIK3CA acted as an oncogene in cervical cancer.17 PIK3CA mutations led to fulvestrant resistance in ER-positive breast cancer.18 Moreover, PIK3CA amplification was associated with the poor prognosis of NPC.19 Therefore, PIK3CA may be an appropriate drug target and novel regulators which modulate PIK3CA expression require to be defined.
In this study, the expression of PVT1, miR-515-5p and PIK3CA in NPC cells and tissues was measured. The potential regulatory mechanism of PVT1 in radioresistance was further investigated by the subsequent experiments.
Materials and Methods
Samples and Cell Culture
Twenty-nine NPC tissues and the paired nearby non-cancerous tissues (within 3 cm around tumors) were collected from the First Affiliate Hospital of Xinjiang Medical University. The clinicopathologic features of these patients are presented in Table 1. The informed consent was acquired from every patient and this research was authorized by the Ethics Committee of the First Affiliate Hospital of Xinjiang Medical University. Human nasopharyngeal epithelial cell line (NP-69) and NPC cell lines (C666-1 and 5–8F) were purchased from Otwo Biotech (Shenzhen, China). McCoy’s 5A medium (Sigma, St Louis, MO, USA) containing 5% CO2 and 10% fetal bovine serum (FBS; Sigma) was utilized to culture cells.
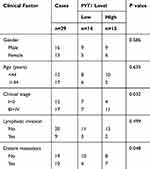 |
Table 1 The Clinicopathological Parameters of NPC Patients |
Cell Transfection
Lentivirus harboring short hairpin RNA targeting PVT1 (named as sh-PVT1) and its negative control (named as sh-Control) were constructed by GeneCopoeia (Rockville, MD, USA). MiR-515-5p mimic (named as miR-515-5p), miR-515-5p inhibitor (named as Anti-miR-515-5p) and the corresponding controls (miR-Control and Anti-Control) were obtained from GenePharma (Shanghai, China). PVT1 overexpression plasmid (named as PVT1), PIK3CA overexpression plasmid (named as PIK3CA) and the corresponding control (named as Vector) were acquired from RiboBio (Guangzhou, China). Cell transfection was executed using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) referring to the given procedures.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted using the TRIzol reagent (Vazyme, Nanjing, China). Then, RNA was reversely transcribed to complementary DNA (cDNA) by PrimeScript™ RT Master Mix kit (Takara, Dalian, China). The qRT-PCR was performed by SYBR Green PCR Master Mix (Vazyme) and data were analyzed using 2−ΔΔCt method. Beta-actin (β-actin) and U6 were introduced as the inner references. Primers in this study were listed as follows: PVT1 (forward 5ʹ-TGCGGCAGTGGTTTTACCCTATG-3ʹ, reverse 5ʹ-CCAGTGCAGGGTCCGAGGT-3ʹ); miR-515-5p (forward, 5ʹ-TTCTCCAAAAGAAAGCACTTTCTG-3ʹ, reverse 5ʹ-TGGTGTCGTGGAGTCG-3ʹ); PIK3CA (forward, 5ʹ-CCACGACCATCATCAGGTGAA-3ʹ, reverse 5ʹ-CCTCACGGAGGCATTCTAAAGT-3ʹ); β-actin (forward 5ʹ-GCACCACACCTTCTACAATG-3ʹ, reverse, 5ʹ-TGCTTGCTGATCCACATCTG-3ʹ); U6 (forward, 5ʹ-TCCGGGTGATGCTTTTCCTAG-3ʹ, reverse, 5ʹ-CGCTTCACGAATTTGCGTGTCAT-3ʹ).
Counting Kit-8 (CCK-8) Assay
After transfection, 3 × 104 C666-1 and 5–8F cells were seeded into 96-well plates and then incubated with 10 μL CCK-8 solution (vazyme) for 2 h. Optical density values were examined at 450 nm wavelength under the microplate reader (Bio-Rad, Richmond, Virginia, USA).
Cell Radiosensitivity Analysis
The survival fractions of C666-1 and 5–8F cells under radiotherapy were checked by the colony formation assay. In brief, cells (1 × 103 cells/well) were seeded into 6-well plates and incubated overnight. Afterwards, cells were exposed to irradiation doses of 0, 2, 4, 6 and 8 Gy. After 12 d of culture, cell colonies were stained with 0.5% crystal violet (Solarbio, Beijing, China). The Image J software was utilized to quantify cell numbers.
