Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Pulmonologists’ Opinion on the Use of Inhaled Corticosteroids in Chronic Obstructive Pulmonary Disease Patients in Spain: A Cross-Sectional Survey
Authors Miravitlles M , González-Torralba F, Represas-Represas C, Pomares X , Márquez-Martín E, González C, Amado C , Forné C , Alonso S, Alcázar B , Barrecheguren M, Jurado Mirete JM, Naval E
Received 18 April 2022
Accepted for publication 4 July 2022
Published 12 July 2022 Volume 2022:17 Pages 1577—1587
DOI https://doi.org/10.2147/COPD.S369118
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Marc Miravitlles,1,2 Fernando González-Torralba,3 Cristina Represas-Represas,4 Xavier Pomares,2,5 Eduardo Márquez-Martín,2,6 Cruz González,7 Carlos Amado,8 Carles Forné,9,10 Soledad Alonso,11 Bernardino Alcázar,12 Miriam Barrecheguren,1 Juan María Jurado Mirete,13 Elsa Naval14
1Pneumology Department, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain; 2CIBER de Enfermedades Respiratorias (CIBERES), ISCIII, Madrid, Spain; 3Pneumology Department, Hospital Universitario del Tajo, Aranjuez, Spain; 4Pneumology Department, Hospital Álvaro Cunqueiro, Vigo, Spain; 5Pneumology Department, Corporació Sanitària Parc Taulí, Sabadell, Spain; 6Medical-Surgical Unit for Respiratory Diseases, Hospital Universitario Virgen del Rocío, Sevilla, Spain; 7Pneumology Department, Hospital Clínico de Valencia, Valencia, Spain; 8Pneumology Department, Hospital Universitario Marqués de Valdecilla, Santander, Spain; 9Heorfy Consulting, Lleida, Spain; 10Basic Medical Sciences Department, University of Lleida, Lleida, Spain; 11Pneumology Department, Hospital Universitario de Torrejón, Torrejón de Ardoz, Spain; 12Pneumology Department, Hospital Universitario Virgen de las Nieves, Granada, Spain; 13Scientific Department, GOC Health Consulting, Barcelona, Spain; 14Pneumology Department, Hospital Universitario de La Ribera, Alzira, Spain
Correspondence: Marc Miravitlles, Pneumology Department, Hospital Universitari Vall d’Hebron, P. Vall d’Hebron 119-129, Barcelona, 08035, Spain, Tel/Fax +34 932746083, Email [email protected]
Introduction: Identifying the variables that guide decision-making in relation to the use of inhaled corticosteroids (ICS) can contribute to the appropriate use of these drugs. The objective of this study was to identify the clinical variables that physicians consider most relevant for prescribing or withdrawing ICS in COPD.
Methods: A cross-sectional survey was conducted in Spain from November 2020 to May 2021. Therapeutic decisions on the use of ICS in 11 hypothetical COPD patient profiles were collected using an online survey answered by specialists with experience in the management of patients with COPD. Mixed-effects logistic regression was used to analyze the impact of patients’ characteristics in the therapeutic decision for prescribing ICS or proceeding to its withdrawal.
Results: A total of 74 pulmonologists agreed to collaborate in the survey and answered the questionnaire. The results showed great variability, with only 2 profiles achieving consensus for starting or withdrawing the treatment. The frequency and severity of exacerbations influenced the decision to prescribe ICS in a dose-response fashion (1 exacerbation odds ratio (OR) = 1.86, 95% confidence interval (CI) 1.02 to 3.43, two exacerbations OR = 11.6, 95% CI: 4.47 to 30.2 and three OR = 123, 95% CI: 25 to 601). Similarly, increasing blood eosinophils and history of asthma were associated with ICS use. On the other hand, pneumonia reduced the probability of initiating treatment with ICS (OR = 0.54 [0.29 to 0.98]). Lung function and dyspnea degree did not influence the clinician’s therapeutic decision. The results for withdrawal of ICS were similar but in the opposite direction.
