Back to Journals » Clinical Interventions in Aging » Volume 16
Pulmonary Pleomorphic Carcinoma Mimicking Primary Sarcoma of the Neck: A Case Report and Literature Review
Authors Ogawa D, Arahata M , Kuriyama M, Shinagawa S, Tomizawa G, Shimizu Y
Received 11 December 2020
Accepted for publication 5 February 2021
Published 23 February 2021 Volume 2021:16 Pages 325—333
DOI https://doi.org/10.2147/CIA.S296875
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Nandu Goswami
Daishi Ogawa,1,2 Masahisa Arahata,2 Masato Kuriyama,3 Shunji Shinagawa,3 Gakuto Tomizawa,4 Yukihiro Shimizu3
1Nanto Community Medical Support Unit, Toyama University Hospital, Toyama, Toyama, Japan; 2Department of General Medicine, Nanto Municipal Hospital, Toyama, Toyama, Japan; 3Department of Internal Medicine, Nanto Municipal Hospital, Nanto, Toyama, Japan; 4Department of Radiology, Nanto Municipal Hospital, Nanto, Toyama, Japan
Correspondence: Masahisa Arahata
Department of General Medicine, Nanto Municipal Hospital, 938 Inami, Nanto, Toyama, 932-0211, Japan
Tel +81-763-82-1475
Fax +81-763-82-1853
Email [email protected]
Abstract: We describe our challenge in diagnosing an unusual and rapidly progressing case of pulmonary pleomorphic carcinoma (PPC)—a rare, poorly differentiated, or undifferentiated non-small-cell carcinoma that can metastasize locally or distantly and has a poor prognosis. Our patient was an elderly man with a one-month history of abdominal pain, anorexia, and weight loss, diagnosed with atrophic gastritis via endoscopy, and treated medically without improvement. A week later, this patient developed pain in the head, neck, and shoulder area, and further examination revealed a thickening of his left neck and shoulder, with no palpable lymph nodes. Computed tomography (CT) of the neck, chest, and abdomen led us to believe that we might be dealing with primary sarcoma of the neck since no lung mass was evident. Further investigation could not be performed because the patient’s status deteriorated rapidly. An autopsy revealed that soft tissue in the left neck and the mesentery was invaded by poorly differentiated polymorphic malignant cells, which were also seen in the lung lesion. Immunohistochemically, these malignant cells were all positive for AE1/AE3, CAM5.2, TTF-1, Napsin-A, and Vimentin. The cells were also positive for programmed death-ligand 1 staining with a low level of tumor proportion score (over 1%). The final diagnosis was PPC with metastases to soft tissues in the left neck and the mesentery. A review of previous case reports of PPC revealed that soft tissue is an uncommon site for metastasis, and that our CT findings were rather unusual. We hereby present our case and review of published case reports, with the hope that an awareness of the heterogeneous features of PPC could prompt timely biopsy and histological diagnosis.
Keywords: pleomorphic carcinoma of the lung, non-small-cell lung cancer, metastases to soft tissues, mesentery, poor prognosis
Introduction
Pulmonary pleomorphic carcinoma (PPC) is a poorly differentiated or undifferentiated non-small cell lung carcinoma. Fishback et al in 1994 originally reported it as a new entity among lung cancers.1 According to the 2015 World Health Organization classification, PPC is a subtype of sarcomatoid carcinoma, containing at least 10% of sarcomatous-like components with spindle and/or giant cells, or consisting only of spindle and giant cells.2
PPC is rare, accounting for less than 1% of all lung tumors.3–5 It usually occurs in men beyond the age of 60 years with a history of heavy smoking.1,5,6 It has an aggressive clinical course, and the prognosis is poor.3–8 Many patients are diagnosed at advanced stages because of its aggressiveness.4 The median overall survival time of PPC has been reported as 6–19 months.1,5–7 PPC can also cause both local and distant metastases occurring in early stages, hence the rapid and tragic clinical course.5,9–11 Treatment is not yet established. Generally, PPC seems to be resistant to cytotoxic agents, but there is no standard treatment strategy except for resection.12,13 Some reports show efficacy of immune checkpoint inhibitors (ICIs) such as nivolumab and pembrolizumab.14,15
We describe a case of PPC that occurred in a patient in our care and reviewed case reports of PPC with metastases. Cases reviewed were searched via PubMed® on October 31, 2020, using the terms “(pleomorphic carcinoma of the lung) OR pulmonary pleomorphic carcinoma” and “case report”, published in English. We limited our focus to cases in which specific sites of metastasis were noted at the time of diagnosis. Through the study, we recognized the fact that PPC could present heterogeneous clinical features, and our case had extremely rare presentations. We must memorize and report this case to diagnose accurately in subsequent patients.