Flow Cytometry
Annexin Apoptosis Detection Kit (Sigma) was hired to analyze cell apoptosis following the given procedures. Briefly, 5 × 104 cells were resuspended using the binding buffer and then 5 μL Annexin V-fluorescein isothiocyanate (Annexin V-FITC) and 5 μL propidium iodide (PI) were added to the buffer to incubate for 5 min in the dark. The stained cells were analyzed by flow cytometry (Countstar, Shanghai, China)
Western Blot
Proteins were isolated using RIPA buffer (Vazyme) and segregated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were then transferred onto the polyvinylidene difluoride (PVDF) membranes (Vazyme) and the membranes were blocked with 5% skimmed milk (Vazyme). After being washed by phosphate-buffered saline (PBS), the membranes were incubated with the primary antibodies: Cyclin D1 (1:1000, ab16663, Abcam, Cambridge, United Kingdom), B-cell lymphoma 2 associated X (Bax) (1:3000, ab32503, Abcam), Cleaved-caspase-3 (Cleaved-casp-3) (1:1000, ab2302, Abcam), phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) (1:2000, ab40776, Abcam), protein kinase B (AKT) (1:1000, ab8805, Abcam) and phosphorylated AKT (p-AKT) (1:1000, ab38449, Abcam) or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:2500, ab9485, Abcam) overnight. After being rewashed, the membranes were incubated with the secondary antibody (1:3000, ab205718, Abcam) for 3 h. The membranes were analyzed by the ChemiDoc™ MP Imaging System (Bio-Rad) after being treated with ECL kit (Vazyme).
Dual-Luciferase Reporter Assay
The potential complementary sequences of miR-515-5p and PVT1 or PIK3CA were forecasted by starBase.20 The luciferase activity was detected by dual-luciferase reporter assay as previously reported.21 The wild type sequence of PVT1 or PIK3CA 3ʹUTR harboring the binding sites of miR-515-5p was inserted into the pGL3 vector (Promega, Madison, WI, USA) to construct the luciferase reporter vector PVT1-WT or PIK3CA-WT. Similarly, PVT1-MUT and PIK3CA-MUT reporter vectors were established by mutating the potential target sites of miR-515-5p. Then, the vectors with miR-515-5p or miR-Control were co-transfected into C666-1 and 5–8F cells using Lipofectamine 2000 (Invitrogen). The Dual-Glo® Luciferase Assay System kit (Promega) was utilized to measure luciferase activity.
RNA Immunoprecipitation (RIP) Assay
RIP was executed using Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, Billerica, MA, USA) referring to the provided protocols. Briefly, the harvested cells were lysed and incubated with magnetic beads conjugated with anti-Argonaute 2 (Anti-Ago2) antibody (Millipore) and anti-immunoglobulin G (Anti-IgG) antibody (Millipore) was used as a negative control. The protein was removed by Proteinase K. The immune precipitated RNA was purified and analyzed by qRT-PCR.22
Xenograft Mice Model
Six-week-old BALB/c nude mice were acquired from Vital River Laboratory Animal Technology (Beijing, China). 2 × 106 C666-1 cells infected with sh-PVT1 or sh-Control were subcutaneously injected into the flank of the nude mice. The tumor volume was calculated every 5 d according to the formula: 0.5 × length × width2. The tumor weight was measured after the mice were euthanized. The mRNA or protein levels of related genes in tumors were checked by qRT-PCR or Western blot. The animal experiment was approved by the Animal Care and Use Committee of the First Affiliate Hospital of Xinjiang Medical University and performed following the instructions of the national animal protection and ethics institute.
Statistical Analysis
Experimental data were calculated by GraphPad Prism (GraphPad, La Jolla, CA, USA) and presented by mean ± standard deviation (SD). Two independent groups were compared by using Student’s t-test. For more than two groups, the one-way analysis of variance (ANOVA) was utilized to assess the difference. The Kaplan-Meier method was utilized to assess overall survival and the Log rank test was utilized to analyze the differences between survival curves. Every experiment was repeated at least three times independently. P < 0.05 represented statistical significance.