Conclusion: In accordance with guidelines, exacerbations, blood eosinophils and history of asthma or pneumonia are the factors considered by pulmonologist for the indication or withdrawal of ICS. However, the agreement in prescription or withdrawal of ICS when confronted with hypothetical cases is very low, suggesting a great variability in clinical practice.
Keywords: COPD, exacerbation, bronchodilators, inhaled corticosteroids, eosinophils, withdrawal
Introduction
Chronic obstructive pulmonary disease (COPD) is a very prevalent disease, in Spain up to 11% of adults over the age of 40 years is affected by this disease.1 Bronchodilator therapy with long-acting muscarinic antagonists (LAMA), long-acting beta-agonists (LABA) or combinations of both, is considered by the different guidelines as the central therapy of COPD.2,3 Additionally, clinical guidelines have recognized the usefulness of inhaled corticosteroids (ICS) in patients with frequent exacerbations despite optimal bronchodilator treatment.2,4 However, despite the evidence suggesting that ICS provide clinical benefit to a subgroup of patients, their use is widespread in COPD treatment.5,6
Spanish guidelines for COPD management (GesEPOC) indicate that ICS should be used initially in patients with frequent or severe exacerbations and a blood eosinophil count of >300 cells/µL or in patients with frequent exacerbations despite dual bronchodilation and a blood eosinophil count of >100 cells/µL specially if exacerbations are not infective in etiology and they previously responded to systemic corticosteroids.3,4,7,8 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2021 strategy2 recommends the use of ICS for group D patients with >300 blood eosinophils/µL, and as continuation therapy for patients with frequent exacerbations despite initial therapy and more than 100 eosinophils/µL.2
Some Spanish surveys indicate that patients fulfilling the criteria for ICS treatment represent approximately between 20% and 30% of patients with COPD in primary and secondary care.9,10 However, over the years, there has been an overuse of ICS5,11 shown in numerous national and international studies carried out at different levels of medical care.12,13 In Spain, different studies have shown that more than 50% of patients with mild COPD received ICS treatment.14,15 It has been observed that most patients initiating treatment with triple therapy (TT) continue on the same treatment over time regardless of severity of disease5 and up to 20% of patients who initiated TT had no previous COPD medication, which indicates a deviation from recommendations.5 A study using data from the UK primary care electronic health-care records found that around three-quarters of patients are prescribed ICS-containing inhalers but ICS withdrawal occurred annually in only approximately 2–3% of patients.6
Our study aimed to identify the clinical variables that pulmonologists consider when prescribing ICS or proceeding to its withdrawal by using hypothetical COPD patient profiles with different clinical characteristics.
Materials and Methods
Study Design
This study used a cross-sectional self-administered online survey to assess pulmonologists’ therapeutic decisions on the use of ICS in COPD patients in Spain. Data was generated from November 2020 through May 2021.
Participants
A panel of pulmonologists from different regions in Spain with experience in the management and treatment of patients with COPD were invited to participate in this survey. They were selected from the COPD task force of the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) based on their participation in research projects and publications about COPD. A criterion of geographic distribution was also considered, inviting experts from 5 areas of the country (North, North-West, East, Center and South). They were not involved in the process of defining the patient’s profiles described below.
Survey
A structured survey, designed by the first author (MM), including 11 hypothetical patient profiles was sent out to the panel of participants. The survey consisted of 2 questions regarding each patient’s profile characteristics, and participants were requested to reply YES or NO to the following questions:
- Q1. If the patient was not treated with ICS, would you start ICS treatment?
- Q2. If the patient was treated with ICS, would you withdraw the ICS treatment?
All the answers were anonymized. Consensus for or against a therapeutic decision for a specific profile was achieved when the same response was obtained in ≥70% or ≤30%, respectively, for Q1 or Q2, and dissent when it was not accomplished.