Case Presentation
Clinical Findings and Course
An 85-year-old man with a 60-pack-year history of smoking presented with epigastric discomfort, loss of appetite, and weight loss of 2 kilograms over the past month. The patient quit smoking in his 50s and had a pacemaker implanted for complete atrioventricular block 7 years earlier. He had been medicated for hypertension, dyslipidemia, diabetes mellitus, hyperuricemia, chronic heart failure, and benign prostatic hypertrophy for more than 5 years, and these diseases were well controlled. During the first visit, upper gastrointestinal endoscopy revealed chronic atrophic gastritis with a small ectopic pancreas. A proton pump inhibitor, mosapride, and digestive enzymes were administered to him for a week. However, his symptoms were not alleviated, and he complained of additional multiple pains appearing in the head, neck, and left shoulder. Pregabalin was additionally administered, but his symptoms gradually worsened. As a result, the patient was admitted to our hospital for further evaluation and treatment.
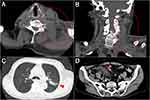
On physical examination, thickness and rigidity of his left neck and shoulder were detected. No lymph nodes were palpable. Wheezes were audible at the end of expiration. Oxyhemoglobin saturation was 98% without oxygen therapy. Laboratory tests revealed hypoproteinemia (total protein 6.5 g/dL), renal dysfunction (serum creatinine 1.44 mg/dL), and mild inflammation (C-reactive protein 0.86 mg/dL). The other common biochemical tests and blood cell counts were within the normal range. Urinalysis revealed no remarkable abnormal findings, and the fecal occult blood test was negative. Computed tomography (CT) revealed an obscure soft tissue in the left supraclavicular fossa, a swollen left levator scapulae muscle, and enlarged lymph nodes in the left neck (Figure 1A and B). CT also detected pulmonary consolidation, with an interlobular pleural shift in the left upper lobe and misty mesentery (Figure 1C and D). Although no tumor mass was detected in the neck, sarcoma or another malignancy was suspected. In addition, coexisting infectious diseases, such as cellulitis of the neck and/or bacterial pneumonia, were also considered.
His general status worsened by the day. A biopsy from the affected lesions of the neck was considered but could not be performed owing to his deteriorating state, which featured high fever, unconsciousness, and circulatory insufficiency. The epithelial tumor marker, carcinoembryonic antigen, was elevated (264.3 ng/mL) on the sixth day of hospitalization. Thereafter, he required steroids and antibiotics, as well as opioids, to relieve his symptoms. He died only 16 days after admission to the hospital.
