Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Pulmonary Rehabilitation Programmes Within Three Days of Hospitalization for Acute Exacerbation of Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis
Authors Zhang D , Zhang H, Li X, Lei S, Wang L, Guo W, Li J
Received 16 September 2021
Accepted for publication 6 December 2021
Published 24 December 2021 Volume 2021:16 Pages 3525—3538
DOI https://doi.org/10.2147/COPD.S338074
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Min Zhang
Dong Zhang,1– 3 Hailong Zhang,1– 3 Xuanlin Li,1– 3 Siyuan Lei,1– 3 Lu Wang,1– 3 Wen Guo,1– 3 Jiansheng Li1– 3
1Co-Construction Collaborative Innovation Center for Chinese Medicine and Respiratory Diseases by Henan & Education Ministry of P.R. China, Henan University of Chinese Medicine, Zhengzhou, 450046, People’s Republic of China; 2Henan Key Laboratory of Chinese Medicine for Respiratory Disease, Henan University of Chinese Medicine, Zhengzhou, 450046, People’s Republic of China; 3Department of Respiratory Diseases, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, Henan, 450003, People’s Republic of China
Correspondence: Jiansheng Li Tel/Fax +86-371-65676568
Email [email protected]
Objective: To evaluate the efficacy and safety of early pulmonary rehabilitation (PR) (ie, < 3 days of hospitalization) in patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD).
Methods: Embase, Web of Science, PubMed and Cochrane Library were searched from their inception to 1 April 2021. Randomized controlled trials were included if they observed the efficacy of early PR in AECOPD patients. Study selection, data extraction, risk of bias and quality of evidence were assessed by two researchers independently. Assessment of the risk of bias and eidence quality were evaluated by the Cochrane Collaboration’s tool and Grading of Recommendations, Assessment, Development and Evaluation system, respectively.
Results: Fourteen trials (829 participants) were identified. Significant improvement was found in the 6-minute walk distance (6MWD; mean difference (MD): 69.64; 95% CI: 40.26 to 99.01; Z = 4.65, P < 0.0001, low quality). In the subgroup analysis, the exercise-training group showed marked improvement (MD: 96.14; 95% CI: 20.24 to 172.04; Z = 2.48, P = 0.001). The Saint George’s Respiratory Questionnaire (SGRQ) total score was low (MD: − 12.77; 95% CI: − 16.03 to − 9.50; Z = 7.67, P < 0.0001, moderate quality). Significant effects were not found for the duration of hospital stay, quadriceps muscle strength or five times sit to stand test. Only one serious adverse event was reported in experimental group, which was not associated with early PR.
Conclusion: PR initiated < 3 days of hospitalization may increase exercise capacity and improve quality of life, but the results should be interpreted prudently and dialectically, and the role of early PR in AECOPD needs further exploration.
Keywords: chronic obstructive pulmonary disease, acute exacerbation, pulmonary rehabilitation, meta-analysis
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive respiratory disease. It carries a high prevalence and is associated with disability and mortality.1,2 COPD is a major public-health challenge facing society today.3,4
An acute exacerbation of chronic obstructive pulmonary disease (AECOPD) is defined as acute exacerbation of respiratory symptoms which results in additional therapy.5 AECOPD can influence disease progression as well as the prevalence of hospitalization and readmission to hospital.3,6–8 More than 20% of AECOPD patients will be hospitalized again with the same diagnosis within 30 days, which increases the economic burden of patients significantly.9 COPD exacerbations can accelerate disease progression by contributing to a decline in lung function of >25%.10 Moreover, the long-term prognosis of severe COPD necessitating hospitalization is poor, with 5-year mortality of ~50%.11 Therefore, reducing the impact of acute exacerbation and preventing its recurrence is an important treatment goal of AECOPD.3,12
Systemic corticosteroids, antibiotics and short-acting bronchodilators are the cornerstones of management of COPD exacerbations. These agents can significantly improve clinical symptoms, accelerate disease recovery, and improve gas exchange.13,14 Although these drug therapies are efficacious, the search for better interventions is ongoing. In recent years, non-drug therapy has garnered increasing attention.
