Back to Journals » Journal of Pain Research » Volume 11
Pulsed radiofrequency of C2 dorsal root ganglion under ultrasound guidance for chronic migraine: a case report
Authors Li J, Yin Y, Ye L , Zuo Y
Received 23 April 2018
Accepted for publication 27 July 2018
Published 21 September 2018 Volume 2018:11 Pages 1915—1919
DOI https://doi.org/10.2147/JPR.S172017
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Jun Li,1,* Yan Yin,1,* Ling Ye,1 Yunxia Zuo2
1Department of Pain Management, West China Hospital, Sichuan University, Chengdu, Sichuan Province 610041, People’s Republic of China; 2Department of Anesthesiology, West China Hospital, Sichuan University, Chengdu, Sichuan Province 610041, People’s Republic of China
*These authors contributed equally to this work
Abstract: Chronic migraine is common but difficult to treat. Most patients respond poorly to drugs. Occipital nerve block such as stellate ganglion block is an effective treatment without continuous effect for migraine. Pulsed radiofrequency (PRF) technique has been shown to be effective in relieving headache and prolonging the effect of nerve block. This case report is about a patient who suffered from chronic migraine with occipital pain and was successfully treated with PRF of C2 (axis) dorsal root ganglion (DRG) under ultrasound guidance confirmed by computed tomograpy scan. The patient did not feel headache after 1-year follow-up. This suggests that C2 DRG PRF might be considered as an alternative treatment for chronic migraine with occipital pain.
Keywords: migraine, chronic facial pain syndrome, ultrasound guidance, pulsed radiofrequency, dorsal root ganglion
Introduction
Headache is a common clinical pain disorder which is the third leading cause of disability in the world. Nearly 95% of people suffer from headache during their life.1 The International Headache Society classifies headaches into the following three major categories: primary headaches, secondary headaches, and painful cranial neuropathies (trigeminal neuropathies, glossopharyngeal, and occipital neuralgias). Primary headaches, the most common and also known as functional headaches, include migraine (including chronic migraine), tension-type headache (including chronic tension-type headache), cluster headaches, and nummular headache. Secondary headaches, also known as symptomatic headaches, are symptoms of local organic damage (cervicogenic headache and headache attributed to craniotomy) or systemic disease.2 Chronic migraine is more likely associated with insomnia, bad mood (such as anxiety and depression), and so on. Migraine can also reduce immune function and even worsen high blood pressure, coronary heart disease, ulcers, and other related diseases.3 Moreover, chronic migraine causes severe burden and quality-of-life impairment.4 Most patients respond poorly to drugs. Reasons for the treatment failure included wrong diagnosis, inadequate analgesia, intolerable side effects, and the development of drug tolerance.5 Even when successfully treated, symptoms can often reoccur. Thus, an innovative treatment with fewer adverse effects and long-term relief was necessary for chronic migraine.
Nerve block is a commonly used method that can relieve the symptoms of pain attacks, prolong the intermission period, and reduce the use of drugs, but without continuous effect.6 In recent years, more and more alternative treatments, such as neuromodulation, have been developed. Neuromodulation can target central or peripheral nerves or dorsal root ganglion (DRG).7 Pulsed radiofrequency (PRF) is a common technique of neuromodulation that has been shown to be effective in regulating neurological function. Occipital nerve regulation can be used to treat chronic migraine.8 Cervical 2 DRG is a common clinical target of occipital nerve regulation, which is often guided by C-arm or computed tomography (CT).9 In recent years, ultrasound (US) has been widely used for the treatment of chronic pain. This case report describes about a patient who suffered from chronic migraine with occipital pain and was successfully treated with PRF of C2 (axis) DRG under US guidance confirmed by CT scan. The patient had been followed-up for 1 year.
Case report
A 34-year-old female with a more than 10-year history of chronic migraine was admitted to our pain department. A MRI of the brain demonstrated an aneurysm of the left internal carotid (carotid-ophthalmic aneurysm, 0.9 cm). She was referred to the neurosurgery department for an interventional therapy twice and successfully treated. But her pain did not resolve and she continued to suffer from daily headaches.
She complained headache over the right orbit and occipitalia, which also spread down to the right side of her neck. She rated her headache as 8/10 based on the VAS. She also complained of autonomic nerve symptoms such as dizziness, vomit, nausea, and photophobia. Nonsteroidal anti-inflammatory agents, triptans, ergot, prophylaxis antidepressants (amitriptyline, 12.5 mg, qn [every night], 2 weeks), and antiepileptic drugs (gabapentin 0.3, three times a day [tid], 2 weeks) were administered to the patient but her pain did not relieve. Stellate ganglion block (SGB) also could not relieve the pain. Then, she was eventually referred to our pain department. Diagnostic right C2 DRG block (1 mL of 2% lidocaine) was administered to the patient and 75%–100% pain relieved for 4 days, but the pain reoccurred. Then, PRF of the C2 DRG was administered to provide a long-term relief.