Patient Profiles
We defined eleven hypothetical COPD patient profiles of the same age (68 years old). We based their characteristics on scenarios relevant in clinical practice to the therapeutic decision about ICS treatment.
The following clinical variables were included: Forced Expiratory Volume in 1 Second (FEV1), Modified Medical Research Council Dyspnea Scale (mMRC dyspnea), the number and severity of exacerbations in the previous year, the treatment of the exacerbations, a record of previous pneumonia, peripheral blood eosinophil count and a record of asthma.
The variables used and their considered values in the questionnaire are shown in Table 1. The variables and their values, which define the 11 patient profiles, are shown in Table 2.
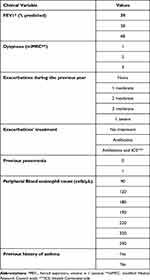 |
Table 1 Clinical Variables and Their Values Included for Patient Profiling |
 |
Table 2 Characteristics of Eleven Hypothetical Patient Profiles Used in the Survey |
Statistical Analysis
The answers from questions Q1 and Q2 were described using percentages. To evaluate the impact of the clinical variables of the profiles defined in the clinical decision on ICS treatment, multivariable mixed-effects logistic regression models were developed and adjusted to the answers to questions Q1 and Q2. The independent variables included in the models were the variables that defined the profiles, previously coded to fit the models: Forced Expiratory Volume in 1 Second (FEV1), number of exacerbations in the previous year, record of previous pneumonia, peripheral eosinophil blood count, and record of asthma. Some variables showed perfect collinearity between them; such as FEV1 and mMRC dyspnea, and number of exacerbations in the previous year and treatment of the exacerbations; consequently, only FEV1 and number of exacerbations were included. The results of the models are presented in odds ratio (OR) and the 95% confidence interval (CI).
Results
Participants Characteristics
A total of 115 pulmonologists were invited to collaborate in the study and 74 (64.3%) of them agreed and answered the questionnaire. All of them work in Spanish hospital centers and have experience in the management of patients with COPD.
Pulmonologists’ Therapeutic Opinion on the Use of ICS
In general, the results showed great variability, with only 2 profiles achieving consensus for starting or withdrawing the treatment (Figures 1 and 2). Patient profile 1 obtained complete agreement (100%) from the respondents to start treatment with ICS, and only one clinician suggested withdrawing the treatment. The other profile with consensus was number 7, where (83.8%) of participants agreed that they would start ICS treatment. In this patient profile, only (17.6%) of the respondents reported that they would withdraw the patient from ICS. The rest of profiles did not achieve consensus in the therapeutic decision. Among them, profile 5 achieved the highest percentage for withdrawing the treatment, which was only 64.9% (Figure 2).
Variables Associated with Indication of ICS
The occurrence of exacerbations during the previous year strongly impacted on the decision, increasing the probability of starting treatment with increasing number of previous episodes (one moderate exacerbation: OR = 1.86 [95% CI, 1.01 to 3.43]; two moderate exacerbations OR = 11.6 [4.47 to 30.20]; three moderate exacerbations OR = 123 [25 to 601]). The severity of exacerbations (OR = 28.9 [8.96 to 93]), higher concentration of blood eosinophils (OR = 3.53 [2.20 to 5.65]) and presence of asthma (OR = 8.36 [4.54 to 15.40]) also increased significantly the probability of starting ICS. On the other hand, pneumonia reduced the probability of initiating treatment with ICS (OR = 0.54 [0.29 to 0.98]). Lung function did not have an impact on the clinicians’ therapeutic decision (Figure 3).