Pulmonary rehabilitation (PR) is a comprehensive intervention that include, but are not limited to, education, exercise training, and behavior change,15 has shown well-established benefits in patients with stable COPD. PR is considered first-line nonpharmacologic treatment for COPD.3 Several studies have indicated that PR for AECOPD patients is safe and efficacious, and can improve clinical symptoms, exercise tolerance and quality of life (QoL).16–31 It has been postulated that comprehensive PR measures should be implemented immediately after acute exacerbation, which can increase exercise capacity and QoL.15,32 Of relevant studies, ~57% have been conducted during hospitalization, 42% of which started within 48 h of hospital admission.33 However, the starting time of PR is in a wide range, from admission to 2–3 weeks after discharge, which hampered judgement of the exact time to initiate PR.33 Moreover, whether comprehensive PR15,33 during hospitalization is safe and clinically effective is still controversial.6,34 This controversy may be related to six main factors: (i) different PR strategies have different effects;33 (ii) the time window for starting PR differs;33 (iii) the safety of initiating PR during hospitalization;6 (iv) insufficient support for medical equipment;35 (v) potential comorbidity instability and the contentious issue of using energy resources for exercise during early illness; (vi) different outcomes and outcome measures are used.36 Given the above considerations, we aimed to explore the efficacy of early PR for AECOPD patients <3 days of hospital admission to provide evidence for clinical practice.
Materials and Methods
This review was conducted in strict accordance with the Cochrane Handbook for Systematic Reviews of Interventions37 and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)38 guidelines. The protocol for this review has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) and the registry number is CRD42021242284.
Search Strategy
A literature search was conducted on 29 December 2020 on four databases (PubMed, Embase, Web of Science, Cochrane Library) and the results were updated on 1 April 2021. To obtain comprehensive results, systematic search strategies were formulated according to the characteristics of each database. The detailed retrieval strategies and steps are presented in the Supplementary Materials Appendix 1. Furthermore, the reference lists of the retrieved articles and relevant systematic reviews were scanned manually for other potentially eligible studies.
Inclusion Criteria
Articles meeting all of the following criteria were included: (i) the study population was AECOPD patients (based on GOLD guidelines or expert consensus); (ii) the intervention was PR15,33 (began <3 days of hospitalization) and at least one of the following interventions was included: exercise training, inspiratory-muscle training, neuromuscular electrical stimulation, nutrition support and self-management; (iii) the comparison was between PR and control (ie, conventional therapy, standard care, no treatment and sham exercise); (iv) the primary outcomes were exercise tolerance (measured by the 6-minute walk distance (6MWD)) and QoL (Saint George’s Respiratory Questionnaire (SGRQ) total score); (v) the secondary outcomes were the duration of hospital stay (DoHS), quadriceps muscle strength (QMS), five times sit to stand test (5STS) and adverse events; (vi) the study design was a randomized controlled trial (RCT).
Exclusion Criteria
The exclusion criteria were: (i) patients had AECOPD combined with other chronic respiratory diseases (asthma, pulmonary fibrosis, bronchiectasis); (ii) conference abstracts, study protocols and grey literature; (iii) duplicate publications; (iv) study design was not RCTs (observational, cohort, retrospective as well as case control studies).
Study Selection
Before the article was screened, we first selected 50 articles for pre-screening, then determined the unified screening criteria, and finally conducted formal screening. Same for data extraction and assessment of risk of bias. Study selection was conducted independently by D Zhang, L Wang, W Guo and SY Lei in two phases to screen which articles were suitable. First, duplicate and irrelevant studies were discarded after examining the titles and abstracts. Then, the full-text of potentially eligible studies was downloaded and reviewed based on the inclusion and exclusion criteria stated above. Disagreements were resolved by HL Zhang, who acted as an arbiter. If there was still a dispute, our team members would discuss it together and finally reach a consensus. NoteExpress software was used for the study screening, and record the reasons why studies were excluded.