Procedure
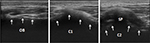
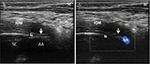
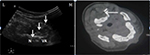
Written informed consent was provided by the patient regarding the publication of their case details and accompanying images before the procedures. All the procedures were accomplished using 2% lidocaine by the same doctor. For the PRF procedure, the patient was placed in prone position with the head slightly flexion. Under US guidance (Philips CX50 ultrasound system), a probe with a frequency of 12 MHz was used. The target site was defined as the medial of the atlantoaxial joint. The spinous process of C2 was located in the short axis view (Figure 1), then the probe was shifted laterally toward the space between C1 and C2, ~1 cm in axial slice, showing an irregular hypoechoic image in the medial of atlantoaxial joint (Figure 2). Doppler was used to avoid vascular injury. A 22G needle was inserted under US guidance, until the needle tip was accurately situated at the target site (Figure 3). Then, the CT scan (Siemens Somatom Sensation CT 64-channel) was used to confirm the accuracy. At the same time, the nerve stimulator (G4; Cosman Medical, Burlington, MA, USA) would induce the sensorimotor movement. Stimulation with 50 Hz of current was applied to determine the electrode position. If the patient complained a tingling sensation of <0.5 V within the nerve distribution area, then the needle electrode was considered to be in the appropriate position. Then, 0.5 mL contrast (iohexol) was injected and contrast media were detected in the medial of atlantoaxial joint by CT scan (Figure 3). After confirming the needle position, PRF was performed for 900 s at 42°C with a duration of 20 ms current (2 Hz, 45 V). Then, 5 mg dexamethasone with 1 mL of 2% lidocaine was injected before the needle was removed. The patient was monitored for 2 h in the ward.
After the procedure, the patient got a complete pain relief and did not complain pain or facial autonomic nerve symptoms during 1-year follow-up. She did not develop any major side effects and she was able to return to a full-time job.
Discussion
This case has reported the successful treatment of chronic migraine by C2 DRG PRF confirmed by CT scan. Chronic migraine is a more challenging and troublesome clinical disorder. There are few therapeutic options. Apart from drugs, nerve blocks have been performed for a long time.10 The occipital nerve block and SGB are most widely used. Previous report demonstrated that the occipital nerve block showed 70%–80% relief in the patients with chronic intractable occipital neuralgia.6,11
According to the anatomical location, occipital nerve blocks could be divided into C2 DRG block, cervical 2,3 paravertebral block, and the second occipital nerve block, (including great occipital, lesser occipital nerve block, and sometimes also the great auricular nerve block).12 Cervical 2,3 medial branch dominates the majority sensory and motion of occipital and affects the meningeal pain conduction. Moreover, C2 DRG block can also affect the primary sensory neurons located in the DRG. Some drugs may also enter the spinal cord through the root sheath (including part of the trigeminal nerve nucleus) in order to play a role in regulating the head sensory conduction.13 It can be used to relieve the symptoms of pain attack, prolong the intermission period, and reduce the use of drugs, but without continuous effect. A recent study have shown that PRF regulation of the C2 DRG is an effective treatment.14 PRF is a non-neurodestructive regulation procedure with temperature not higher than 42°C, whereas radiofrequency (RF) thermocoagulation can cause nerve destruction (temperature higher than 60°C–80°C). Recent experimental studies have shown that PRF would cause an increase of c-fos expression15,16 and synaptic change in transmission, as well as minimize the neuritis and lessen the neural damage.17,18 However, the exact analgesic mechanism is yet to be known. PRF has proved to get long-term pain relief in various chronic pain conditions.19 The PRF procedure was often performed under the guidance of C-arm or CT. In recent years, US is more widely used in the treatment of chronic pain without radiation.
There was one case that reported cervical medial branch RF neurotomy by US guide,20 but there are no reports on C2 DRG RF guided by US. Compared to other methods, US guidance could greatly compensate the shortcomings of traditional methods and improve the safety and effectiveness of neuro interventional therapy. Through US, the target nerve can be visualized, so the needle can be introduced directly to the nerve in real time, and this method can avoid the damage of important structures and tissues. Studies have shown that US could be used for the posterior branch of the C2 spinal nerve block and electrode implantation.21,22 Due to the anatomic characteristics of the cervical DRG, US is also suitable for this interventional treatment. C2 DRG locates on the coverage of bones that passes through the middle part of the atlantoaxial joint, and then it travels between obliquus capitis inferior muscle and semispinalis capitis muscle, continuing as the great occipital nerve. Under the guidance of X-ray or CT, the target is the atlantoaxial joint. US guidance can not only locate the C2 dorsal root nerve and atlantoaxial joints but also identify the blood vessels accompanying the nerve and the vertebral artery that runs along the lateral side of the atlantoaxial joint by color Doppler.23 This new method can avoid vascular injury, especially vertebral artery injury. In addition, the intervertebral space between the C1 (atlas) and C2 is wide, and there is no skeletal protection on the spinal cord. US can identify the dural sac to avoid the puncture injury.