Variables Associated with ICS Withdrawal
An increased number of exacerbations in the previous year were significantly associated with a reduced probability of a decision to withdraw ICS (one moderate exacerbation: OR = 0.65 [95% CI, 0.4 to 1.06]; two moderate exacerbations OR = 0.19 [0.08 to 0.44]; three moderate exacerbations OR = 0.03 [0.01 to 0.11]). A greater severity of exacerbations (OR = 0.11 [0.04 to 0.28]), higher concentration of blood eosinophils (OR = 0.35 [0.23 to 0.53]) and presence of asthma (OR = 0.17 [0.09 to 0.33]) also reduced the probability of withdrawing patients from ICS treatment. Conversely, if the patient suffered from pneumonia, the probability of withdrawing ICS treatment increased (OR = 2 [1.15 to 3.47]). As for Q1, lung function had no significant impact on the therapeutic decision for ICS withdrawal (Figure 4).
Discussion
According to the answers from the pulmonologists included in the survey, the number of exacerbations in the previous year and their severity, a higher concentration of blood eosinophils and the coexistence of asthma and pneumonia were the most relevant clinical variables when deciding to start or withdraw the patient from the treatment with ICS. Despite the strong association of these variables with the decision to prescribe or withdrawn ICS, when combined in different case scenarios, the degree of agreement among specialists in the use of ICS was very low. These results reflect the difficulties in translating recommendations of guidelines to the usual clinical practice.
Inhaled corticosteroids combined with bronchodilators can reduce the frequency of exacerbations in some patients with COPD.16 Nevertheless, the chronic use of ICS is associated with a series of adverse effects, such as the appearance of pneumonia, osteoporosis, tuberculosis or poor control of diabetes.17–19 Several observational studies have found poor adherence to guidelines regarding treatment, with overuse of ICS13,20,21 resulting in extensive use in patients where ICS is not indicated and leading to inappropriate use.10–12 Although studies show that ICS withdrawal does not carry a greater risk of exacerbation in most patients with stable COPD,22–24 and that withdrawal has been recommended by international guidelines in order to prevent side effects in patients in whom ICS are not indicated,25,26 there is a tendency to keep patients on treatment with ICS irrespective of the previous history of exacerbations.27 The withdrawal of ICSs is still subject to controversy and variability in clinical practice.6
Exacerbations in the previous year and their severity are considered by the guidelines for starting treatment with ICS.2,4 Treatment with ICS in combination with bronchodilation is recommended in case of two or more moderate or one severe exacerbation in the previous year. Conversely, the absence of a history of frequent exacerbations is an indication for considering ICS withdrawal.25,28
We found a very low level of agreement among specialists for using ICS in the different patient profiles, regardless of the exacerbation frequency. These results could be explained because the profiles include other relevant clinical variables that could have had a significant influence in the decision-making. In particular, the decision about ICS treatment in patient profiles with only one moderate exacerbation in the previous year may be influenced by several other variables such as peripheral blood eosinophil count and coexistent asthma, among others.7 Interestingly, experiencing one severe exacerbation seems to carry less value in the decision-making process of prescribing ICS than experiencing three moderate exacerbations. Thus, the frequency of previous exacerbations becomes a more important factor on the therapeutic decision than severity. On the other hand, we have found that the variable of one moderate exacerbation in the previous year, although it decreases the probability of withdrawing ICS, is not statistically significant compared with two or more moderate exacerbations or the presence of one severe exacerbation.
Peripheral blood eosinophil count is also considered a relevant variable by the guidelines2,4,26 when evaluating starting treatment with ICS on top of primary therapy with LABA or LABA/LAMA. Our results showed that the probability of initiating ICS treatment increases significantly with a higher eosinophil count, while decreasing the probability of withdrawing ICS.
Patient profile 7, with an eosinophil blood count of 100–300 cells/µL, reached the second highest number of answers for starting treatment with ICS. This profile also has the comorbidity of asthma, a variable that might have influenced the final therapeutic decision of the clinician. The patient profiles with higher variability in the decision (6, 9 and 10) had a blood eosinophil count of 100–300 cells/µL but no other relevant variable to guide the use of ICS, such as asthma or pneumonia. In these cases, it is important to note that as the eosinophil levels may vary through time, the clinician faces considerable uncertainty regarding using this biomarker.29 We observed that for patient profiles with a blood eosinophil count of 100–300 cells/µL, the coexistence of other important variables such as pneumonia (profile 5) or asthma (profile 7) was decisive for the therapeutic decision.