Data Extraction
Data extraction was carried out independently by D Zhang and XL Li from eligible studies using a standardized data-extraction form. The latter was based on the title, author information, year of publication, country, experimental design, participant characteristics (age, sex, sample size), type and duration of intervention, comparators and outcomes. The outcomes were collected at baseline (<3 days of hospitalization) and before discharge. JS Li resolved any disagreements.
Assessment of Risk of Bias
The Risk of Bias tool37 was used for quality assessment. This tool contains seven items. Before the literature was assessed, we would conduct a training session, then D Zhang and XL Li would assess each study independently to see if it had a “high”, “unclear” or “low” risk of bias. If opinions differed, HL Zhang intervened until a consensus was reached.
Statistical Analyses
Review Manager 5.3 (https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/) was used for meta-analysis.39 The mean difference (MD) was employed for continuous outcomes. The odds ratio (OR) was used for dichotomous outcomes. The 95% confidence interval (CI) was calculated for each outcome. If heterogeneity was absent (P ≥ 0.1, I2 ≤ 50%), the fixed-effect model was used for analyses, otherwise the random-effect model was chosen. In order to reduce heterogeneity and address potential confounding, we conducted a subgroup analysis of 6MWD based on different interventions. Sensitivity analysis was undertaken to estimate the consistency of the results by removing each study separately.
Evidence Assessment
Grading of Recommendations, Assessment, Development and Evaluation (GRADE)40 was used to assess the quality of evidence of primary outcomes. Five aspects were focused upon: “risk of bias”, “inconsistency”, “indirectness”, “imprecision”, and “publication bias”. The quality of evidence was assessed as “high”, “moderate”, “low” or “very low”.
Results
Identification of Studies
A total of 3580 articles were identified through manual searching and electronic searching, of which 1226 were duplicates. Fifty-one articles were considered potentially eligible for further assessment after screening of the title and abstract. After reading the full-text carefully, 37 articles were excluded for the following reasons: (i) meeting abstracts (n = 3), (ii) not RCTs (n = 2); (iii) study protocol (n = 4); (iv) irrelevant outcomes (n = 1); (v) inclusion of participants >3 days after AECOPD onset (n = 27). A list of excluded literature studies and reasons for exclusion are provided in the Supplementary Materials Appendix 2. Finally, 14 studies were included in this review. The process of study selection is shown as Figure 1.
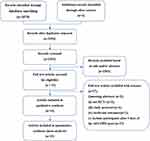 |
Figure 1 Study selection process for this review. |
 |
Figure 2 Continue. |
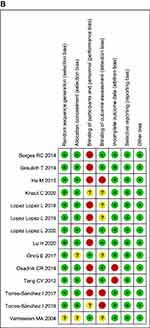 |
Figure 2 (A) Risk of bias graph. (B) Risk of bias summary. |
Study Characteristics
Fourteen RCTs41–54 from 2004 to 2020 in seven countries were included in this review, of which 57.14% were published in the previous 5 years. The sample size of the experimental group and control group in the included studies was 471 and 358, respectively, with the maximum sample size being 94 and the minimum being 26. The basic characteristics of included studies are shown in Table 1.
 |  |  |
Table 1 Basic Characteristics of Included Studies |
Risk of Bias
All included studies were RCTs and had similar characteristics of the study cohort at baseline, and only one study41 blinded the study protocol from patients. Twelve studies42–47,49–54 identified the method of allocation concealment, and the outcome assessment was blinded in six studies.42–45,52,54 A detailed assessment of risk of bias is provided in Figure 2A and B.