Results
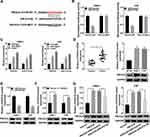
PVT1 Was Highly Expressed in NPC and Was Associated with the Poor Prognosis
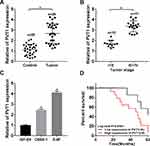
To explore the role of PVT1 in NPC, we first measured its expression patterns. The qRT-PCR data showed that PVT1 was significantly upregulated in NPC tissues compared with the paired non-tumorous tissues (Figure 1A). Afterwards, the correlation between PVT1 expression and NPC progression was evaluated and the results showed that the level of PVT1 was higher in advanced stages (III + IV) compared with early stages (I + II) (Figure 1B). Besides, PVT1 was also upregulated in NPC cells (Figure 1C), and the higher level of PVT1 resulted in the lower survival rate of NPC patients (Figure 1D). Altogether, these results demonstrated that PVT1 might act as an oncogene in NPC and its high expression led to the poor prognosis.
Knockdown of PVT1 Inhibited Proliferation, Promoted Apoptosis and Suppressed Radioresistance in vitro
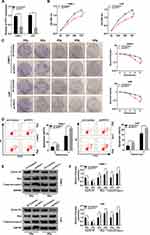
To study the function of PVT1 in NPC, NPC cells were first infected with sh-PVT1 or sh-Control and the knockdown efficiency was confirmed (Figure 2A). Then, CCK8 assay was performed and the result showed that downregulation of PVT1 significantly inhibited proliferation of NPC cells (Figure 2B). Besides, colony formation assay indicated that PVT1 silencing clearly promoted radiosensitivity in NPC cells (Figure 2C). Meanwhile, flow cytometry assay showed that downregulation of PVT1 notably induced apoptosis in NPC cells after radiotherapy (Figure 2D). Afterwards, the protein levels of Cyclin D1 (related to proliferation), Bax and Cleaved-casp-3 (related to apoptosis) were measured by Western blot and the data showed that silence of PVT1 markedly decreased the level of Cyclin D1 and increased the expression of Bax and Cleaved-casp-3 in NPC cells after radiotherapy (Figure 2E and F). Collectively, these results illuminated that PVT1 silencing handicapped proliferation and radioresistance and induced apoptosis in NPC cells in vitro.
PVT1 Targeted miR-515-5p and Negatively Regulated miR-515-5p in NPC Cells
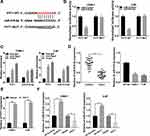
The interaction between lncRNAs and miRNAs was documented in various human cancers.6,23 By using starBase, we found that miR-515-5p harbored the target sites of PVT1 (Figure 3A). The dual-luciferase reporter assay was carried out to verify the interaction and the result showed that miR-515-5p significantly diminished the luciferase activity of PVT1-WT in NPC cells, rather than PVT1-MUT (Figure 3B). Also, RIP assay indicated that the relative enrichment of PVT1 and miR-515-5p was higher in Anti-Ago2 group than in Anti-IgG group (Figure 3C). Thereafter, the expression of miR-515-5p was checked and the data disclosed that miR-515-5p was apparently declined in NPC tissues and cells (Figure 3D). To investigate the regulatory relationship between PVT1 and miR-515-5p, PVT1 or Vector was introduced to NPC cells and the overexpression efficiency was corroborated (Figure 3E). Our results showed that knockdown of PVT1 markedly increased the level of miR-515-5p, while overexpression of PVT1 clearly decreased the expression of PVT1 in NPC cells (Figure 3F). All in all, these results illustrated that miR-515-5p was a target of PVT1 and negatively modulated by PVT1 in NPC cells in vitro.
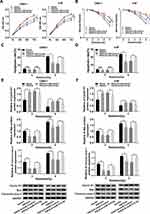
Overexpression of PVT1 Reversed miR-515-5p-Mediated Effects on Proliferation, Apoptosis and Radioresistance in vitro
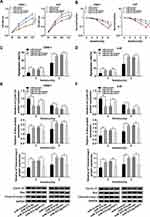
To further dissect the function of PVT1 and miR-515-5p in NPC, NPC cells were transfected with miR-515-5p, miR-515-5p + PVT1 or the matched controls. Cell proliferation was analyzed and the result indicated that miR-515-5p mimic significantly repressed the proliferation of NPC cells, whereas upregulation of PVT1 reversed this effect (Figure 4A). Also, colony formation assay showed that overexpression of PVT1 clearly inverted miR-515-5p-mediated inhibitory effect on radioresistance (Figure 4B). Simultaneously, flow cytometry assay indicated that the high apoptosis rate of NPC cells, treated with radiation and transfected with miR-515-5p, was transposed following the additional transfection with PVT1 (Figure 4C and D). Analogously, the declined protein level of Cyclin D1 and increased level of Bax and Cleaved-casp-3 in miR-515-5p group were inverted following the infection with PVT1 (Figure 4E and F). From these results, it could be concluded that PVT1 mediated the proliferation, apoptosis and radioresistance in NPC cells by interacting with miR-515-5p.
