Back to Journals » Nutrition and Dietary Supplements » Volume 13
Purposeful Review to Identify the Benefits, Mechanism of Action and Practical Considerations of Omega-3 Polyunsaturated Fatty Acid Supplementation for the Management of Diabetes Mellitus
Authors Abdissa D
Received 25 December 2020
Accepted for publication 19 February 2021
Published 5 March 2021 Volume 2021:13 Pages 53—65
DOI https://doi.org/10.2147/NDS.S298870
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gary Johanning
Daba Abdissa
Department of Biomedical Sciences, College of Medical Sciences, Institute of Health Science, Jimma University, Jimma, Ethiopia
Correspondence: Daba Abdissa Email [email protected]
Abstract: Despite advances in medical management diabetes mellitus (DM), it remains on the rise and it is the major cause of morbidity and mortality. Its etiology is multifactorial involving environmental, genetic and behavioral origins. It is closely linked to sedentary life and inappropriate food intake. Patients with DM should know about the uses of correct nutritional habits, which is the key in the regulation of blood glucose. Despite the promising experimental investigations, currently, the clinical evidence for the usage of omega-3 supplementation for the management of DM and its complications is both conflicting and limited. In this narrative review, I will summarize recent findings about dietary sources, the potential mechanisms, benefits, and practical considerations on omega-3 polyunsaturated fatty acid supplementation for the management of DM. The search of literature for this narrative review was done comprehensively by using appropriate search terms and different electronic databases.
Keywords: review, benefits, omega-3 polyunsaturated fatty acid, diabetes mellitus
Introduction
Diabetes mellitus (DM) is one of the most prevalent metabolic disorders globally. It is a highly inflammatory disorder with increased blood concentrations of numerous inflammatory biomarkers.1,2 It is closely related to sedentary life and inappropriate food intake.3 Patients with DM should know about the uses of correct nutritional habits, which is the key in the regulation of blood glucose.4 Dietary strategies are vital to the treatment of DM and risk factors for cardiovascular disease (CVD) development.5
Eating patterns like the Mediterranean style, Dietary Approaches to Stop Hypertension and monitored carbohydrate diet are effective for lowering CVD risk factors and controlling glycemia.6 Mediterranean-style diet is the most comprehensive diet, characterized by olive oil as the chief source of fat and high consumption of vegetables, monounsaturated fatty acids and a low consumption of red or processed meat.7 The American Diabetes Association (ADA) approves a mediterranean-style diet and long-chain omega-3 fatty acids without supplements. Oily fish intake, without supplementation, is recommended in the United Kingdom for DM patients.8 In addition, the national lipid association recommends adults ≥2 servings of fish/seafood per week.9
CVD is the principal cause of mortality in patients with DM.10 According to some studies omega-3 polyunsaturated fatty acid (O-3 PUFA) therapy helps in the prevention of CVD among DM patients. O-3 PUFAs helps in the improvement of coagulation, lipid profile and inflammatory parameters.11 Moreover, O-3PUFA long-term supplementation helps in the reduction in pulse pressure and blood pressure.12,13
Animal studies revealed the plasma level of O-3 fatty acid is decreased among diabetes.14 The blood levels of O-3fatty acids can differ based on diet habits and geography. For instance, Japanese living in Japan have higher blood o-3fatty acid levels than whites in living in Pennsylvania and Japanese Americans living in Honolulu.15 A low level of O-3 fatty acid promotes inflammation, on the contrary, higher intake of O-3 fatty acid and their high concentration in the erythrocyte membrane is related to a lower risk of inflammation.16
Evidences regarding those issues were relatively deficient with few summarized studies. Hence, in this review, I will provide comprehensive summarized evidence regarding the benefit, types, dietary sources and mechanism of action of O-3PUFA for DM patients using available evidence.
Types and Dietary Sources of O-3PUFA
There are three types of Omega‐3 fatty acids: Those include; Alpha‐linoleic acid (ALA), Eicosapentaenoic acid (EPA) and Docosahexaenoic acid (DHA). EPA and DHA are derived predominantly from fish and seafood and ALA is derived from plant sources, such as seeds, particularly flaxseed and leafy greens.17 DHA and EPA are found in predominantly in fish and other seafood, and thus they may be together referred to as marine O−3 fatty acids.18
In human diets, ALA is frequently derived from botanical sources such as green leaves, flaxseed, pecans and kiwifruit with chia seed and flax seed being the richest sources. Linseeds and their oil typically contain 45–55% of fatty acids as ALA, while rapeseed oil, soybean oil, and walnuts all typically contain ~10% of fatty acids as ALA. There is small ALA from sunflower oil and corn oil.19 The principal food sources of omega-3 fatty acids are oily fish (Table 1). But, it should be noted that the omega-3 content of fish differs by the fish’s diet and species. The best sources of omega-3 fatty acids are herring, salmon, anchovies, rainbow trout and sardines.20
 |
Table 1 Summary on the Sources of Omega-3 Polyunsaturated Fatty Acid and Their Concentration |
Marine fishes have more O−3 PUFAs than farmed ones since most marine fishes feed on phytoplankton and zooplankton that are rich in O−3 PUFAs. Similarly, cold-water fishes accumulate much proportions of long-chain O−3 PUFAs that aid them to adapt to cold environment than warm water fishes. Omega-3 fatty acid products are available as prescription formulations (icosapent ethyl, omega-3-acid ethyl esters A, omega-3-acid ethyl esters, Omega-3-carboxylic acids) and dietary supplements (predominantly fish oils). Fish, fish oil supplements, and other seafoods primarily account for the DHA and EPA in human diets.21
Once eaten, the body converts ALA to EPA and then to DPA and lastly to DHA, but this conversion is inadequate, with less than 15%. As this conversion is not efficient enough to fulfill health requirements, DHA and EPA are considered essential fatty acid as well and,22 thus consuming them is the only practical way to get them in the body.23 The consumption of O−3 PUFAs is usually inadequate because of their limited sources.24 EPA and DHA are also available in O-3 fortified foods, including pastas, breads, cereals, eggs, dairy products, meats, juices, salad dressings, spreads, and oils. Consumption of O-3 fortified foods is a potential option to increase EPA and DHA intake in vegetarians or individuals who dislike fish/seafood.25
Mechanism of Action
T2DM is closely linked with obesity, and adipose tissue produces numerous hormone-like compounds that can raise insulin resistance (IR).26 Adipose tissue is involved in hormone secretion, such as leptin, adiponectin and visfatin, and could stimulate insulin signals. Adiponectin has a role in the modulation of lipid and glucose metabolism together with insulin-sensitive tissues.27
In humans, levels of adiponectin are lower in IR states, as well as in T2DM. Experimental studies revealed supplementation with O-3 PUFA improved insulin sensitization, thru increased levels of adiponectin and reduced inflammation.28–30 It raises adiponectin synthesis by inhibiting transient receptor potential canonical calcium ion channels, which can control adiponectin production.31 Besides, in animal model adiponectin prevents T2DM and atherosclerosis.32 Agonism of G-protein coupled receptor 120 (GPR120) which is a receptor for O‐3 fatty acids, has potent anti‐inflammatory effects by blocking the signaling of many pro‐inflammatory mediators.33