Effects of Interventions
3.3.1 6-minute walk distance (6MWD)
Six studies43,45,46,48,53,54 (319 participants) were included in this part of the meta-analysis. Significant heterogeneity was found (χ2 = 44.24, P < 0.0001; I2 = 89%), so a random-effects model was employed. There was a remarkable improvement on 6MWD (MD: 69.64; 95% CI: 40.26 to 99.01; Z = 4.65, P < 0.0001). In the subgroup analysis, the improvement was more obvious in the exercise-training group (MD: 96.14; 95% CI: 20.24 to 172.04; Z = 2.48, P = 0.01) (Figure 3). The quality of evidence for early PR to improve 6MWD in AECOPD patients was low.
 |
Figure 3 Experimental group versus control group, 6MWD. |
Saint George’s Respiratory Questionnaire (SGRQ)
Three studies43,45,53 (95 participants) were included in this part of the meta-analysis. Significant heterogeneity was not observed (χ2 = 1.49, P = 0.47; I2 = 0%) and a fixed-effects model was chosen. There was a significant treatment effect on the total score of SGRQ (MD: −12.77; 95% CI: −16.03 to −9.50; Z = 7.67, P < 0.0001) (Figure 4). The quality of evidence for early PR to reduce the total score of SGRQ in AECOPD patients was moderate.
 |
Figure 4 Experimental group versus control group, SGRQ. |
Duration of Hospital Stay (DoHS)
Eight studies43–45,49,50,52–54 (434 participants) provided numerical data for DoHS and were included in this part of the meta-analysis. Significant heterogeneity was not found (χ2 = 6.95, P = 0.43; I2 = 0%) and a fixed-effects model was used. Early PR could not reduce the DoHS (MD: 0.26; 95% CI: −0.08 to 0.61; Z = 1.49, P = 0.14) (Figure 5).
 |
Figure 5 Experimental group versus control group, DoHS. |
Quadriceps Muscle Strength (QMS)
Three studies41,47,51 (149 participants) were included in this part of the meta-analysis. Significant heterogeneity was found (χ2 = 11.80, P = 0.003; I2 = 83%) so a random-effects model was chosen. There was no significant treatment effect on QMS (MD: 17.54; 95% CI: −4.46 to 39.55; Z = 1.56, P = 0.12) (Figure 6).
 |
Figure 6 Experimental group versus control group, QMS. |
Five Times Sit to Stand Test (5STS)
Four studies43,50–52 (193 participants) were included in this part of the meta-analysis. Significant heterogeneity was not observed (χ2 = 2.46, P = 0.48; I2 = 0%) so a fixed-effects model was chosen. A positive influence on reducing 5STS was not documented after PR (MD: −3.83; 95% CI: −8.78 to 1.12; Z = 1.52, P = 0.13) (Figure 7).
 |
Figure 7 Experimental group versus control group, 5 STS. |
Quality of Evidence
The GRADE system was used to evaluate the evidence level of 6MWD and SGRQ. The quality of evidence was low for 6MWD, because of unclear allocation concealment, lack of blinding and high heterogeneity. The quality of evidence was downgraded to moderate for SGRQ because of lack of blinding or unclear allocation concealment. The detail of GRADE evaluation is shown in Table 2.
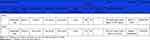 |
Table 2 Quality of Evidence |
Adverse Events
Only two of the included studies42,44 reported adverse events. Eight patients were involved in 15 adverse events related to PR. Only one patient had a serious adverse event, and this patient had a history of atrial fibrillation. Without medical intervention, the chest pain disappeared and this patient continued to complete the remaining tasks. Other events were nonserious and tolerable.
Sensitivity Analysis
Each article was removed individually and then sensitivity analysis was conducted to ascertain the stability of the results. There were no considerable changes among most of the outcomes. However, a large change was noted for QMS when one study was excluded41 (Table 3).
 |
Table 3 Results of Sensitivity Analysis |
Discussion
Our systematic review was based on 14 RCTs involving 829 patients with AECOPD. The meta-analysis was based on 13 RCTs with 791 patients. Compared with the control group, for AECOPD patients, the early-PR group had improved exercise endurance and QoL. Serious adverse events related to early PR were not observed.