The coexistence of asthma is also one of the most influencing variables for prescribing ICS. This is in line with the COPD guidelines, which recommend using LABA with ICS in patients with COPD and a history of concomitant asthma.4 On the other hand, it is not recommended to withdraw ICS when the patient has asthma.25 Our results showed a statistically significant increase in the probability of starting ICS treatment in the presence of asthma and a reduction in the probability of withdrawing ICS. Furthermore, the only patient profile in which asthma comorbidity was included (profile 7) shows one of the highest consensus for initiating therapy with ICS. If we compare profile 7 and profile 9, the most remarkable difference between them is the presence of asthma in profile 7, which might have guided the experts’ decision.
The evidence shows that ICS treatment increases the risk of pneumonia.19 Furthermore, it has been shown that the withdrawal of ICS significantly reduces the risk of pneumonia.30 Our results showed alignment with these findings, with a statistically significant reduction in the probability of initiating ICS and an increase in the probability of withdrawing ICS when the patient profile indicates a history of pneumonia. However, the only patient in whom pneumonia was included (patient 5) did not achieve consensus for withdrawing ICS treatment.
Even though the variables identified in our survey coincide with the ones featured in the recommendations from the guidelines, at the moment of deciding in a case scenario, we found great variability, highlighting low consensus in real clinical practice.
Our study has some limitations. The study reflects the opinion of a selected sample of expert pulmonologists who may not represent all health professionals involved in managing COPD patients. In addition, the patients are hypothetical, so we do not have objective data to confirm that these therapeutic decisions correspond to the real prescription patterns of the experts. Only one case included a previous diagnosis of pneumonia and another a previous diagnosis of asthma; we recognized that both can be very relevant for the decision to prescribe or withdraw ICS, but we were more interested in the variables of COPD itself and we did not want to make the survey too long. On the other hand, the credibility of our study was supported by the consistency with the current guidelines. Our study contributes to the knowledge regarding variables influencing the therapeutic decision on COPD and the adherence to the current guidelines. Additional studies should be conducted to determine these findings’ external validity and generalizability.
Conclusions
In accordance with guidelines, exacerbations, blood eosinophils and history of asthma or pneumonia are the factors considered by pulmonologist for the indication or withdrawal of ICS. However, the agreement in prescription or withdrawal of ICS when confronted with hypothetical cases is very low, suggesting a great variability in clinical practice.
Acknowledgments
The authors would like to thank the respondents for their participation in the study.
Funding
The study was supported and funded by Boehringer Ingelheim (BI) Spain. BI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Disclosure
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive payment related to the development of the manuscript. MM has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, Sandoz, Zambon, Kamada, CSL Behring, Grifols and Novartis, consulting fees from AstraZeneca, Atriva Therapeutics, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, Spin Therapeutics, ONO Pharma, pH Pharma, Palobiofarma SL, Takeda, Novartis, Sanofi and Grifols and research grants from Grifols. FG has received economic compensation for consulting, lectures at various congresses and scientific training sessions, manuscript