EPA is metabolized to the thromboxanes, prostaglandins and leukotrienes, which had anti-coagulant and anti-inflammatory effects.28 Besides, the metabolic products of O‐3 fatty acids, namely protectins, resolvins and maresins are also anti‐inflammatory in nature and thus counteract inflammatory responses during CVD.34
Generally, proposed mechanisms of O-3PUFAs protection against several diseases including DM, were: (i) They increase the production of anti-inflammatory eicosanoids that can help in phagocytosis and resolution of inflammation; (ii) they can inhibit the production of adhesion molecules (iii) they can limit the activity and production of inflammatory mediators (iv) they can inhibit sterol regulatory element-binding protein 1c nuclear factor which mediates lipid degradation and decreases lipid biosynthesis; and (v) they can improve hypothalamic regulation and glucose uptake.35
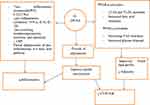
Furthermore, O-3 PUFAs can potentially reduce inflammation via many mechanisms, including inhibition of nuclear factor kappa B activation, inhibition of the arachidonic acid pathway, and initiation of anti-inflammatory signaling through GPR120 and decrease C-reactive protein (C-RP) concentration (Figure 1).36–38
Benefits on Diabetic Complications
On Glycaemic Control
O-3PUFA supplementation was postulated to improve glycaemic control which is the cornerstone of DM management. Possible mechanisms for this include improved hepatic insulin sensitivity through reducing lipogenesis and hepatic fatty acid oxidation39 and modulation of incretin hormones, which are participated in glucose-stimulated insulin secretion.40 Furthermore, in animal studies, supplementation with O-3PUFA improved insulin sensitization, potentially via increased levels of adiponectin, an emerging protective risk factor, and reduced inflammation.33,41
According to some studies O-3 PUFA supplementation had beneficial effect on glucose level, Hb1Ac, reduces pro-inflammatory cytokine levels and improves glycaemia.42,43 A review exploring the impact of PUFA intake on glycaemic control in T2DM populations concluded that PUFA supplementation of 0.42–5.2 g/day for at least 2months may benefit glycaemic control, particularly in Asian populations.44 DPA supplementation was shown to be effective in reducing blood glucose levels and improving homeostasis model assessment of insulin resistance in a rodent model.45
However, a meta-analysis of 20 randomized controlled trials (RCTs) with T2DM patients reported that there were no significant differences in markers of glycaemic control, including fasting blood glucose, postprandial plasma glucose, fasting insulin and HbA1c with O-3 PUFA supplementation (0.52 to 3.89 g/day EPA and up to 3.69 g/day of DHA, 65 duration ranged 2–48 weeks) in comparison to control groups.46 Human trials showed that O-3 PUFA supplementation appears to have a negligible effect on insulin sensitivity and markers of glycaemic control including HbA1c and fasting glucose.47,48 Furthermore, one RCT has suggested no benefits of low-dose O-3 PUFAs in dysglycemia.49 Generally, so far there are inadequate studies that recommend the usage of O-3 PUFA for glycaemic control.
On Cardiovascular Disease
CVD is the most common cause of morbidity and mortality in T2DM patients and its risk factors are common in patients with T2DM.50 It has been revealed that O-3 fatty acids can decrease cholesterol and prevent IR.51 They switch off the genes participated in lipid synthesis, diminish the hormones associated with obesity and inhibit omega-6 fatty acids production.52 Furthermore, O-3 PUFA has been widely used for the management of hypertriglyceridemia53,54 and according to recent systematic review of RCTs O-3PUFAs can be recommended for ameliorating CVD risk factors.55
Suggested mechanisms for the protective role of O‐3 fats against CVDs include: modulating arterial lipoprotein lipase levels; altering the lipid profile, lowering the blood pressure, reducing thrombotic tendency; producing anti‐inflammatory effects and improving vascular endothelial function.56 Furthermore, it helps against CVD by disrupting the c-Jun N-terminal kinase (JNK) signaling by reducing the expression of tumour necrosis factor‐alpha.57
A meta-analysis of 5 trials in T2DM revealed that O-3 PUFA supplementation considerably reduced diastolic blood pressure.58 In a similar way, a clinical trial with O-3PUFA supplementation in women with T2DM revealed a major mean reduction in blood pressure.59 Consistent O-3PUFA supplementation may decrease the risks of sudden cardiac death and myocardial infarction (MI). In addition, some evidence proposed that it may improve blood circulation and increase the breakdown of fibrin.60
EPA at a pharmacologic dose can lower fasting triglyceride and interfere with atherosclerosis which causes reduced CVD.61 It has also been reported that O-3PUFA supplementation inhibits platelet aggregation and decreases oxidative stress.62 A meta-analysis demonstrated that O-3 PUFA supplementation improves endothelial dysfunction and arterial stiffness in T2DM patients.63 Furthermore, according to meta-analysis by Wang et al supplementation with long-chain PUFAs significantly improves the endothelial function.64
However, over three months of high-dose O-3 PUFA treatment in very high-risk patients with atherosclerotic CVD and T2DM did not improve the endothelial function indices.65 A recent meta-analysis among type one DM patients indicated that daily high dose bolus of O-3PUFA supplementation for six-months does not improve glucose homeostasis, vascular health or metabolic parameters.66 Furthermore, other study demonstrated that in patients with long-standing, well-controlled T2DM and atherosclerotic disease treatment with a high dose of O-3 PUFAs for three months does not improve coagulation and inflammation.67
According to the result from the ORIGIN (Outcome Reduction with Initial Glargine Intervention) trial, which evaluated the effects of 0.9g/day of O-3 fatty acids ethyl esters on cardiovascular outcomes in patients with DM, found no reductions in deaths from CVD causes.49 Besides, according to ASCEND (A Study of Cardiovascular Events in Diabetes) revealed that among patients with DM but without evidence of CVD at baseline, there was no significant difference in the incidence of serious vascular events between those who received O−3 fatty acids and those who received placebo.68
However, Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)- -Prevenzion study, showed that the early administration of low-dose (1 g/day) O-3 PUFA reduces total mortality and sudden death by CVD.69 According to JELIS (Japan Eicosapentaenoic Acid Lipid Intervention Study) among patients with hypercholesterolemia received statins alone or in combination with highly purified EPA (1.8 g/day) after 5 years patients receiving the combined treatment experienced a 19% relative reduction in major coronary events.70 Furthermore, based on results from REDUCE-IT (Reduction of Cardiovascular Events with EPA Intervention Trial), the addition of 4 g/d of EPA plus statin resulted in a significantly lower occurrence of CVD events.71 This finding provides a strong rationale for prescribing icosapent ethyl for patients with hypertriglyceridemia who are on a statin.