As one of the most used methods to measure the clinical outcome of COPD, the 6MWD has an important role in evaluating exercise capacity as well as estimating the prognosis and treatment response of COPD patients.36,55 In our review, the 6MWD in the early PR group was longer than that in the control group, with an MD of 69.64 m, which exceeded the minimum clinically important difference of 6MWD by 54 m.56 However, the quality of evidence was low, which should be interpreted prudently and dialectically. Compared with that in previous studies, the 6MWD was longer in our study, which may be related to the different time window for starting PR. This result suggests that early PR has potential for improving exercise tolerance.27 In the subgroup analysis, improvement in the 6MWD was more remarkable in the exercise-training group, with an MD of 96.14 m. This change demonstrates that, as the cornerstone of PR, exercise training can improve exercise endurance in terms of muscle function. High heterogeneity was found in the included studies, which may be related to the type, intensity and duration of intervention measures.
QoL measurements have become a vital outcome measure in COPD management.57 SGRQ, as one of the indicators for evaluating the QoL of patients, can reflect the overall status of the patient.58 The higher the SGRQ score, the worse is the QoL related to health. The total score of SGRQ in the early-PR group was lower than that of the control group, with an MD of −12.77 (95% CI, −16.03 to −9.50), and the quality of evidence was moderate. Compared with previous studies, the total score of SGRQ was lower.17,18,27 This result implies that PR initiated <3 days of hospitalization can improve the QoL of AECOPD patients significantly.
The DoHS not only reflects disease severity directly, it also indirectly reflects the efficacy of clinical interventions. Eight articles focused on the DoHS as an outcome measure. The DoHS in the PR group was longer than that in the control group (MD = 0.26 days) so early PR did not shorten the DoHS.
Often, patients with COPD have dysfunction and atrophy of skeletal muscle, which not only severely affects function of the lower limbs, it also affects function of the respiratory muscles and aggravates respiratory symptoms.59 5STS and QMS are simple, effective and portable clinical tools that are often used to assess the function, balance and mobility of the lower limbs in older patients.60 The 5STS and QMS of the early-PR group were greater compared with those of the control group, and the MD of all AECOPD patients included was −3.83 s and 17.54 N, respectively. However, there was no significant improvement in 5STS or QMS between the two groups. When one study41 was excluded, the results were reversed, which may have been related to different interventions. Compared with nutritional support, exercise training can improve lower-limb muscle strength significantly in a short time. Due to the small sample size of the included studies, this result needs to be verified further.
Studies have focused more on the role of PR in improving the QoL and exercise tolerance of AECOPD patients. The prevalence of mortality and hospital readmission are convincing endpoints to judge the efficacy of treatment methods. Studies have shown that PR during hospitalization can reduce the prevalence of hospital readmission, but cohort studies have suggested that, for some patients, this approach may increase the risk of future hospitalization.26 Healthcare facilities should provide early PR and pay attention to its long-term effect in AECOPD patients, and whether it can reduce the prevalence of hospital readmission and mortality.
Our study had three main advantages compared with previous studies. First, this is the first systematic review to analyze the efficacy and safety of early PR started <3 days of hospitalization in patients with AECOPD. Second, articles were from seven countries on four continents, which reduces any regional bias. Third, we used a combination of electronic retrieval and manual retrieval without language restrictions, which reduced the selection bias.
Our study had four main limitations in. First, evaluation of the quality of each study based on Review Manager 5.3 is a subjective process. Although the quality of each study was evaluated by two researchers independently and checked by a third researcher, some biases would have remained. Second, the studies we included involved PR being initiated <3 days of hospital admission; we did not compare the effects with studies conducted >3 days after AECOPD. Third, the number of studies that could undergo meta-analysis for each outcome indicator was limited, so funnel plots were not suitable, hence we could not fully assess publication bias. Fourth, the effect of a single intervention for early PR was not evaluated.