writing and support for attending meetings from Boehringer Ingelheim, GSK, Novartis, Astrazeneca, Chiesi, Pfizer, Esteve and Rovi. CRR reports no disclosure related to this paper. XP has received speaker fees from Boehringer Ingelheim, GlaxoSmithKline, Rovi and Chiesi. EMM has received economic compensation for consulting, lectures, presentations, manuscript writing, educational events, support for attending meetings, participation in data safety monitoring or advisory board from Boehringer Ingelheim, Glaxo Smith Kline, Novartis, Aflofarm, Bial, Chiesi, Meranini and GSK. CG has received economic compensation for consulting, support for attending meetings from Chiesi, Boehringer Ingelheim, Glaxo Smith Kline, Novartis, Meranini, Ferrer and Teva. CA has received speaker fees and/or consulting fees from Boehringer Ingelheim, Pfizer, AstraZeneca, Novartis, Chiesi, Faes farma, Esteve and GlaxoSmithKline. CF has received economic compensation by GOC Health Consulting for statistical analysis. SA has received economic compensation for support for attending meetings, other sessions and consulting from Novartis, Astra Zeneca, Menarini, GSK, Boeringer Ingelheim, Chiesi, Mundipharma, FAES, Pfizer and Teva. BA reports personal fees from GSK, grants, personal fees and non-financial support from Novartis, personal fees and non-financial support from Boehringer Ingelheim, personal fees and non-financial support from Chiesi, grants, personal fees and non-financial support from Laboratorios BIAL, Menarini, personal fees from Gebro, Astra- Zeneca, and Gilead, outside the submitted work. In addition, has a patent P201730724 pending. EN has received economic compensation for consulting, lectures, presentations, manuscript writing and educational events from Novartis, Boehringer Ingelheim, Chiesi and Glaxo Smith Kline. MB reports personal fees from Grifols, Menarini, CSL Behring, GSK, Boehringer Ingelheim, and Novartis, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Soriano JB, Alfageme I, Miravitlles M, et al. Prevalence and determinants of COPD in Spain: EPISCAN II. Arch Bronconeumol. 2021;57(1):61–69. doi:10.1016/j.arbres.2020.07.024
2. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease (GOLD); 2021.
3. Miravitlles M, Calle M, Soler-Cataluña JJ. GesEPOC 2021: one more step towards personalized treatment of COPD. Arch Bronconeumol. 2021;57(1):9–10. doi:10.1016/j.arbres.2020.08.002
4. Miravitlles M, Calle M, Molina J, et al. Spanish COPD Guidelines (GesEPOC) 2021: updated pharmacological treatment of stable COPD. Arch Bronconeumol. 2022;58(1):69–81. doi:10.1016/j.arbres.2021.03.005
5. Monteagudo M, Nuñez A, Solntseva I, et al. Treatment pathways before and after triple therapy in COPD: a population-based study in primary care in Spain. Arch Bronconeumol. 2021;57(3):205–213. doi:10.1016/j.arbres.2020.07.032
6. Bloom CI, Douglas I, Usmani OS, Quint JK. Inhaled corticosteroid treatment regimens and health outcomes in a UK COPD population study. Int J Chron Obstruct Pulmon Dis. 2020;15:701–710. doi:10.2147/COPD.S241568
7. Stolz D, Miravitlles M. The right treatment for the right patient with COPD: lessons from the IMPACT trial. Eur Respir J. 2020;55(5):2000881. doi:10.1183/13993003.00881-2020
8. Pascoe S, Barnes N, Brusselle G, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med. 2019;7(9):745–756. doi:10.1016/S2213-2600(19)30190-0
9. Miravitlles M, Monteagudo M, Solntseva I, Alcázar B. Blood eosinophil counts and their variability and risk of exacerbations in COPD: a population-based study. Arch Bronconeumol. 2021;57(1):13–20. doi:10.1016/j.arbres.2019.12.015
10. Izquierdo JL, Morena D, González Y, et al. Clinical management of COPD in a real-world setting. A big data analysis. Arch Bronconeumol. 2021;57(2):94–100. doi:10.1016/j.arbres.2019.12.025