This is the first report to describe US-guided PRF regulation of the C2 DRG for the treatment of chronic migraine. The patient got significant pain relief without serious adverse effects and she returned to a full-time job.
Conclusion
Our report demonstrated a new method of PRF on C2 DRG under US guidance for chronic migraine with occipital pain, which might be considered as an alternative method. More studies can be carried out to confirm the new method.
Acknowledgment
This report was funded by Grant 81200865 to Ling Ye from National Natural Science Foundation of China (NSFC) and Grant 17PJ370 from Health and Family Planning Commission of Sichuan Province.
Disclosure
The authors report no conflicts of interest in this work.
References
Jensen R, Stovner LJ. Epidemiology and comorbidity of headache. Lancet Neurol. 2008;7(4):354–361. | ||
Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephaalgia. 2013;33:629–808. | ||
Voigt AW, Gould HJ. Chronic daily headache: mechanisms and principles of management. Curr Pain Headache Rep. 2016;20(2):10. | ||
Linde M, Gustavsson A, Stovner LJ, et al. The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol. 2012;19(5):703–711. | ||
Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache. 2013;53(4):644–655. | ||
Dach F, Éckeli ÁL, Ferreira KS, Speciali JG. Nerve block for the treatment of headaches and cranial neuralgias: a practical approach. Headache. 2015;55(Suppl 1):59–71. | ||
Lee PB, Horazeck C, Nahm FS, Huh BK. Peripheral nerve stimulation for the treatment of chronic intractable headaches: long-term efficacy and safety study. Pain Physician. 2015;18(5):505–516. | ||
Palamar D, Uluduz D, Saip S, Erden G, Unalan H, Akarirmak U. Ultrasound-guided greater occipital nerve block: an efficient technique in chronic refractory migraine without aura? Pain Physician. 2015;18(2):153–162. | ||
Kim ED, Kim YH, Park CM, Kwak JA, Moon DE. Ultrasound-guided pulsed radiofrequency of the third occipital nerve. Korean J Pain. 2013;26(2):186–190. | ||
Cho S-J, Song T-J, Chu MK. Treatment update of chronic migraine. Curr Pain Headache Rep. 2017;21:26. | ||
Zipfel J, Kastle A, Tatu L, Behr J, Kechidi R, Kastler B. Ultrasound-guided intermediate site greater occipital nerve infiltration: a technical feasibility study. Pain Physician. 2016;19:E1027–E1034. | ||
Cesmebasi A, Muhleman MA, Hulsberg P, et al. Occipital neuralgia: anatomic considerations. Clin Anat. 2015;28(1):101–108. | ||
Biondi DM. Cervicogenic headache: mechanisms, evaluation, and treatment strategies. J Am Osteopath Assoc. 2000;100(9 Suppl):S7–S14. | ||
Hamer J, Purath T. Response of cervicogenic headaches and occipital neuralgia to radiofrequency ablation of the C2 dorsal root ganglion and/or third occipital nerve. Headache. 2014;54(3):500–510. | ||
Podhajsky RJ, Sekiguchi Y, Kikuchi S, Myers RR. The histologic effects of pulsed and continuous radiofrequency lesions at 42°C to rat dorsal root ganglion and sciatic nerve. Spine. 2005;30(9):1008–1013. | ||
Nagar VR, Birthi P, Grider JS, Asopa A. Systematic review of radiofrequency ablation and pulsed radiofrequency for management of cervicogenic headache. Pain Physician. 2015;18(2):109–130. | ||
Fang L, Tao W, Jingjing L, Nan J. Comparison of high-voltage with standard-voltage pulsed radiofrequency of Gasserian ganglion in the treatment of idiopathic trigeminal neuralgia. Pain Pract. 2015;15(7):595–603. | ||
Rohof OJ. Caudal epidural of pulsed radiofrequency in post herpetic neuralgia (PHN): report of three cases. Anesth Pain Med. 2014;4(3): e16369. | ||
Zhang X, Xu Y, Zhou J, et al. Ultrasound-guided alcohol neurolysis and radiofrequency ablation of painful stump neuroma: effective treatments for post-amputation pain. J Pain Res. 2017;10:295–302. | ||
Hamer JF, Purath TA. Repeat RF ablation of C2 and third occipital nerves for recurrent occipital neuralgia and cervicogenic headaches. World J Neurosci. 2016;06(04):236–242. | ||
Zipfel J, Kastler A, Tatu L, Behr J, Kechidi R, Kastler B. Ultrasound-guided intermediate site greater occipital nerve infiltration: a technical feasibility study. Pain Physician. 2016;19(7):E1027–E1034. | ||
Eldrige JS, Obray JB, Pingree MJ, Hoelzer BC. Occipital neuromodulation: ultrasound guidance for peripheral nerve stimulator implantation. Pain Pract. 2010;10(6):580–585. | ||
Atlas of ultrasound-guided procedures in interventional pain management. Narouze SN, editor. Springer. 2011;29:353–356. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.