Conclusions
PR within three days of hospitalization may increase the exercise capacity and improve the QoL of patients with AECOPD. Our meta-analysis did not show that early PR shortened the DoHS or improve the QMS and 5STS of AECOPD patients. Only one serious adverse event was reported in the experimental group, which was not associated with early PR. Our meta-analysis demonstrates that early PR during hospitalization is safe and efficacious, but the results should be interpreted prudently and dialectically, and the role of early PR in AECOPD needs further exploration.
Acknowledgments
The authors really appreciate the help of people from Henan University of Traditional Chinese Medicine.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This article was sponsored by The Key Program of Specialized Research for National TCM Clinical Research Bases from Traditional Chinese Medicine Administration of Henan Province, China (Grant No. 2018JDZX007) and the construction project of the characteristic backbone discipline of Chinese medicine in Henan province, China (Grant No. STG-ZYXKY-2020005).
Disclosure
The authors report no conflicts of interest in this work.
References
1. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788.
2. GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706.
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (updated 2020). Available from: https://goldcopd.org/gold-reports/.
4. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study[J]. Lancet. 2018;391(10131):1706–1717.
5. Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796.
6. Wedzicha JA, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;49(3):1600791.
7. Montagnani A, Mathieu G, Pomero F, et al. Hospitalization and mortality for acute exacerbation of chronic obstructive pulmonary disease (COPD): an Italian population-based study. Eur Rev Med Pharmacol Sci. 2020;24(12):6899–6907.
8. Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931.
9. Bhatt SP, Wells JM, Iyer AS, et al. Results of a medicare bundled payments for care improvement initiative for chronic obstructive pulmonary disease readmissions. Ann Am Thorac Soc. 2017;14(5):643–648.
10. MacLeod M, Papi A, Contoli M, et al. Chronic obstructive pulmonary disease exacerbation fundamentals: diagnosis, treatment, prevention and disease impact. Respirology. 2021;26(6):532–551.
11. Hoogendoorn M, Hoogenveen RT, Rutten-van Mölken MP, et al. Case fatality of COPD exacerbations: a meta-analysis and statistical modelling approach. Eur Respir J. 2011;37(3):508–515.
12. Vogelmeier, Claus F, Criner, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Am J Respir Crit Care Med. 2017;195(5):557–582.
13. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365.
14. Dobler CC, Morrow AS, Farah MH, et al. Pharmacologic and Nonpharmacologic Therapies in Adult Patients with Exacerbation of COPD: A Systematic Review. Report No.: 19(20)-EHC024-EF. Rockville (MD): Agency for Healthcare Research and Quality (US); 2019.
15. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64.
16. Lightowler JV, Wedzicha JA, Elliott MW, et al. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: cochrane systematic review and meta-analysis. BMJ. 2003;326(7382):185.
17. Puhan MA, Scharplatz M, Troosters T, et al. Respiratory rehabilitation after acute exacerbation of COPD may reduce risk for readmission and mortality – a systematic review. Respir Res. 2005;6(1):54.
18. Puhan M, Scharplatz M, Troosters T, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2009;1:CD005305.
19. Tang CY, Taylor NF, Blackstock FC. Chest physiotherapy for patients admitted to hospital with an acute exacerbation of chronic obstructive pulmonary disease (COPD): a systematic review. Physiotherapy. 2010;96(1):1–13.
20. Hill K, Patman S, Brooks D. Effect of airway clearance techniques in patients experiencing an acute exacerbation of chronic obstructive pulmonary disease: a systematic review. Chron Respir Dis. 2010;7(1):9–17.
21. Walters JA, Turnock AC, Walters EH, et al. Action plans with limited patient education only for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010;5:CD005074.