In general, despite the substantial body of evidence that has investigated O-3PUFA supplementation on CVD risk factors within T2DM populations; the effect of O-3PUFAs on clinical CVD endpoints is not conclusive. Thus, O-3 PUFA supplementation is therefore not recommended by the AHA for the prevention of CVD in patients with T2DM.72
On Diabetic Retinopathy
Omega‐3 fatty acids have a great role to avoid the progression of diabetic retinopathy, because of their anti‐angiogenic, anti‐inflammatory and antioxidant properties.73 They reduce the formation of free radicals and inducing the expressions of endogenous antioxidant enzymes. Also, they prevent the initiation of retinal angiogenesis remarkably by down-regulating the expressions of various angiogenic agents such as Matrix metalloproteinases (MMPs), Cyclooxygenase-2 (COX‐2) and Vascular endothelial growth factor (VEGF).74,75 Furthermore, since they form an vital constituent of cell membranes they control cell membrane fluidity.76
Animal studies revealed that O-3PUFAs inhibit hyperoxia-induced premature retinopathy.77 A prospective observational study among older patients with T2DM who consumed Mediterranean diet and had a dietary O-3 PUFA intake equivalent to at least two weekly servings of oily fish had a significantly lower risk of diabetic retinopathy.78 However, a beneficial effect of omega-3 PUFAs in human retinopathies is unclear, possibly due to the paucity of human studies in the area.
On Diabetic Nephropathy
Recent meta-analysis revealed that O-3PUFA supplementation could help improve proteinuria and maintain renal function among T2DM.79,80 The effects of O-3 PUFA supplementation in subjects in DM patients are dependent on the dose of O-3Fatty acid supplementation.81 Other study showed that higher dietary O-3PUFA consumption was related with a lower risk of proteinuria among DM patients.82 For people with T2DM, the European Prospective Investigation of Cancer study showed that consuming at least two servings of fish per week lowered their risk of macro-albuminuria.83 In the CKD population specifically, a 12-week intervention study showed omega-3 PUFA supplementation (3.6 g daily) to reduce triglyceride levels, retard CKD progression, and having the capacity to reduce inflammation and oxidative stress.84
Early rodent models suggest a higher O-3 PUFAs intake, particularly omega-3 PUFAs (from fish oil), to reduce albuminuria in diabetic nephropathy.85 In human trials, however, the effects are far from conclusive, likely owing to the short durations and small sample sizes of current studies.86 Currently, O-3PUFA supplementation should not be advocated for avoiding kidney complications in diabetic nephropathy and existing literature were unable to draw conclusions.
The mechanisms via which O-3 fatty acids diminish proteinuria are not clear up to now. One of the suggested hypotheses is that O-3 fatty acids may decrease urine protein excretion via anti-inflammatory and oxidative stress. As hyperglycemia amongst diabetic sufferers induces podocyte injury as well as endothelial cell and tubulointerstitial harm through the activation of protein kinase C, formation of advanced glycation end‐products, and generation of reactive oxygen species, which performs a pivotal function in initiation and progression of proteinuria and diabetic nephropathy.87
For Prevention of DM
Omega-3 polyunsaturated fatty acids are an increasing number of being used to prevent CVD, along with DM. However, long-term effects of PUFA on development and management of DM remain inconclusive. Some studies suggest that O‐3fatty acids supplementation was either positively, negatively or insignificantly associated with DM development.88,89 Recent systematic review and meta-analysis suggests that increasing O-3 fatty acids has little or no effect on prevention and management of T2DM.90 A recent meta-analysis of RCTs established that O-3PUFA supplementation has little effect on the prevention of T2DM in humans and evidence for preventing T1DM remains preliminary and limited to animal studies.91
However, a meta‐analysis revealed that the appropriate dosage and compositions of omega‐3, optimized cooking method, and early omega‐3 supplementation might be beneficial for T2DM prevention.92 Epidemiological studies suggest that inadequate O-3PUFA intake is related with an increased risk of developing both T2DM93 and type 1 DM.16
Other Functions
DHA helps in maintaining membrane fluidity of the brain which is vital for proper neurological and cognitive functions.94 O-3PUFAs are involved in the development and maintenance of healthy nerves. Experimental study revealed in peripheral nerve injury, increased endogenous levels of O-3 PUFAs have been shown to improve sciatic nerve blood flow and speed up the recovery.95 Furthermore, O-3 PUFAs helps in the prevention of heart disease4 and prevent oxidative stress among DM patients.96
Moreover, it has been proposed that O-3 Fatty acid treatment partially blocks the development of experimental diabetic cardiomyopathy by affecting sarcoplasmic reticulum calcium transport activity.97 Supplementation of O-3 FUFAs is also a promising novel nutritional approach to decrease obesity and associated metabolic disorders. They are effective in protecting against obesity by activating brown adipose tissue which aids energy expenditure thru its specialized thermogenic function.98 In general, there is limited clinical evidence which support O-3 PUFA supplement use in DM management and prevention (Table 2).
 |
Table 2 Current State of Evidence for the Effects of Omega-3 PUFA Intake Regarding Diabetes Complications |
Nutrition Guidelines for T2DM Treatment
Dietary intervention is a key factor in the management of DM and the goals of nutrition therapy to adults with diabetes is to promote and support healthful eating patterns, focusing a variety of nutrient dense diet in appropriate portion sizes, to improve overall health (Table 3). Medical Nutrition Therapy for diabetes aims to achieve the following objectives:
- Achieve and maintain near normal blood glucose goals
- Achieve and/or maintain normal blood pressure
- Achieve and/or maintain optimal blood lipid levels
- Prevent, delay or treat nutrition-related complications
- Provide adequate kilocalories for achievement of reasonable body weight
- Provide optimal nutrition for maximizing health and for growth, development, pregnancy, and lactation.99
 |
Table 3 American Diabetes Association 2016 Recommendations for Diabetes Patients |
Evidence does not support recommending O-3PUFA supplements for people with DM for the prevention or treatment of CVD. As recommended for the general public, an increase in foods containing long-chain O-3 fatty acids is recommended for patients with DM due to their beneficial effects on the prevention of heart disease, lipoproteins and associations with positive health outcomes.100 If the triglyceride concentration is not controlled with statins or fibrates O-3 fatty acids (4g/day) EPA can be used in patients with T2DM and in general CVD population.71
Practical Considerations of O-3 PUFA Supplementation
So far, there is no similar scientific guideline on the ideal O-3 PUFA intake. Things need to be considered include the issues related to supplement dose, purity, cost of obtaining O-3 PUFAs thru supplementation versus food and possibility of adverse effects. Adverse effects are likely dose-dependent. Due to their structure, O-3 PUFAs are susceptible to oxidation if exposed to excess heat. Improper storage of O-3PUFA supplements may cause their damage and affect their beneficial health effects.