Autopsy
With the consent of his proxy, an autopsy was performed. Grossly, multiple tumors and tumor-like lesions with lymphadenopathies were found in the left upper lobe of the lung, the mesentery of the small bowel, and the left neck (Figure 2). These findings were compatible with the results of the enhanced CT scan (Figure 1). Microscopically, the lung tumors were composed of poorly differentiated polymorphic cells mixed with spindle cells (Figure 3A). Thickened soft tissue in the left neck was invaded by atypical malignant cells similar to those in the lung (Figure 3B). The invasion had spread to the subclavian vessels, the common carotid artery/vein, and the sternocleidomastoid muscles. The mesentery had also been invaded by malignant cells similar to those in the lung and neck (Figure 3C). Although these three malignant tumors were detected, the origin was unclear by the findings of hematoxylin–eosin stain alone. Immunohistochemically, these cells in multiple organs were all positive for AE1/AE3, CAM5.2, TTF-1, Napsin-A, and Vimentin (Figure 4A–D), but negative for P40/CK14, PAX8, Thyroglobulin, Desmin, MyoD1, and S100 (data not shown). Based on these findings, the tumors had their primary origin in the lung; therefore, they were diagnosed as PPC with an adenocarcinoma component with metastasis. The expression of programmed death-ligand 1 (PD-L1, clone 22C3) was detected with a low level of tumor proportion score (TPS) in the lung tumor cells (Figure 5). The autopsy revealed no evidence of infection and concluded that the cause of death was circulatory insufficiency induced by cachexia due to advanced PPC.
 |
Figure 2 Gross findings of the autopsy. The tumors are shown inside the red circles. (A) Left lung. (B) Soft tissue, muscles, and carotid artery (arrowhead) of the left neck. (C) Mesentery. |
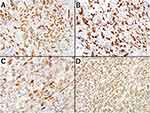 |
Figure 4 Immunohistochemical findings of the tumors. The malignant pleomorphic cells are positive for all stains. (A) AE1/AE3. (B) CAM5.2. (C) TTF-1/Napsin-A. (D) Vimentin. |
 |
Figure 5 PD-L1 staining of the lung tumor. The tumor cells were weakly stained by the anti-PD-L1 antibody clone 22C3 with a low expression level (TPS was slightly over 1%). |
Review of Case Reports
Our review of the literature revealed 33 case reports of PPC having distant metastasis at the time of diagnosis (Table 1).15–46 The common sites of metastases were the adrenal glands (11 cases), bones (9 cases), gastrointestinal tract (7 cases), and brain (5 cases); there was only one case (other than ours) with metastasis to the soft tissue, including the muscle (Case 1, Table 1). Typical CT findings consistently showed one or more distinct lung masses, versus our unusual CT findings (no mass lesion but focal consolidation). Immunohistochemically, it is interesting to note that some cases had positive results for Vimentin and extremely short survival (Cases 5, 9, 18, 23, and 33, Table 1), and they are similar to our case. However, there are no clear correlations of immunohistochemical markers across all case reports. Various treatments were employed, usually in combination, and the range in survival was very broad, from days and weeks to beyond 7 years. No clear correlations between treatment and survival can be drawn in this small collection of cases; however, most patients treated with ICIs were alive at the time of each publication. Most ICIs were adopted as adjuvants to conventional chemotherapy.
 |
Table 1 Published Case Reports of Pleomorphic Carcinoma with Metastasis |
Discussion
Our patient was an elderly man with severe and rapidly progressing PPC who died before the final diagnosis. Death before diagnosis often causes confusion in clinical situations. There were some reasons for the delay in diagnosis. First, his initial symptoms were epigastric discomfort and neck pain, whereas respiratory symptoms were absent. Furthermore, symptoms indicating lung cancer were never seen in his entire clinical course. In contrast, typical symptoms of PPC include chest pain, cough, fever, and hemoptysis.1,5–7 Second, the abnormal lesion detected by the chest CT could not be recognized as a primary lung cancer because it did not form a nodule or mass; it rather formed a consolidation in the limited area. We initially considered this consolidation as focal pneumonia based on the evidence of mild inflammation in the patient’s blood tests. Radiological findings of PPC by CT scan are usually observed as masses or nodules but rarely as consolidations or interstitial pneumonia.5,47 Meanwhile, his clinical findings were more remarkable in the neck muscles. Therefore, we suspected primary sarcoma of the left neck. Third, a planned biopsy could not be performed because of his deteriorating condition after non-invasive examinations. We had performed gastrointestinal endoscopy, but biopsy here would not be revealing since the mucous tissue of his intestinal tract was not invaded by PPC. So, the final diagnosis came from the autopsy and gave us a great impression.