22. Puhan MA, Gimeno-Santos E, Scharplatz M, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;10:CD005305.
23. Reid WD, Yamabayashi C, Goodridge D, et al. Exercise prescription for hospitalized people with chronic obstructive pulmonary disease and comorbidities: a synthesis of systematic reviews. Int J Chron Obstruct Pulmon Dis. 2012;7:297–320.
24. Harrison SL, Janaudis-Ferreira T, Brooks D, et al. Self-management following an acute exacerbation of COPD: a systematic review. Chest. 2015;147(3):646–661.
25. Echevarria C, Brewin K, Horobin H, et al. Early supported discharge/hospital at home for acute exacerbation of chronic obstructive pulmonary disease: a review and meta-analysis. COPD. 2016;13(4):523–533.
26. Moore E, Palmer T, Newson R, et al. Pulmonary rehabilitation as a mechanism to reduce hospitalizations for acute exacerbations of COPD: a systematic review and meta-analysis. Chest. 2016;150(4):837–859.
27. Puhan MA, Gimeno-Santos E, Cates CJ, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016;12(12):CD005305.
28. Howcroft M, Walters EH, Wood-Baker R, et al. Action plans with brief patient education for exacerbations in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016;12(12):CD005074.
29. Ryrsø CK, Godtfredsen NS, Kofod LM, et al. Lower mortality after early supervised pulmonary rehabilitation following COPD-exacerbations: a systematic review and meta-analysis. BMC Pulm Med. 2018;18(1):154.
30. Torres-Sánchez I, Cruz-Ramírez R, Cabrera-Martos I, et al. Results of physiotherapy treatments in exacerbations of chronic obstructive pulmonary disease: a systematic review. Physiother Can. 2017;69(2):122–132.
31. Lindenauer PK, Stefan MS, Pekow PS, et al. Association between initiation of pulmonary rehabilitation after hospitalization for COPD and 1-year survival among medicare beneficiaries. JAMA. 2020;323(18):1813–1823.
32. Burtin C, Decramer M, Gosselink R, et al. Rehabilitation and acute exacerbations. Eur Respir J. 2011;38(3):702–712.
33. Machado A, Matos Silva P, Afreixo V, et al. Design of pulmonary rehabilitation programmes during acute exacerbations of COPD: a systematic review and network meta-analysis. Eur Respir Rev. 2020;29(158):200039.
34. Greening NJ, Williams JE, Hussain SFet al. An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trial. BMJ. 2014;349:g4315.
35. Valenzuela PL, Saco-Ledo G, Rivas-Baeza B, et al. Safety of in-hospital early rehabilitation in chronic obstructive pulmonary disease exacerbations: a systematic review and meta-analysis. Ann Phys Rehabil Med. 2021;65:101528.
36. Oliveira AL, Marques AS. Outcome measures used in pulmonary rehabilitation in patients with acute exacerbation of chronic obstructive pulmonary disease: a systematic review. Phys Ther. 2018;98(3):191–204.
37. Higgins J, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions (Version 6.2). The cochrane collaboration; 2021. Available from: https://training.cochrane.org/handbook.
38. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
39. Review Manager (RevMan) [Computer program], “Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration; 2014.
40. Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ. 2008;336(7650):924–926.
41. Vermeeren MA, Wouters EF, Geraerts-Keeris AJ, et al. Nutritional support in patients with chronic obstructive pulmonary disease during hospitalization for an acute exacerbation; a randomized controlled feasibility trial. Clin Nutr. 2004;23(5):1184–1192.
42. Tang CY, Blackstock FC, Clarence M, et al. Early rehabilitation exercise program for inpatients during an acute exacerbation of chronic obstructive pulmonary disease: a randomized controlled trial. J Cardiopulm Rehabil Prev. 2012;32(3):163–169.
43. Greulich T, Nell C, Koepke J, et al. Benefits of whole body vibration training in patients hospitalised for COPD exacerbations - a randomized clinical trial. BMC Pulm Med. 2014;14(1):60.