O-3 PUFA supplements are a cost-effective way of attaining therapeutic doses. The common therapeutic dosages range from 1–4g/day.101 But, due to the beneficial effect of dietary sources in improving diet quality and improve intake of other beneficial nutrients, the food sources have to be prioritized.102
There are limited studies to draw exact suggestions regarding dosage; duration and the interaction of dosage O-3 PUFA intake and the available information are inconsistent. Probably, the consistent their cardiovascular effects might be likely only with doses above 2000 mg daily.103 Currently, dietary recommendations for individuals with and without DM supports increased consumption of foods rich in O-3PUFA but do not recommend supplementation.104 The Global Burden of Disease Study suggests that optimal intake of long-chain O-3 fatty acid is 0.25 g/d.105
The International Diabetes Federation Global Guideline for T2DM recommends that in patients with T2DM who are unable to achieve lipid-lowering targets with or are intolerant of conventional medications should be considered as candidates for other medications for dyslipidemia, including high-dose O-3PUFAs.106 The 2012 Endocrine Society clinical practice guideline on evaluation and treatment of hypertriglyceridemia, though not specific to patients with T2DM, recommends that drug therapies like fibrates, or O-3PUFAs (alone or in combination with statins) be considered for treatment of moderate to severe triglyceride levels.107
According to a systematic review by Brown et al supplemental long-chain O-3 fatty acids would not be encouraged for the prevention or treatment of DM. In case, supplementary long-chain omega-3 fatty acid is used to reduce triglyceride concentrations, or people with or at risk of T2DM choose to take supplementary long-chain O-3, doses below 4.4g/d should be encouraged.90 On the other hand, according to some studies, there is an increased risk of T2DM with the intake of long-chain O-3 fatty acids, particularly with higher intakes (≥0.20g O-3/d).108 The Food and Drug Administration (FDA) recommends that the intake for consumers not exceeds 3g/day of EPA plus DHA with no more than 2 g/day from dietary supplementation.109
According to recent clinical trial for the general population and those with DM, about 1 g/d of omega-3 fatty acids reduced the risk of from CVD. A higher dose of omega-3 fatty acids (approximately 4 g/d of EPA and likely 4 g/d of EPA+DHA, as well) is also an effective adjunct for cardiovascular treatment in those with high triglycerides who take statins.110 According to one experimental study O-3 fatty acids can be given in conjunction with metformin to decrease triglyceride levels in diabetic dyslipidaemia. Two grams of O-3 fatty acids were more effective than 1 gram of omega-3 fatty acids in decreasing triglyceride levels.111
Based on results from REDUCE-IT, the addition of 4 g/d of EPA should be considered for statin-treated patients who have cardiovascular disease or diabetes and elevated triglycerides. But, the STRENGTH (Statin Residual Risk Reduction with Epanova in High Risk Patients with Hypertriglyceridemia) trial showed no benefit on cardiovascular event rates of a high-dose combination of EPA and DHA and do not support use of this omega-3 fatty acid formulation to reduce major adverse cardiovascular events in high risk patients.112
O-3 PUFA supplementation is not suggested by the American Heart Association (AHA) for the avoidance of CVD in T2DM patients. However, for the secondary prevention of CVD in the general population, the AHA considers O-3 PUFA supplementation reasonable.72 AHA does not recommend supplementation with O-3 PUFAs for individuals with T2DM to prevent coronary artery disease.113,114 Furthermore, the ADA does not recommend O-3PUFA supplements to treat or prevent CVD in patients with DM, even though the consumption of foods containing O-3PUFAs is recommended.115 The AHA recommends supplementation for adults not eating enough oily fish.116
In general, there are limited data on the use of O-3 PUFA supplementation among DM patients. However, for clinical practice, evidence from the most current clinical trials supports the recommendation to consume at least one to two servings of fish/seafood per week, with additional primary prevention benefits conferred by consuming ~1 g/d of DHA and EPA.
Approaches to Enhance O-3PUFAs Diet
Several strategies have been suggested to rise the intake of O−3 fatty acids in the body; including; (i) Increased consumption of fatty fish and other O−3 PUFAs-rich foods, (ii) Fortification of food products with fish oil or ALA, (iii) Enhancement of O−3 PUFAs in animal products by feeding O−3 PUFAs-rich diets, and (iv) Fortification of O−3 PUFAs in oilseed crops by genetic engineering.117
It has been demonstrated that feeding animals with ALA-rich diet can increase the content of O−3PUFA in animal-derived products. However, the magnitude of increase in O−3PUFA content appears to be dependent on the type of diet supplementation.24 For instance, two- to five-fold higher DHA and EPA has been recorded in breast and thigh meat of broilers fed with high ALA-rich flaxseed oil for 6 weeks.118 Moreover, O−3 PUFAs enriched eggs produced by feeding laying hens with a fish meal or canola/linseed oil are commercially viable as a good source of O−3 PUFA.118
FUFAs Metabolically engineered oilseed crops are also beneficial to improve the content of O−3 FAs in seed oil. They have certain advantages over conventional sources of PUFAs. For example, pollution of marine ecosystems has resulted in high accumulation of toxic dioxins, polychlorinated biphenyls, heavy metals, and organochlorine pesticides in fish.119 Additionally, low content of n−3 PUFAs in farmed fishes and wild fish stock are insufficient in order to satisfy recommended EPA and DHA intake levels. Thus, genetically modified crops can serve as future sources of O−3 PUFAs.120
Enhancement of O−3 FAs in animal products by feeding O−3 FAs rich diets or by genetic engineering fatty acid biosynthetic pathways has revealed potential to improve the content of O−3 PUFAs in the diet. However, these strategies need to be fine-tuned. In addition, the releasing and approval of genetically modified crops are challenging due to social concerns.35
Fish oil dietary supplements are widely available and commonly used by consumers; however, there are critical distinctions between these dietary supplements and the FDA-approved O-3PUFA drugs that can be obtained only by prescription. Prescription O-3PUFA products are highly purified, subject to quality control regulations, and required to demonstrate both safety and efficacy in clinical studies to achieve approval by the FDA.
In order to provide adequate PUFAs, the level of their fortification into foods needs extensive consideration. At least 0.5 g/d of O-3 PUFAs EPA and DHA are recommended for daily consumption.121 Dietary consumption of O-3 PUFAs via incorporation into foods is ultimately the most effective mechanism of providing them to the average consumer.122
Future Directions
There is considerable evidence indicated that the consumption of O-3 PUFA is associated with substantial health benefits among DM patients. However, despite the promising experimental investigations, currently, the clinical evidence for the usage of omega-3 supplementation for the management of DM and its complications is both conflicting and inconsistent. Hence, further study is required to ascertain the effects of O-3 PUFA supplementation in the management of diabetes.
Conclusion
Diabetes mellitus especially type 2 is a significant health challenge with multiple associated comorbidities. Diet and lifestyle factors are central to its prevention and control. There are three types of Omega‐3 fatty acids; those include ALA which is derived from plant oils and EPA and DHA which are derived primarily from fish oil.
There is limited clinical evidence that supports O-3 PUFA supplement use in DM management and so far, there is no similar scientific guideline on the ideal O-3 PUFA intake. There are limited studies to draw exact suggestions regarding dosage; duration and the interaction of dosage O-3 PUFA intake and the available information are inconsistent. Several strategies have been suggested to raise the intake of O−3 fatty acids and dietary approach to increase omega-3 fatty acid intake is preferable.