miR-515-5p Bound to the 3ʹUTR of PIK3CA and Negatively Modulated PIK3CA in NPC Cells
To further probe the regulatory mechanism of miR-515-5p, we found its target gene PIK3CA using starBase (Figure 5A). Subsequently, the dual-luciferase reporter assay and RIP assay were conducted to confirm the interaction. The dual-luciferase reporter assay showed that miR-515-5p significantly reduced the luciferase activity of PIK3CA-WT in NPC cells (Figure 5B). RIP assay indicated that PIK3CA and miR-515-5p were apparently enriched in Anti-Ago2 group (Figure 5C). Next, the mRNA and protein levels of PIK3CA were measured and the data indicated that PIK3CA was clearly increased in NPC tissues and cells (Figure 5D). We then checked the protein level of PIK3CA in NPC cells infected with miR-515-5p, PIK3CA or the matched controls, and the result indicated that PIK3CA was notably downregulated in miR-515-5p group (Figure 5E), while its expression was strikingly elevated in PIK3CA group (Figure 5F). Further analysis disclosed that the increased protein level of PIK3CA in PIK3CA group was reversed following the transfection with miR-515-5p mimic (Figure 5G). To sum up, these results manifested that miR-515-5p targeted PIK3CA and negatively regulated PIK3CA expression in vitro.
Upregulation of miR-515-5p Inverted PIK3CA-Mediated Impacts on Proliferation, Apoptosis and Radioresistance in vitro
To further explore the roles of miR-515-5p and PIK3CA in NPC, the proliferation of NPC cells infected with PIK3CA, PIK3CA + miR-515-5p or corresponding controls was checked. The data showed that miR-515-5p mimic inverted PIK3CA-mediated promoted effect on cell proliferation (Figure 6A). In addition, colony formation assay indicated that upregulation of miR-515-5p significantly rescued PIK3CA-mediated enhanced impact on radioresistance (Figure 6B). Also, the flow cytometry assay demonstrated that overexpression of PIK3CA inhibited the apoptosis of NPC cells treated with radiation, whereas the effect was abolished by miR-515-5p mimic (Figure 6C and D). Similarly, the increased protein level of Cyclin D1 and declined level of Bax and Cleaved-casp-3 in PIK3CA group were reversed following the transfection with miR-515-5p mimic (Figure 6E and F). In summary, these results suggested that miR-515-5p and PIK3CA played opposite roles in regulating proliferation, apoptosis and radioresistance in NPC cells.
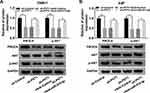
PVT1/miR-515-5p Axis Regulated AKT Pathway in NPC Cells
A previous report indicated that AKT pathway activation was associated with PIK3CA mutations24 and AKT signaling pathway functioned in radioresistance in many human cancers.25,26 To figure out whether PVT1 could affect AKT pathway, we checked the expression of PIK3CA, AKT, p-AKT in NPC cells infected with sh-PVT1, sh-PVT1 + Anti-miR-515-5p or the matched controls. The results indicated that downregulation of PVT1 markedly decreased the levels of PIK3CA and p-AKT, while miR-515-5p inhibitor transposed the effect (Figure 7A and B). Overall, these results manifested that PVT1 mediated AKT pathway by interacting with miR-515-5p in NPC cells in vitro.