Previous observational studies indicated that PPC predominantly occurs in heavily smoking males at an average age of approximately 60 years and present an aggressive clinical course.1,5,6 The clinical aggressiveness of PPC, such as an early metastasis, might be associated with smoking history. Lung cancer metastasis is promoted by long intergenic noncoding RNA (linc00152) and mediators of inflammation, such as transforming growth factor-β1, Smad2, matrix metalloproteinase 3, and hypoxia inducible factor-1, all of which are induced by cigarette smoking extracts.48,49 In our patient, therefore, the heavy-smoking habit in his youth, followed by pulmonary inflammation, might have induced these mediators and have been promoting carcinogenesis and metastasis for many years.
As the PPC cells were positive for PD-L1, our patient might have been successfully treated using ICIs such as pembrolizumab. However, previous studies indicated that the high efficacy of ICIs was observed only in patients with advanced PPC with a high level of TPS.14,15 Therefore, even if our patients had a chance to receive ICI therapy, its efficacy could not be fully expected because the TPS of PD-L1 was relatively low. However, an examination of the PD-L1 expression in PPC cells is particularly important because treatment with ICIs such as a PD-L1 or PD-1 inhibitors is expected to be the most effective on PPC for now.14,50 In addition, PD-L1 expression could be a more specific marker of PPC compared with other subtypes of non-small-cell lung cancer.51
In our review of PPC case reports, we confirmed that metastases to soft tissues (particularly to the muscles) is extremely rare and that our patient’s CT findings (focal consolidations rather than an obvious tumor) were rather unusual (Table 1). The expression of Vimentin on the PPC cells might be associated with a poor prognosis.
Conclusion
We presented an elderly patient with PPC presenting unusual clinical findings, which was diagnosed only by autopsy. Awareness of the heterogeneous features and metastatic locations of PPC might have raised clinical suspicion and prompted timely biopsy and histological diagnosis, possibly providing the appropriate interventions and solving the confusion.
Ethics Statement
Ethical approval for publication was obtained from The Institutional Review Board of Nanto Municipal Hospital (reference number 183-2).
Consent for Publication
Written informed consent was obtained from the next of kin of the patient for the publication of this report and any accompanying images.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors declare that this case report has received no financial support.
Disclosure
The authors report no conflicts of interest.
References
1. Fishback NF, Travis WD, Moran CA, Guinee DG, McCarthy WF, Koss MN. Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer. 1994;73(12):2936–2945. doi:10.1002/1097-0142(19940615)73:12<2936::AID-CNCR2820731210>3.0.CO;2-U
2. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260. doi:10.1097/JTO.0000000000000630
3. Yendamuri S, Caty L, Pine M, et al. Outcomes of sarcomatoid carcinoma of the lung: a surveillance, epidemiology, and end results database analysis. Surgery. 2012;152(3):397–402. doi:10.1016/j.surg.2012.05.007