Disclosure
The author reports no conflicts of interest for this work.
References
1. Gómez JM, Vila R, Catalina P, Soler J, Badimón L, Sahún M. The markers of inflammation and endothelial dysfunction in correlation with glycated haemoglobin are present in type 2 diabetes mellitus patients but not in their relatives. Glycoconj J. 2008;25(6):573–579. doi:10.1007/s10719-008-9118-8
2. Yaturu S, Rains J, Jain SK. Relationship of elevated osteoprotegerin with insulin resistance, CRP, and TNF-α levels in men with type 2 diabetes. Cytokine. 2008;44(1):168–171. doi:10.1016/j.cyto.2008.07.471
3. Bagchi D, Nair S, editors. Nutritional and Therapeutic Interventions for Diabetes and Metabolic Syndrome. Academic Press; 2018 May, 25.
4. Steyn NP, Mann J, Bennett PH, et al. Diet, nutrition and the prevention of type 2 diabetes. Public Health Nutr. 2004;7(1a):147–165. doi:10.1079/PHN2003586
5. Hall RM, Strong AP, Krebs JD. Importance of low carbohydrate diets in diabetes management. Nutr Diet Suppl. 2016;8:9–19.
6. American Diabetes Association. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2007;30(suppl 1):S48–65. doi:10.2337/dc07-S048
7. Schröder H. Protective mechanisms of the Mediterranean diet in obesity and type 2 diabetes. J Nutr Biochem. 2007;18(3):149–160. doi:10.1016/j.jnutbio.2006.05.006
8. American Diabetes Association. 5. Lifestyle management: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Supplement 1):S46–S60. doi:10.2337/dc19-S005
9. Jacobson TA, Maki KC, Orringer CE, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 2. J Clin Lipidol. 2015;9(6):S1–22.
10. American Diabetes Association. 9. Cardiovascular disease and risk management. Diabetes Care. 2017;40(Supplement 1):S75–87. doi:10.2337/dc17-S012
11. Derosa G, Maffioli P, D’Angelo A, et al. Effects of long chain ω-3 fatty acids on metalloproteinases and their inhibitors in combined dyslipidemia patients. Expert Opin Pharmacother. 2009;10(8):1239–1247. doi:10.1517/14656560902865601
12. Cicero AF, Derosa G, Gregori VD, Bove M, Gaddi AV, Borghi C. Omega 3 polyunsaturated fatty acids supplementation and blood pressure levels in hypertriglyceridemic patients with untreated normal-high blood pressure and with or without metabolic syndrome: a retrospective study. Clin Exp Hypertens. 2010;32(2):137–144. doi:10.3109/10641960903254448
13. Hajianfar H, Paknahad Z, Bahonar A. The effect of omega-3 supplements on antioxidant capacity in patients with type 2 diabetes. Int J Prev Med. 2013;(Suppl 2):S234.
14. McLennan PL, Raederstorff D. Diabetes puts myocardial n-3 fatty acid status at risk in the absence of supplementation in the rat. Lipids. 1999;34(1):S91–2. doi:10.1007/BF02562242
15. Sekikawa A, Curb JD, Ueshima H, et al. Marine-derived n-3 fatty acids and atherosclerosis in Japanese, Japanese-American, and white men: a cross-sectional study. J Am Coll Cardiol. 2008;52(6):417–424. doi:10.1016/j.jacc.2008.03.047
16. Norris JM, Yin X, Lamb MM, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007;298(12):1420–1428. doi:10.1001/jama.298.12.1420
17. Gebauer SK, Psota TL, Harris WS, Kris-Etherton PM. n− 3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am J Clin Nutr. 2006;83(6):1526S–35S. doi:10.1093/ajcn/83.6.1526S
18. Kaur G, Cameron-Smith D, Garg M, Sinclair AJ. Docosapentaenoic acid (22: 5n-3): a review of its biological effects. Prog Lipid Res. 2011;50(1):28–34. doi:10.1016/j.plipres.2010.07.004
19. Burdge GC, Calder PC. Dietary α-linolenic acid and health-related outcomes: a metabolic perspective. Nutr Res Rev. 2006;19(1):26–52. doi:10.1079/NRR2005113
20. Richter CK, Skulas-Ray AC, Kris-Etherton PM. Recommended intake of fish and fish oils worldwide. In: Fish and Fish Oil in Health and Disease Prevention. Academic Press; January 1, 2016:27–48.
21. Lee JH, O’keefe JH, Lavie CJ, Harris WS. Omega-3 fatty acids: cardiovascular benefits, sources and sustainability. Nat Rev Cardiol. 2009;6(12):753–758. doi:10.1038/nrcardio.2009.188
22. Office of Dietary Supplements - Omega-3 Fatty Acids [Internet]; [cited December 8, 2020]. Available from: https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/.
23. Encyclopedia of Dietary Supplements [Internet]. Routledge & CRC Press; [cited December 11, 2020]. Available from: https://www.routledge.com/Encyclopedia-of-Dietary-Supplements/Coates-Betz-Blackman-Cragg-Levine-Moss-White/p/book/9781439819289.
24. Moghadasian MH. Advances in dietary enrichment with n-3 fatty acids. Crit Rev Food Sci Nutr. 2008;48(5):402–410. doi:10.1080/10408390701424303
25. Whelan J, Rust C. Innovative dietary sources of n-3 fatty acids. Annu Rev Nutr. 2006;26(1):75–103. doi:10.1146/annurev.nutr.25.050304.092605
26. Jackson MB, Ahima RS. Neuroendocrine and metabolic effects of adipocyte-derived hormones. Clin Sci. 2006;110(2):143–152. doi:10.1042/CS20050243
27. Ramel A, Martinez A, Kiely M, Morais G, Bandarra NM, Thorsdottir I. Beneficial effects of long-chain n-3 fatty acids included in an energy-restricted diet on insulin resistance in overweight and obese European young adults. Diabetologia. 2008;51(7):1261. doi:10.1007/s00125-008-1035-7
28. De Caterina R, Madonna R, Bertolotto A, Schmidt EB. n-3 fatty acids in the treatment of diabetic patients: biological rationale and clinical data. Diabetes Care. 2007;30(4):1012–1026. doi:10.2337/dc06-1332
29. Kalupahana NS, Claycombe K, Newman SJ, et al. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J Nutr. 2010;140(11):1915–1922. doi:10.3945/jn.110.125732
30. Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci. 2006;110(3):267–278. doi:10.1042/CS20050182
31. Sukumar P, Sedo A, Li J, et al. Constitutively active TRPC channels of adipocytes confer a mechanism for sensing dietary fatty acids and regulating adiponectin. Circ Res. 2012;111(2):191–200. doi:10.1161/CIRCRESAHA.112.270751
32. Park M, Sweeney G. Direct effects of adipokines on the heart: focus on adiponectin. Heart Fail Rev. 2013;18(5):631–644. doi:10.1007/s10741-012-9337-8
33. Talukdar S, Bae EJ, Imamura T, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–698. doi:10.1016/j.cell.2010.07.041
34. Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: new pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta Mol Cell Biol Lipids. 2015;1851(4):397–413.