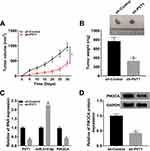
Knockdown of PVT1 Hampered Tumor Growth in vivo
To verify the function of PVT1 in vivo, we established the xenograft mouse model using C666-1 cells transfected with sh-PVT1 or sh-Control. The data showed that knockdown of PVT1 contributed to the obvious shrink in tumor volume (Figure 8A) and decline in tumor weight (Figure 8B). Meanwhile, the levels of PVT1 and PIK3CA were remarkably declined in sh-PVT1 group (Figure 8C), opposite to the expression of miR-515-5p. Besides, knockdown of PVT1 significantly reduced the expression of PIK3CA in tumors (Figure 8D). Taken together, these results suggested that PVT1 silencing suppressed tumor growth in vivo.
Discussion
NPC is a growing threat to humans and radiotherapy is thought to be the only curative treatment.1 Yet, radioresistance greatly discourages the therapeutic efficacy for NPC.2,3 Hence, it is essential to find the new molecular targets and investigate potential mechanisms. Lately, lncRNAs were reported to play roles in radioresistance in many human cancers. For instance, lncRNA TP73-AS1 silencing reduced radioresistance in hepatocellular carcinoma via the PTEN/Akt signaling pathway.5 LncRNA LINC02582 promoted radioresistance in breast cancer cells.6 Moreover, knockdown of lncRNA ANRIL enhanced radiosensitivity in NPC cells.7 Also, a recent paper showed that lncRNA PVT1 induced radioresistance in NPC cells.9 In order to investigate the role of PVT1 in NPC, we checked its expression level and found that PVT1 was strikingly upregulated in NPC tissues and cells and contributed to the poor prognosis, which was supported by a previous study.9 In-depth investigation indicated that PVT1 silencing repressed proliferation and radioresistance and promoted apoptosis in NPC cells. In addition, in vivo experiment indicated that downregulation of PVT1 inhibited tumor growth. Altogether, these results demonstrated that PVT1 might function as an oncogene and could be a potential therapeutic target in NPC.
Emerging evidence has shed light on the fact that lncRNAs could regulate radioresistance by interacting with miRNAs in lots of human cancers.27,28 In our study, miR-515-5p was predicted to be a target of PVT1 and this interaction was verified. We then checked its expression and found that miR-515-5p was clearly downregulated in NPC tissues and cells. Besides, PVT1 negatively modulated the expression of miR-515-5p in NPC cells in vitro. Further studies indicated that overexpression of PVT1 reversed miR-515-5p-mediated repressive effects on cell proliferation and radioresistance, as well as the promotion impact on cell apoptosis in NPC cells. In addition, upregulation of PVT1 inverted the decreased protein level of Cyclin D1 and the elevated expression of Bax in NPC cells treated with radiation. Our results suggested that PVT1 targeted miR-515-5p to regulate cell proliferation, apoptosis and radioresistance in NPC cells in vitro.
To further figure out the regulatory mechanism of miR-515-5p, its target gene PIK3CA was predicated by bioinformatic analysis and the interaction was confirmed. In our research, the level of PIK3CA was apparently elevated in NPC tissues and cells, which was in line with a previous report.29 Meanwhile, PIK3CA was negatively regulated by miR-515-5p in NPC cells. Moreover, upregulation of miR-515-5p rescued PIK3CA-mediated promotion effects on cell proliferation and radioresistance, as well as the inhibitory effect on cell apoptosis in NPC cells in vitro. Recently, PIK3CA was reported to be involved in the activation of AKT pathway,24 which played a pivotal role in regulating radioresistance in human cancers.25,26 In this research, we found that PVT1 silencing notably decreased the expression of PIK3CA and p-AKT, whereas miR-515-5p depletion reversed this effect, indicating that PVT1 might modulate cell radioresistance by affecting AKT pathway via interacting with miR-515-5p in NPC cells. Taken together, these results illustrated that PVT1 mediated cell proliferation, apoptosis, radioresistance and AKT pathway via miR-515-5p/PIK3CA axis in NPC cells.
In conclusion, our research disclosed that PVT1 was strikingly upregulated in NPC tissues and cells. Moreover, knockdown of PVT1 restrained cell proliferation, radioresistance and induced cell apoptosis via miR-515-5p/PIK3CA axis in NPC cells. The novel mechanism may contribute to the improvement of radiotherapy for NPC.
Highlights
- PVT1 was upregulated in nasopharyngeal carcinoma tissues and cells.
- PVT1 silencing inhibited cell proliferation, radioresistance and induced cell apoptosis in nasopharyngeal carcinoma.