4. Steuer CE, Behera M, Liu Y, et al. Pulmonary sarcomatoid carcinoma: an analysis of the national cancer data base. Clin Lung Cancer. 2017;18(3):286–292. doi:10.1016/j.cllc.2016.11.016
5. Ito K, Oizumi S, Fukumoto S, et al. Clinical characteristics of pleomorphic carcinoma of the lung. Lung Cancer. 2010;68(2):204–210. doi:10.1016/j.lungcan.2009.06.002
6. Rossi G, Cavazza A, Sturm N, et al. Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements: a clinicopathologic and immunohistochemical study of 75 cases. Am J Surg Pathol. 2003;27(3):311–324. doi:10.1097/00000478-200303000-00004
7. Huang SY, Shen SJ, Li XY. Pulmonary sarcomatoid carcinoma: a clinicopathologic study and prognostic analysis of 51 cases. World J Surg Oncol. 2013;11:252. doi:10.1186/1477-7819-11-252
8. Tamura Y, Fujiwara Y, Yamamoto N, et al. Retrospective analysis of the efficacy of chemotherapy and molecular targeted therapy for advanced pulmonary pleomorphic carcinoma. BMC Res Notes. 2015;8:800. doi:10.1186/s13104-015-1762-z
9. Yuki T, Sakuma T, Onbayashi C, et al. Pleomorphic carcinoma of the lung: a surgical outcome. J Thorac Cardiovasc Surg. 2007;134(2):399–404. doi:10.1016/j.jtcvs.2007.04.018
10. Koh H, Chiyotani A, Tokuda T, et al. Pleomorphic carcinoma showing rapid growth, multiple metastases, and intestinal perforation. Ann Thorac Cardiovasc Surg. 2014;20:669–673. doi:10.5761/atcs.cr.13-00167
11. Chang YL, Lee YC, Shih JY, Wu CT. Pulmonary pleomorphic (spindle) cell carcinoma: peculiar clinicopathologic manifestations different from ordinary non-small cell carcinoma. Lung Cancer. 2001;34(1):91–97. doi:10.1016/S0169-5002(01)00224-0
12. Bae HM, Min HS, Lee SH, et al. Palliative chemotherapy for pleomorphic carcinoma. Lung Cancer. 2007;58(1):112–115. doi:10.1016/j.lungcan.2007.05.006
13. Hong JY, Choi MK, Uhm JL, et al. The role of palliative chemotherapy for advanced pulmonary pleomorphic carcinoma. Med Oncol. 2009;26(3):287–291. doi:10.1007/s12032-008-9117-4
14. Sukrithan V, Sandler J, Gucalp R, Gralla R, Halmos B. Immune checkpoint blockade is associated with durable responses in pulmonary sarcomatoid carcinoma. Clin Lung Cancer. 2019;20(3):e242–e246. doi:10.1016/j.cllc.2018.12.013
15. Senoo S, Ninomiya T, Makimoto G, et al. Rapid and long-term response of pulmonary pleomorphic carcinoma to nivolumab. Intern Med. 2019;58(7):985–989. doi:10.2169/internalmedicine.0890-18
16. Chang HC, Hsu CL, Chang YL, Yu CJ. Pulmonary pleomorphic carcinoma with pembrolizumab monotherapy. Respirol Case Rep. 2020;8(6):e0597. doi:10.1002/rcr2.597
17. Ota T, Niho S, Kirita K, Ishii G, Tsuboi M, Goto K. Impressive response to nivolumab of non-small-cell lung cancer containing sarcomatoid components. Respirol Case Rep. 2019;7(8):e00477.
18. Tokuyasu H, Ishikawa S, Sakai H, Ikeuchi T, Miura H. Single pembrolizumab treatment causing profound durable response in a patient with pulmonary pleomorphic carcinoma. Respir Med Case Rep. 2019;28:100879. doi:10.1016/j.rmcr.2019.100879
19. Basu A, Moreira AL, Simms A, Brandler TC. Sarcomatoid carcinoma in cytology: report of a rare entity presenting in pleural and pericardial fluid preparations. Diagn Cytopathol. 2019;47(8):813–816. doi:10.1002/dc.24183