35. Saini RK, Keum YS. Omega-3 and omega-6 polyunsaturated fatty acids: dietary sources, metabolism, and significance—A review. Life Sci. 2018;203:255–267. doi:10.1016/j.lfs.2018.04.049
36. Lin N, Shi JJ, Li YM, et al. What is the impact of n-3 PUFAs on inflammation markers in type 2 diabetic mellitus populations?: a systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2016;15(1):133. doi:10.1186/s12944-016-0303-7
37. Fedor D, Kelley DS. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2009;12(2):138–146. doi:10.1097/MCO.0b013e3283218299
38. Horia E, Watkins BA. Complementary actions of docosahexaenoic acid and genistein on COX-2, PGE2 and invasiveness in MDA-MB-231 breast cancer cells. Carcinogenesis. 2007;28(4):809–815. doi:10.1093/carcin/bgl183
39. Wu JH, Micha R, Imamura F, et al. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr. 2012;107(S2):S214–27. doi:10.1017/S0007114512001602
40. Flachs P, Rossmeisl M, Kopecky J. The effect of n-3 fatty acids on glucose homeostasis and insulin sensitivity. Physiol. Res. 2014;63:S93. doi:10.33549/physiolres.932715
41. Berquin IM, Edwards IJ, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008;269(2):363–377. doi:10.1016/j.canlet.2008.03.044
42. Toorang F, Djazayery A, Djalali M. Effects of omega-3 fatty acids supplement on antioxidant enzymes activity in type 2 diabetic patients. Iran J Public Health. 2016;45(3):340.
43. O’Mahoney LL, Matu J, Price OJ, et al. Omega-3 polyunsaturated fatty acids favourably modulate cardiometabolic biomarkers in type 2 diabetes: a meta-analysis and meta-regression of randomized controlled trials. Cardiovasc Diabetol. 2018;17(1):98. doi:10.1186/s12933-018-0740-x
44. Coelho OG, da Silva BP, Rocha DM, Lopes LL, Alfenas RD. Polyunsaturated fatty acids and type 2 diabetes: impact on the glycemic control mechanism. Crit Rev Food Sci Nutr. 2017;57(17):3614–3619. doi:10.1080/10408398.2015.1130016
45. Guo XF, Sinclair AJ, Kaur G, Li D. Differential effects of EPA, DPA and DHA on cardio-metabolic risk factors in high-fat diet fed mice. Prostaglandins Leukot Essent Fatty Acids. 2018;136:47–55. doi:10.1016/j.plefa.2017.09.011
46. Chen C, Yu X, Shao S. Effects of omega-3 fatty acid supplementation on glucose control and lipid levels in type 2 diabetes: a meta-analysis. PLoS One. 2015;10(10):e0139565. doi:10.1371/journal.pone.0139565
47. Hartweg J, Perera R, Montori VM, Dinneen SF, Neil AH, Farmer AJ. Omega‐3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008;(1). doi:10.1002/14651858.CD003205.pub2.
48. Chauhan S, Kodali H, Noor J, Ramteke K, Gawai V. Role of omega-3 fatty acids on lipid profile in diabetic dyslipidaemia: single blind, randomised clinical trial. J Clin Diagn Res. 2017;11(3):OC13. doi:10.7860/JCDR/2017/20628.9449
49. ORIGIN Trial Investigators. n–3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367(4):309–318. doi:10.1056/NEJMoa1203859
50. Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6(13):1246. doi:10.4239/wjd.v6.i13.1246
51. Shah M, Adams-Huet B, Brinkley L, Grundy SM, Garg A. Lipid, glycemic, and insulin responses to meals rich in saturated, cis-monounsaturated, and polyunsaturated (n-3 and n-6) fatty acids in subjects with type 2 diabetes. Diabetes Care. 2007;30(12):2993–2998. doi:10.2337/dc07-1026
52. Tribole E. The Ultimate Omega-3 Diet. McGraw-Hill Professional Publishing; 2007.
53. Abdelhamid AS, Brown TJ, Brainard JS, et al. Omega‐3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2020;(3).
54. Adkins Y, Kelley DS. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem. 2010;21(9):781–792. doi:10.1016/j.jnutbio.2009.12.004
55. Rangel-Huerta OD, Gil GA. Omega 3 fatty acids in cardiovascular disease risk factors: an updated systematic review of randomised clinical trials. Clin Nutr. 2018;37(1):72–77. doi:10.1016/j.clnu.2017.05.015
56. Chang CL, Deckelbaum RJ. Omega-3 fatty acids: mechanisms underlying “protective effects” in atherosclerosis. Curr Opin Lipidol. 2013;24(4):345. doi:10.1097/MOL.0b013e3283616364
57. Cintra DE, Ropelle ER, Moraes JC, et al. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS One. 2012;7(1):e30571. doi:10.1371/journal.pone.0030571
58. Hartweg J, Farmer AJ, Holman RR, Neil HA. Meta-analysis of the effects of n-3 polyunsaturated fatty acids on haematological and thrombogenic factors in type 2 diabetes. Diabetologia. 2007;50(2):250–258. doi:10.1007/s00125-006-0486-y
59. Atar MJ, Hajianfar H, Bahonar A. The effects of omega-3 on blood pressure and the relationship between serum visfatin level and blood pressure in patients with type II diabetes. ARYA Atheroscler. 2012;8(1):27.