44. Osadnik CR, McDonald CF, Miller BR, et al. The effect of positive expiratory pressure (PEP) therapy on symptoms, quality of life and incidence of re-exacerbation in patients with acute exacerbations of chronic obstructive pulmonary disease: a multicentre, randomised controlled trial. Thorax. 2014;69(2):137–143.
45. Borges RC, Carvalho CR. Impact of resistance training in chronic obstructive pulmonary disease patients during periods of acute exacerbation. Arch Phys Med Rehabil. 2014;95(9):1638–1645.
46. He M, Yu S, Wang L, et al. Efficiency and safety of pulmonary rehabilitation in acute exacerbation of chronic obstructive pulmonary disease. Med Sci Monit. 2015;21:806–812.
47. Torres-Sánchez I, Valenza MC, Cabrera-Martos I, et al. Effects of an exercise intervention in frail older patients with chronic obstructive pulmonary disease hospitalized due to an exacerbation: a randomized controlled trial. COPD. 2017;14(1):37–42.
48. Öncü E, Zincir H. The effect of transcutaneous electrical nerve stimulation in patients with acute exacerbation of chronic obstructive pulmonary disease: randomised controlled trial. J Clin Nurs. 2017;26(13–14):1834–1844.
49. Torres-Sánchez I, Valenza MC. Cebriá I Iranzo MDÀ, et al. Effects of Different Physical Therapy Programs on Perceived Health Status in Acute Exacerbation of Chronic Obstructive Pulmonary Disease Patients: a Randomized Clinical Trial. Disabil Rehabil. 2018;40(17):2025–2031.
50. Lopez Lopez L, Granados Santiago M, Donaire Galindo M, et al. Efficacy of combined electrostimulation in patients with acute exacerbation of COPD: randomised clinical trial. Med Clin (Barc). 2018;151(8):323–328.
51. Lopez-Lopez L, Torres-Sanchez I, Rodriguez-Torres J, et al. Randomized feasibility study of twice a day functional electrostimulation in patients with severe chronic obstructive pulmonary disease hospitalized for acute exacerbation. Physiother Theory Pract. 2019;37:1–8.
52. Lopez-Lopez L, Valenza MC, Rodriguez-Torres J, et al. Results on health-related quality of life and functionality of a patient-centered self-management program in hospitalized COPD: a randomized control trial. Disabil Rehabil. 2020;42(25):3687–3695.
53. Knaut C, Bonfanti Mesquita C, Dourado VZ, et al. Evaluation of inflammatory markers in patients undergoing a short-term aerobic exercise program while hospitalized due to acute exacerbation of COPD. Int J Inflam. 2020;2020:6492720.
54. Lu H, Liu N, Hu JY, et al. The effectiveness, safety and compliance of Zheng’s supine rehabilitation exercise as a rehabilitation programme among elderly patients with AECOPD. Clin Respir J. 2020;14(6):533–540.
55. Holland AE, Nici L. The return of the minimum clinically important difference for 6-minute-walk distance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(4):335–336.
56. Redelmeier DA, Bayoumi AM, Goldstein RS, et al. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155(4):1278–1282.
57. Weldam SW, Schuurmans MJ, Liu R, et al. Evaluation of Quality of Life instruments for use in COPD care and research: a systematic review. Int J Nurs Stud. 2013;50(5):688–707.
58. Janson C, Marks G, Buist S, et al. The impact of COPD on health status: findings from the BOLD study. Eur Respir J. 2013;42(6):1472–1483.
59. Evans RA, Kaplovitch E, Beauchamp MK, et al. Is quadriceps endurance reduced in COPD?: a systematic review. Chest. 2015;147(3):673–684.
60. Alcazar J, Losa-Reyna J, Rodriguez-Lopez C, et al. The sit-to-stand muscle power test: an easy, inexpensive and portable procedure to assess muscle power in older people. Exp Gerontol. 2018;112:38–43.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