20. Kim MJ, Lee HN, Kim JI, Kim GY. Pleomorphic carcinoma of the lung mimicking synchronous pulmonary adenocarcinoma and small bowel sarcoma. Ann Thorac Med. 2018;13(4):251–253. doi:10.4103/atm.ATM_362_17
21. Noguchi S, Kawachi H, Yoshida H, et al. Sarcoid-like granulomatosis induced by nivolumab treatment in a lung cancer patient. Case Rep Oncol. 2018;11(2):562–566. doi:10.1159/000492383
22. Ando T, Goto Y, Mano K, et al. Paraneoplastic autoimmune encephalitis associated with pleomorphic lung carcinoma: an autopsy case report. Neuropathology. 2018;38(4):448–454. doi:10.1111/neup.12477
23. Masuda K, Tokito T, Azuma K, et al. Pulmonary pleomorphic carcinoma: a case harboring EGFR mutation treated with EGFR-TKIs. Thorac Cancer. 2018;9(6):754–757. doi:10.1111/1759-7714.12646
24. Ustaoğlu M, Koçak G, Siviloğlu Ç, Cengiz E. An unusual case of duodenal metastasis of pulmonary pleomorphic carcinoma. Turk J Gastroenterol. 2018;29(1):136–137. doi:10.5152/tjg.2018.17288
25. Okamura K, Fukuda Y, Soda H, et al. Pulmonary pleomorphic carcinoma with few PD-1-positive immune cells and regulatory T cells that showed a complete response to nivolumab. Thorac Cancer. 2018;9(1):193–196. doi:10.1111/1759-7714.12557
26. Marchand L, Paulus V, Fabien N, et al. Nivolumab-induced acute diabetes mellitus and hypophysitis in a patient with advanced pulmonary pleomorphic carcinoma with a prolonged tumor response. J Thorac Oncol. 2017;12(11):e182–e184. doi:10.1016/j.jtho.2017.07.021
27. Matsumoto Y, Miura T, Horiuchi H, Usui K. The successful treatment of pulmonary pleomorphic carcinoma with pembrolizumab: a case report. Case Rep Oncol. 2017;10(2):752–757. doi:10.1159/000479552
28. Arshad HS, Dudekula RA, Niazi M, Malik S, Khaja M. A rare case of sarcomatoid carcinoma of the lung with spine metastasis, including a literature review. Am J Case Rep. 2017;18:760–765. doi:10.12659/AJCR.904584
29. Murai T, Yonetsu T, Isobe M, Kakuta T. Coronary embolization caused by pleomorphic lung carcinoma. Intern Med. 2016;55(24):3607–3609. doi:10.2169/internalmedicine.55.7571
30. Ito K, Hataji O, Katsuta K, Kobayashi T, Gabazza EC, Yatabe Y. “Pseudoprogression” of pulmonary pleomorphic carcinoma during nivolumab therapy. J Thorac Oncol. 2016;11(10):e117–e119. doi:10.1016/j.jtho.2016.05.002
31. Oda T, Sekine A, Kato T, Baba T, Okudela K, Ogura T. Promising effect of chemotherapy with bevacizumab for patients with pulmonary pleomorphic carcinoma: two case reports and a literature review. Respir Investig. 2015;53(6):296–299. doi:10.1016/j.resinv.2015.05.004
32. Murakami Y, Saka H, Oki M. Response to crizotinib and clinical outcome in ALK-rearranged pulmonary pleomorphic carcinoma. J Thorac Oncol. 2015;10(5):e28–e29. doi:10.1097/JTO.0000000000000450
33. Yamanashi K, Marumo S, Miura K, Kawashima M. Long-term survival in a case of pleomorphic carcinoma with a brain metastasis. Case Rep Oncol. 2014;7(3):799–803. doi:10.1159/000368186
34. Shimizu Y, Iguchi T, Nitta Y, Machida Y, Kuratsukuri K, Kawashima H. A case of pulmonary pleomorphic carcinoma with renal metastasis. Urol Case Rep. 2014;2(6):179–180. doi:10.1016/j.eucr.2014.07.007
35. Rice B, Ross J, Todd N, Caputo L, Rush S, Shrestha B. Pulmonary pleomorphic carcinoma metastasis to the midfoot. J Foot Ankle Surg. 2015;54(3):483–486. doi:10.1053/j.jfas.2014.04.018