60. Cicero AF, Ertek S, Borghi C. Omega-3 polyunsaturated fatty acids: their potential role in blood pressure prevention and management. Curr Vasc Pharmacol. 2009;7(3):330–337. doi:10.2174/157016109788340659
61. Mason RP. New insights into mechanisms of action for omega-3 fatty acids in atherothrombotic cardiovascular disease. Curr Atheroscler Rep. 2019;21(1):2. doi:10.1007/s11883-019-0762-1
62. Raghu B, Venkatesan P. Effect of n-3 fatty acid supplementation on blood glucose, lipid profile and cytokines in humans: a pilot study. Indian J Clin Biochem. 2008;23(1):85–88. doi:10.1007/s12291-008-0020-8
63. Pase MP, Grima NA, Sarris J. Do long-chain n-3 fatty acids reduce arterial stiffness? A meta-analysis of randomised controlled trials. Br J Nutr. 2011;106(7):974–980. doi:10.1017/S0007114511002819
64. Wang Q, Liang X, Wang L, et al. Effect of omega-3 fatty acids supplementation on endothelial function: a meta-analysis of randomized controlled trials. Atherosclerosis. 2012;221(2):536–543. doi:10.1016/j.atherosclerosis.2012.01.006
65. Siniarski A, Haberka M, Mostowik M, et al. Treatment with omega-3 polyunsaturated fatty acids does not improve endothelial function in patients with type 2 diabetes and very high cardiovascular risk: a randomized, double-blind, placebo-controlled study (Omega-FMD). Atherosclerosis. 2018;271:
66. O’Mahoney LL, Dunseath G, Churm R, et al. Omega-3 polyunsaturated fatty acid supplementation versus placebo on vascular health, glycaemic control, and metabolic parameters in people with type 1 diabetes: a randomised controlled preliminary trial. Cardiovasc Diabetol. 2020;19(1):1. doi:10.1186/s12933-020-01094-5
67. Poreba M, Mostowik M, Siniarski A, et al. Treatment with high-dose n-3 PUFAs has no effect on platelet function, coagulation, metabolic status or inflammation in patients with atherosclerosis and type 2 diabetes. Cardiovasc Diabetol. 2017;16(1):1. doi:10.1186/s12933-017-0523-9
68. ASCEND Study Collaborative Group. Effects of n− 3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018;379(16):1540–1550. doi:10.1056/NEJMoa1804989
69. Marchioli R, Barzi F, Bomba E, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105(16):1897–1903. doi:10.1161/01.CIR.0000014682.14181.F2
70. Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. lancet. 2007;369(9567):1090–1098. doi:10.1016/S0140-6736(07)60527-3
71. Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22. doi:10.1056/NEJMoa1812792
72. Siscovick DS, Barringer TA, Fretts AM, et al. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2017;135(15):e867–84. doi:10.1161/CIR.0000000000000482
73. Watson H, Mitra S, Croden FC, et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut. 2018;67(11):1974–1983. doi:10.1136/gutjnl-2017-314968
74. Behl T, Kotwani A. Omega‐3 fatty acids in prevention of diabetic retinopathy. J Pharm Pharmacol. 2017;69(8):946–954. doi:10.1111/jphp.12744
75. Zhuang W, Wang G, Li L, Lin G, Deng Z. Omega-3 polyunsaturated fatty acids reduce vascular endothelial growth factor production and suppress endothelial wound repair. J Cardiovasc Transl Res. 2013;6(2):287–293. doi:10.1007/s12265-012-9409-0
76. Larson MK, Tormoen GW, Weaver LJ, et al. Exogenous modification of platelet membranes with the omega-3 fatty acids EPA and DHA reduces platelet procoagulant activity and thrombus formation. Am J Physiol Cell Physiol. 2013;304(3):C273–9. doi:10.1152/ajpcell.00174.2012
77. Tikhonenko M, Lydic TA, Opreanu M, et al. N-3 polyunsaturated fatty acids prevent diabetic retinopathy by inhibition of retinal vascular damage and enhanced endothelial progenitor cell reparative function. PLoS One. 2013;8(1):e55177. doi:10.1371/journal.pone.0055177
78. Sala-Vila A, Díaz-López A, Valls-Pedret C, et al. Dietary marine ω-3 fatty acids and incident sight-threatening retinopathy in middle-aged and older individuals with type 2 diabetes: prospective investigation from the PREDIMED trial. JAMA Ophthalmol. 2016;134(10):1142–1149. doi:10.1001/jamaophthalmol.2016.2906
79. Hu J, Liu Z, Zhang H. Omega-3 fatty acid supplementation as an adjunctive therapy in the treatment of chronic kidney disease: a meta-analysis. Clinics. 2017;72(1):58–64. doi:10.6061/clinics/2017(01)10
80. Chewcharat A, Chewcharat P, Rutirapong A, Papatheodorou S. The effects of omega-3 fatty acids on diabetic nephropathy: a meta-analysis of randomized controlled trials. PLoS One. 2020;15(2):e0228315. doi:10.1371/journal.pone.0228315
81. Elajami TK, Alfaddagh A, Lakshminarayan D, Soliman M, Chandnani M, Welty FK. Eicosapentaenoic and docosahexaenoic acids attenuate progression of albuminuria in patients with type 2 diabetes mellitus and coronary artery disease. J Am Heart Assoc. 2017;6(7):e004740. doi:10.1161/JAHA.116.004740
82. Navaneethan SD, Virani SS. Omega-3 fatty acids (Fish Oil) supplementation and albuminuria: not a slam dunk. J Am Heart Assoc. 2017;6(7). doi:10.1161/JAHA.117.006020
83. Lee CC, Sharp SJ, Wexler DJ, Adler AI. Dietary intake of eicosapentaenoic and docosahexaenoic acid and diabetic nephropathy: cohort analysis of the diabetes control and complications trial. Diabetes Care. 2010;33(7):2010.
84. Guebre-Egziabher F, Debard C, Drai J, et al. Differential dose effect of fish oil on inflammation and adipose tissue gene expression in chronic kidney disease patients. Nutrition. 2013;29(5):730–736. doi:10.1016/j.nut.2012.10.011
85. Shapiro H, Theilla M, Attal-Singer J, Singer P. Effects of polyunsaturated fatty acid consumption in diabetic nephropathy. Nat Rev Nephrol. 2011;7(2):110. doi:10.1038/nrneph.2010.156
86. Huang X, Lindholm B, Stenvinkel P, Carrero JJ. Dietary fat modification in patients with chronic kidney disease: n-3 fatty acids and beyond. J Nephrol. 2013;26(6):960–974. doi:10.5301/jn.5000284
87. Cao Z, Cooper ME. Pathogenesis of diabetic nephropathy. J Diabetes Investig. 2011;2(4):243–247. doi:10.1111/j.2040-1124.2011.00131.x
88. Zhou Y, Tian C, Jia C. Association of fish and n-3 fatty acid intake with the risk of type 2 diabetes: a meta-analysis of prospective studies. Br J Nutr. 2012;108(3):408–417. doi:10.1017/S0007114512002036
89. Zhang M, Picard-Deland E, Marette A. Fish and marine omega-3 polyunsaturated fatty acid consumption and incidence of type 2 diabetes: a systematic review and meta-analysis. Int J Endocrinol. 2013;8:2013.
90. Brown TJ, Brainard J, Song F, Wang X, Abdelhamid A, Hooper L. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. BMJ. 2019;21(366):l4697. doi:10.1136/bmj.l4697
91. Bi X, Li F, Liu S, et al. ω-3 polyunsaturated fatty acids ameliorate type 1 diabetes and autoimmunity. J Clin Invest. 2017;127(5):1757–1771. doi:10.1172/JCI87388
92. Chen C, Yang Y, Yu X, Hu S, Shao S. Association between omega‐3 fatty acids consumption and the risk of type 2 diabetes: a meta‐analysis of cohort studies. J Diabetes Investig. 2017;8(4):480–488. doi:10.1111/jdi.12614
93. Brostow DP, Odegaard AO, Koh WP, et al. Omega-3 fatty acids and incident type 2 diabetes: the Singapore Chinese Health Study. Am J Clin Nutr. 2011;94(2):520–526. doi:10.3945/ajcn.110.009357
94. Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126(1):1–27. doi:10.1016/S0009-3084(03)00101-4
95. Gladman SJ, Huang W, Lim SN, et al. Improved outcome after peripheral nerve injury in mice with increased levels of endogenous omega-3 polyunsaturated fatty acids. J Neurosci. 2012;32(2):563–571. doi:10.1523/JNEUROSCI.3371-11.2012
96. Azizi-Soleiman F, Jazayeri S, Eghtesadi S, et al. Effects of pure eicosapentaenoic and docosahexaenoic acids on oxidative stress, inflammation and body fat mass in patients with type 2 diabetes. Int J Prev Med. 2013;4(8):922.