- MiR-515-5p was a target of PVT1 and targeted PIK3CA in nasopharyngeal carcinoma.
- PVT1 regulated nasopharyngeal carcinoma progression via miR-515-5p/PIK3CA axis.
- Downregulation of PVT1 inhibited tumor growth in vivo.
Abbreviations
NPC, nasopharyngeal carcinoma; PVT1, plasmacytoma variant translocation 1; qRT-PCR, quantitative real-time polymerase chain reaction; PIK3CA, phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha; CCK8, Cell counting kit-8; RIP, RNA immunoprecipitation.
Data Sharing Statement
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Ethics Approval
The present study was approved by the ethical review committee of the First Affiliate Hospital of Xinjiang Medical University.
Author Contributions
All authors made substantial contribution to conception and design, acquisition of the data, or analysis and interpretation of the data; take part in drafting the article or revising it critically for important intellectual content; gave final approval of the revision to be published; and agree to be accountable for all aspect of the work.
Funding
This work was supported by the Natural Science Foundation of Xinjiang Uygur Autonomous Region (Grant No.2017D01A19).
Disclosure
The authors declare that they have no competing interests for this work.
References
1. Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012–1024. doi:10.1016/S0140-6736(15)00055-0
2. Qu C, Zhao Y, Feng G, et al. RPA3 is a potential marker of prognosis and radioresistance for nasopharyngeal carcinoma. J Cell Mol Med. 2017;21(11):2872–2883. doi:10.1111/jcmm.13200
3. Zhang Z, Yu X, Zhou Z, et al. LMP1-positive extracellular vesicles promote radioresistance in nasopharyngeal carcinoma cells through P38 MAPK signaling. Cancer Med. 2019;8(13):6082–6094. doi:10.1002/cam4.2506
4. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. doi:10.1038/nrg2521
5. Song W, Zhang J, Xia Q, Sun M. Down-regulated lncRNA TP73-AS1 reduces radioresistance in hepatocellular carcinoma via the PTEN/Akt signaling pathway. Cell Cycle. 2019;18(22):3177–3188. doi:10.1080/15384101.2019.1671089
6. Wang B, Zheng J, Li R, et al. Long noncoding RNA LINC02582 acts downstream of miR-200c to promote radioresistance through CHK1 in breast cancer cells. Cell Death Dis. 2019;10(10):764. doi:10.1038/s41419-019-1996-0
7. Hu X, Jiang H, Jiang X. Downregulation of lncRNA ANRIL inhibits proliferation, induces apoptosis, and enhances radiosensitivity in nasopharyngeal carcinoma cells through regulating miR-125a. Cancer Biol Ther. 2017;18(5):331–338. doi:10.1080/15384047.2017.1310348
8. Li G, Liu Y, Liu C, et al. Genome-wide analyses of long noncoding RNA expression profiles correlated with radioresistance in nasopharyngeal carcinoma via next-generation deep sequencing. BMC Cancer. 2016;16:719. doi:10.1186/s12885-016-2755-6
9. He Y, Jing Y, Wei F, et al. Long non-coding RNA PVT1 predicts poor prognosis and induces radioresistance by regulating DNA repair and cell apoptosis in nasopharyngeal carcinoma. Cell Death Dis. 2018;9(2):235. doi:10.1038/s41419-018-0265-y
10. Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199–227. doi:10.1146/annurev.pathol.4.110807.092222
11. Huang X, Taeb S, Jahangiri S, et al. miRNA-95 mediates radioresistance in tumors by targeting the sphingolipid phosphatase SGPP1. Cancer Res. 2013;73(23):6972–6986. doi:10.1158/0008-5472.CAN-13-1657
12. Wang P, Zhang J, Zhang L, et al. MicroRNA 23b regulates autophagy associated with radioresistance of pancreatic cancer cells. Gastroenterology. 2013;145(5):1133–1143.e1112. doi:10.1053/j.gastro.2013.07.048
13. Zhang P, Wang L, Rodriguez-Aguayo C, et al. miR-205 acts as a tumour radiosensitizer by targeting ZEB1 and Ubc13. Nat Commun. 2014;5:5671. doi:10.1038/ncomms6671
14. Qiao K, Ning S, Wan L, et al. LINC00673 is activated by YY1 and promotes the proliferation of breast cancer cells via the miR-515-5p/MARK4/hippo signaling pathway. J Exp Clin Cancer Res. 2019;38(1):418. doi:10.1186/s13046-019-1421-7
15. Zhang H, Wang X, Huang H, Wang Y, Zhang F, Wang S. Hsa_circ_0067997 promotes the progression of gastric cancer by inhibition of miR-515-5p and activation of X chromosome-linked inhibitor of apoptosis (XIAP). Artif Cells Nanomed Biotechnol. 2019;47(1):308–318. doi:10.1080/21691401.2018.1553787
16. Zhang X, Zhou J, Xue D, Li Z, Liu Y, Dong L. MiR-515-5p acts as a tumor suppressor via targeting TRIP13 in prostate cancer. Int J Biol Macromol. 2019;129:227–232. doi:10.1016/j.ijbiomac.2019.01.127
17. Ma YY, Wei SJ, Lin YC, et al. PIK3CA as an oncogene in cervical cancer. Oncogene. 2000;19(23):2739–2744. doi:10.1038/sj.onc.1203597
18. Huang D, Tang L, Yang F, Jin J, Guan X. mutations contribute to fulvestrant resistance in ER-positive breast cancer. Am J Transl Res. 2019;11(9):6055–6065.