11. Izquierdo Alonso JL, Rodríguez Glez-Moro JM. The excessive use of inhaled corticosteroids in chronic obstructive pulmonary disease. Arch Bronconeumol. 2012;48(6):207–212. doi:10.1016/j.arbres.2012.01.002
12. Calle Rubio M, Rodríguez Hermosa JL, Soler-Cataluña JJ, et al. Medical care according to risk level and adaptation to Spanish COPD guidelines (Gesepoc): the Epoconsul Study. Arch Bronconeumol. 2018;54(5):270–279. doi:10.1016/j.arbres.2017.11.015
13. Casas A, Montes de Oca M, Menezes AM, et al. Respiratory medication used in COPD patients from seven Latin American countries: the LASSYC study. Int J Chron Obstruct Pulmon Dis. 2018;13:1545–1556. doi:10.2147/COPD.S154097
14. Izquierdo Alonso JL, Rodríguez González-Moro JM, de Lucas Ramos P, Martín Centeno A, Gobartt Vázquez E. ¿Ha cambiado el manejo de la EPOC en España? Resultados de un estudio multicéntrico comunitario (VICE). [Has the treatment of COPD changed in Spain? Results of a community multicenter study (VICE)]. Rev Clin Esp. 2008;208(1):18–25. Spanish. doi:10.1157/13115003
15. Barrecheguren M, Monteagudo M, Ferrer J, et al. Treatment patterns in COPD patients newly diagnosed in primary care. A population-based study. Respir Med. 2016;111(111):47–53. doi:10.1016/j.rmed.2015.12.004
16. Halpin DM, Miravitlles M, Metzdorf N, Celli B. Impact and prevention of severe exacerbations of COPD: a review of the evidence. Int J Chron Obstruct Pulmon Dis. 2017;12:2891–2908. doi:10.2147/COPD.S139470
17. Loke YK, Cavallazzi R, Singh S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax. 2011;66(8):699–708. doi:10.1136/thx.2011.160028
18. Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7). doi:10.1002/14651858.CD002991.pub3
19. Miravitlles M, Auladell-Rispau A, Monteagudo M, et al. Systematic review on long-term adverse effects of inhaled corticosteroids in the treatment of COPD. Eur Respir Rev. 2021;30(160):210075. doi:10.1183/16000617.0075-2021
20. Tsiligianni I, Kampouraki M, Ierodiakonou D, Poulonirakis I, Papadokostakis P. COPD patients’ characteristics, usual care, and adherence to guidelines: the Greek UNLOCK study. Int J Chron Obstruct Pulmon Dis. 2019;14:547–556. doi:10.2147/COPD.S185362
21. Price D, West D, Brusselle G, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis. 2014;9:889–904. doi:10.2147/COPD.S62750
22. Chapman KR, Hurst JR, Frent SM, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198(3):329–339. doi:10.1164/rccm.201803-0405OC
23. Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294. doi:10.1056/NEJMoa1407154
24. Rossi A, van der Molen T, Del Olmo R, et al. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44(6):1548–1556. doi:10.1183/09031936.00126814
25. Chalmers JD, Laska IF, Franssen FME, et al. Withdrawal of inhaled corticosteroids in COPD: a European Respiratory Society guideline. Eur Respir J. 2020;55:2000351. doi:10.1183/13993003.00351-2020
26. Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56–e69. doi:10.1164/rccm.202003-0625ST
27. Magnussen H, Lucas S, Lapperre T, et al. Withdrawal of inhaled corticosteroids versus continuation of triple therapy in patients with COPD in real life: observational comparative effectiveness study. Respir Res. 2021;22(1):25. doi:10.1186/s12931-021-01615-0
28. Miravitlles M, Cosio BG, Arnedillo A, et al. A proposal for the withdrawal of inhaled corticosteroids in the clinical practice of chronic obstructive pulmonary disease. Respir Res. 2017;18(1):198. doi:10.1186/s12931-017-0682-y
29. Golpe R, Dacal D, Sanjuán-López P, Martín-Robles I, Pérez-de-llano LA. Plasma eosinophil count and patient-centered events in chronic obstructive pulmonary disease in real-life clinical practice. Arch Bronconeumol. 2020;56(2):129–130.
30. Suissa S, Coulombe J, Ernst P. Discontinuation of inhaled corticosteroids in COPD and the risk reduction of pneumonia. Chest. 2015;148(5):1177–1183. doi:10.1378/chest.15-0627
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