97. Black SC, Katz S, McNeill JH. Cardiac performance and plasma lipids of omega-3 fatty acid-treated streptozocin-induced diabetic rats. Diabetes. 1989;38(8):969–974. doi:10.2337/diab.38.8.969
98. Pahlavani M, Razafimanjato F, Ramalingam L, et al. Eicosapentaenoic acid regulates brown adipose tissue metabolism in high-fat-fed mice and in clonal brown adipocytes, J. Nutr Biochem. 2017;39:101–109. doi:10.1016/j.jnutbio.2016.08.012
99. Franz MJ, Bantle JP, Beebe CA, et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2002;25(1):148–198. doi:10.2337/diacare.25.1.148
100. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36(11):3821–3842. doi:10.2337/dc13-2042
101. Radcliffe JE, Thomas J, Bramley AL, et al. Controversies in omega-3 efficacy and novel concepts for application. J Nutr Intermed Metab. 2016;5:11–22. doi:10.1016/j.jnim.2016.05.002
102. Itsiopoulos C, Marx W, Mayr HL, et al. The role of omega-3 polyunsaturated fatty acid supplementation in the management of type 2 diabetes mellitus: a narrative review. J Nutr Intermed Metab. 2018;14:42–51. doi:10.1016/j.jnim.2018.02.002
103. Tenenbaum A, Fisman EZ. Omega-3 polyunsaturated fatty acids supplementation in patients with diabetes and cardiovascular disease risk: does dose really matter? Cardiovasc Diabetol. 2018;17(1):1–3. doi:10.1186/s12933-018-0766-0
104. NIfHaC E. Cardiovascular Disease: Risk Assessment and Reduction, Including Lipid Modification. London: National Institute for Health and Care Excellence; 2016.
105. Afshin A, Sur PJ, Fay KA, et al. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;393(10184):1958–1972. doi:10.1016/S0140-6736(19)30041-8
106. Clinical Guidelines Task Force. Global Guideline for Type 2 Diabetes. Brussels, Belgium: International Diabetes Federation; 2012.
107. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969–2989. doi:10.1210/jc.2011-3213
108. Djousse L, Gaziano JM, Buring JE, Lee IM. Dietary omega-3 fatty acids and fish consumption and risk of type 2 diabetes. Am J Clin Nutr. 2011;93(1):143–150. doi:10.3945/ajcn.110.005603
109. FDA announces qualified health claims for omega-3 fatty acids.
110. Kris-etherton PM, Richter CK, Bowen KJ, et al. Recent clinical trials shed new light on the cardiovascular benefits of omega-3 fatty acids. Methodist Debakey Cardiovasc J . 2019;15(3):171–178. doi:10.14797/mdcj-15-3-171
111. Article O, Chauhan S, Kodali H, Noor J, Ramteke K, Gawai V. Role of omega-3 fatty acids on lipid profile in diabetic dyslipidaemia: single blind, randomised clinical trial. J Clin Diagn Res. 2017;11(3):13–16.
112. Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk. JAMA. 2020;324(22):2268. doi:10.1001/jama.2020.22258
113. Siscovick DS, Barringer TA, Fretts AM, Wu JH, Lichtenstein AH, Costello RB. American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2017;135(15):e867–84.
114. A.H.A.S. Advisory. Omega-3 polyunsaturated fatty acid (Fish Oil) supplementation and the prevention of clinical cardiovascular disease. Circulation. 2017;135(15):e867–84. doi:10.1161/CIR.0000000000000482.
115. American Diabetes Association. Standards of medical care in diabetes – 2016. Diabetes Care. 2016;39(Suppl1):S1– S112.
116. American Heart Association. Fish and omega-3 fatty acids; 2017. Available from: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/fats/fish-and-omega-3-fatty-acids.
117. Lane KE, Derbyshire EJ. Omega-3 fatty acids–A review of existing and innovative delivery methods. Crit Rev Food Sci Nutr. 2018;58(1):62–69. doi:10.1080/10408398.2014.994699
118. Kanakri K, Carragher J, Hughes R, Muhlhausler B, Gibson R. A reduced cost strategy for enriching chicken meat with omega-3 long chain polyunsaturated fatty acids using dietary flaxseed oil. Br Poult Sci. 2017;58(3):283–289. doi:10.1080/00071668.2017.1293798
119. Nøstbakken OJ, Hove HT, Duinker A, et al. Contaminant levels in Norwegian farmed Atlantic salmon (Salmo salar) in the 13-year period from 1999. Environ Int. 2015;74:274–80.
120. Sprague M, Dick JR, Tocher DR. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon, 2006–2015. Sci Rep. 2016;6. doi:10.1038/srep21892
121. Harris WS, Mozaffarian D, Lefevre M, et al. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J Nutr. 2009;139(4):804S– 819S. doi:10.3945/jn.108.101329
122. Taylor P, Ganesan B, Brothersen C, Mcmahon DJ, Ganesan B, Brothersen C. fortification of foods with omega-3 polyunsaturated fatty acids. Crit Rev Food Sci Nutr. 2014;54(1):37–41.
123. USDA National Nutrient Database for Standard Reference, Release 28; 2015. Available from: http://ndb.nal.usda.gov/ndb/search.
124. Kurowska EM, Dresser GK, Deutsch L, Vachon D, Khalil W. Bioavailability of omega-3 essential fatty acids from perilla seed oil. Prostaglandins Leukot Essent Fatty Acids. 2003;68(3):207–212. doi:10.1016/S0952-3278(02)00271-5
125. Rallidis LS, Paschos G, Papaioannou ML, et al. The effect of diet enriched with α-linolenic acid on soluble cellular adhesion molecules in dyslipidaemic patients. Atherosclerosis. 2004;174(1):127–132. doi:10.1016/j.atherosclerosis.2004.01.013
126. Bell JG, Henderson RJ, Tocher DR, Sargent JR. Replacement of dietary fish oil with increasing levels of linseed oil: modification of flesh fatty acid compositions in Atlantic salmon (Salmo salar) using a fish oil finishing diet. Lipids. 2004;39(3):223–232. doi:10.1007/s11745-004-1223-5
127. Kris-Etherton PM, Taylor DS, Yu-Poth S, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71(1):179S–88S. doi:10.1093/ajcn/71.1.179S
128. USDA Nutrient Data Lab; 2004. Available from: http://www.nal.usda.gov/fnic/foodcomp/search/.
129. Wheeler ML, Dunbar SA, Jaacks LM, et al. Macronutrients, food groups, and eating patterns in the management of diabetes: a systematic review of the literature, 2010. Diabetes Care. 2012;35(2):434445. doi:10.2337/dc11-2216
130. El-Sayed E, Ibrahim K. Effect of the types of dietary fats and non-dietary oils on bone metabolism. Crit Rev Food Sci Nutr. 2017;57(4):653–658. doi:10.1080/10408398.2014.914889
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.