36. Lin MW, Wu CT, Chang YL. Intussusception caused by intestinal metastasis from lung pleomorphic carcinoma. Ann Thorac Cardiovasc Surg. 2014;20:635–638. doi:10.5761/atcs.cr.13-00099
37. Ioncica AM, Atiq M, Lee JH, Saftoiu A, Bhutani MS. Gastric and pancreatic metastases from pleomorphic lung carcinoma. Endoscopy. 2011;43:
38. Rashid S, Rajan D, Jacob R, et al. Colonic metastases from pleomorphic carcinoma of the lung presenting as an ileocecal intussusception. ISRN Gastroenterol. 2011;2011:137139. doi:10.5402/2011/137139
39. Saitoh M, Niijima M, Takiguchi Y, et al. An early event of EGFR mutation in pleomorphic carcinoma of the lung. Int J Clin Oncol. 2011;16(6):770–773. doi:10.1007/s10147-011-0247-x
40. Lee KW, Kim YJ, Kim JH, Bang SM, Chung JH, Lee JS. Two consecutive cases of platinum-refractory pulmonary pleomorphic carcinoma that showed dramatic responses to MAID (mesna, doxorubicin, ifosfamide and dacarbazine) chemotherapy. Jpn J Clin Oncol. 2011;41(3):430–433. doi:10.1093/jjco/hyq180
41. Shi B, Gaebelein G, Hildebrandt B, Weichert W, Glanemann M. Adult jejunojejunal intussusception caused by metastasized pleomorphic carcinoma of the lung: report of a case. Surg Today. 2009;39(11):984–989. doi:10.1007/s00595-008-3954-9
42. Nomura R, Yoshida D, Kim K, Kobayashi S, Teramoto A. Intracerebral hemorrhage caused by a neoplastic aneurysm from pleomorphic lung carcinoma. Neurol Med Chir (Tokyo). 2009;49(1):33–36. doi:10.2176/nmc.49.33
43. Ushiki A, Koizumi T, Kobayashi N, et al. Genetic heterogeneity of EGFR mutation in pleomorphic carcinoma of the lung: response to gefitinib and clinical outcome. Jpn J Clin Oncol. 2009;39(4):267–270. doi:10.1093/jjco/hyn155
44. Aokage K, Yoshida J, Ishii G, et al. Long-term survival in two cases of resected gastric metastasis of pulmonary pleomorphic carcinoma. J Thorac Oncol. 2008;3(7):796–799. doi:10.1097/JTO.0b013e31817c925c
45. Yilmaz A, Sulu E, Arinç S, et al. Pleomorphic carcinoma of the lung: a report of six cases. Tuberk Toraks. 2007;55(3):290–294.
46. Burnette RE, Ballard BR. Metastatic pleomorphic carcinoma of lung presenting as abdominal pain. J Natl Med Assoc. 2004;96(12):1657–1660.
47. Fujisaki A, Aoki T, Kasai T, et al. Pleomorphic carcinoma of the lung: relationship between CT findings and prognosis. AJR Am J Roentgenol. 2016;207(2):289–294. doi:10.2214/AJR.15.15542
48. Pezzuto A, Citarella F, Croghan I, Tonini G. The effects of cigarette smoking extracts on cell cycle and tumor spread: novel evidence. Future Sci OA. 2019;5(5):FSO394. doi:10.2144/fsoa-2019-0017
49. Pezzuto A, Carico E. Role of HIF-1 in cancer progression: novel insights. Curr Mol Med. 2018;18(6):343–351. doi:10.2174/1566524018666181109121849
50. Lee J, Choi Y, Jung HA, et al. Outstanding clinical efficacy of PD-1/PD-L1 inhibitors for pulmonary pleomorphic carcinoma. Eur J Cancer. 2020;132:150–158. doi:10.1016/j.ejca.2020.03.029
51. Ancoš V, Farkašová A, Kviatkovská Z, et al. Non-small cell lung carcinomas with a minor sarcomatoid component and pleomorphic carcinomas are associated with high expression of programmed death ligand 1. Pathol Res Pract. 2020;216(12):153238. doi:10.1016/j.prp.2020.153238
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.