19. Fendri A, Khabir A, Mnejja W, et al. PIK3CA amplification is predictive of poor prognosis in Tunisian patients with nasopharyngeal carcinoma. Cancer Sci. 2009;100(11):2034–2039. doi:10.1111/j.1349-7006.2009.01292.x
20. Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42(Database issue):D92–D97. doi:10.1093/nar/gkt1248
21. Wang B, Xu L, Zhang J, et al. LncRNA NORAD accelerates the progression and doxorubicin resistance of neuroblastoma through up-regulating HDAC8 via sponging miR-144-3p. Biomed Pharmacother. 2020;129:110268. doi:10.1016/j.biopha.2020.110268
22. Long X, Song K, Hu H, et al. Long non-coding RNA GAS5 inhibits DDP-resistance and tumor progression of epithelial ovarian cancer via GAS5-E2F4-PARP1-MAPK axis. J Exp Clin Cancer Res. 2019;38(1):345. doi:10.1186/s13046-019-1329-2
23. Tan J, Qiu K, Li M, Liang Y. Double-negative feedback loop between long non-coding RNA TUG1 and miR-145 promotes epithelial to mesenchymal transition and radioresistance in human bladder cancer cells. FEBS Lett. 2015;589(20 Pt B):3175–3181. doi:10.1016/j.febslet.2015.08.020
24. Sonnenblick A, Venet D, Brohée S, Pondé N, Sotiriou C. pAKT pathway activation is associated with mutations and good prognosis in luminal breast cancer in contrast to p-mTOR pathway activation. NPJ Breast Cancer. 2019;5:7. doi:10.1038/s41523-019-0102-1
25. Tomioka A, Tanaka M, De Velasco MA, et al. Delivery of PTEN via a novel gene microcapsule sensitizes prostate cancer cells to irradiation. Mol Cancer Ther. 2008;7(7):1864–1870. doi:10.1158/1535-7163.MCT-07-2198
26. Tanno S, Yanagawa N, Habiro A, et al. Serine/threonine kinase AKT is frequently activated in human bile duct cancer and is associated with increased radioresistance. Cancer Res. 2004;64(10):3486–3490. doi:10.1158/0008-5472.CAN-03-1788
27. Zou Y, Yao S, Chen X, et al. LncRNA OIP5-AS1 regulates radioresistance by targeting DYRK1A through miR-369-3p in colorectal cancer cells. Eur J Cell Biol. 2018;97(5):369–378. doi:10.1016/j.ejcb.2018.04.005
28. Liu L, Zhu Y, Liu AM, Feng Y, Chen Y. Long noncoding RNA LINC00511 involves in breast cancer recurrence and radioresistance by regulating STXBP4 expression via miR-185. Eur Rev Med Pharmacol Sci. 2019;23(17):7457–7468. doi:10.26355/eurrev_201909_18855
29. Wang X, Huang Y, Guo R, et al. Clinicopathological significance of ROCK1 and PIK3CA expression in nasopharyngeal carcinoma. Exp Ther Med. 2017;13(3):1064–1068. doi:10.3892/etm.2017.4076
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.