Back to Journals » International Medical Case Reports Journal » Volume 16
Pulmonary Edema After Intracranial Aneurysm Clipping in Kyphosis: A Case Report
Authors Ye Y, Wang W, Yang L, He M
Received 22 February 2023
Accepted for publication 27 May 2023
Published 1 June 2023 Volume 2023:16 Pages 333—337
DOI https://doi.org/10.2147/IMCRJ.S409578
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Yuancai Ye,1 Weisi Wang,1 Lei Yang,1 Min He2
1Department of Anesthesiology, West China Hospital of Sichuan University, Chengdu City, Sichuan, People’s Republic of China; 2Department of Critical Care Medicine, West China Hospital of Sichuan University, Chengdu City, Sichuan, People’s Republic of China
Correspondence: Lei Yang; Min He, Email [email protected]; [email protected]
Introduction: A 56-year-old female patient was admitted to the hospital for “ 10+days of right eye droop and 1 day of aggravation”. After admission, the physical examination found that the patient had severe scoliosis. 3D reconstruction and enhanced CT scan of the head vessels showed that the right internal carotid artery C6 aneurysms were clipped under general anesthesia. After the operation, the patient had increased airway pressure, with a large number of pink foam sputum attracted from the trachea catheter, and the lungs were scattered with moist rales during auscultation, After the treatment of anti-heart failure, the patient returned to the ICU through the trachea catheter. Eight hours later, the trachea catheter was pulled out and the patient was released from the ventilator. The symptoms were relieved on the fifth day after the operation. This case report describes the perioperative management of intracranial aneurysm with severe scoliosis. After strict monitoring and timely treatment during the perioperative period, the patient turned from crisis to safety, providing some reference for colleagues who encounter such patients in the future.
Conclusion: In patients with scoliosis, due to long-term compression of the thorax, pulmonary restrictive ventilation dysfunction, small airway function and diffusion function are reduced, and cardiac function is decreased. Therefore, during the operation of intracranial aneurysms, fluid infusion should be careful, and volume monitoring should be done at all times to maintain the effective circulating blood volume of the body and prevent the aggravation of cardiac insufficiency and pulmonary edema.
Keywords: intracranial aneurysm, scoliosis, pulmonary edema
Introduction
Intracranial Aneurysm (IA) is a common vascular anomaly, which is characterized by the expansion of intracranial artery, often leading to rupture of cerebral vessels, subarachnoid hemorrhage (SAH) and serious consequences.1 Scoliosis is a skeletal muscle disease accompanied by spinal deflection or rotation; It is a generic term for a group of different diseases of the shape and position of the spine, thorax and trunk caused by changes in composition.2 Nearly two-thirds of scoliosis patients are usually accompanied by restrictive abnormal breathing patterns, leading to external malformations and reducing respiratory muscle contraction, which limits the movement of ribs and trunk and leads to pulmonary dysfunction and respiratory dysfunction and complications such as pulmonary edema, atelectasis, lung infection and heart failure may occur.3 Therefore, intracranial aneurysm with scoliosis is rare, which brings great challenges to anesthesia management.
Case Presentation
The patient, a 56-year-old female, was admitted for “10+days of right eye droop and 1 day of aggravation”. 10+days ago, the patient had no obvious inducement of right eye face droop without limb movement restriction, accompanied by right parietal and occipital pain, no nausea, vomiting, dizziness, walking unsteadily, feeling of cotton stepping, no slurred words, no choking or coughing of drinking water. The patient was treated outside the hospital, and improved relevant examinations to consider the diagnosis of “cerebral aneurysm”. 1+days ago, the patient’s right eye droop was aggravated, so she went to the neurosurgery department of our hospital. History of previous diseases: No history of hypertension, diabetes, family history of aneurysms, respiratory or neurological diseases. Physical examination after admission: the patient’s vital signs were stable, and her mind was clear. The left pupil, with a diameter of about 3mm, is sensitive to light reflection; the right pupil, with a diameter of about 5mm, is slow to light reflection; the right eye has a drooping face; the right eye has limited eye movement; the right eye has decreased vision; the left eye has no obvious abnormality in vision and eye movement; the limbs have normal muscle strength and muscle tension; the bilateral pathological signs are negative; the meningeal stimulation sign is positive; and the spine is bent (Figure 1). After admission, 3D reconstruction and enhanced scanning of CT head vessels showed that the end of C7 segment of the right internal carotid artery was swollen and two saccular processes with a length of about 0.4 cm were seen, which were multiple aneurysms with daughter aneurysms (Figure 2). Diagnosis after admission: 1. C6 aneurysm of the right internal carotid artery 2. Ophthalmoplegia of the right side 3. Anesthesia and surgical procedure: sevoflurane was given to retain spontaneous respiration induction and after successful tracheal intubation with visual laryngoscope, the mechanical ventilation was normal. Subsequently, craniotomy aneurysm clipping was successful performed. During the 3.6-hour surgery, 3200mL liquid (1600mL crystal and 1300mL of colloid) was input, 1700mL of urine was urinated, and 200mL of blood was bled. The last blood gas analysis (FiO2 50%) during the operation showed that pH was 7.45, PaCO2 was 40.3mmHg, PaO2 was 212.7mmHg, Na was 137.9mmol/L, K was 3.24 mmol/L, and Ca was 1.0mmol/L. After the operation, the patient was transferred to the small recovery room with a tube. SpO2 99%, BP 100/50 mmHg, HR 68 bpm, EtCO2 32 mmHg at the time of admission. One hour after entering the anesthesia recovery room, the patient recovered from spontaneous breathing, but the tidal volume was low, about 90mL. Half an hour later, the patient developed dyspnea in the inspiratory phase, severe triple concave sign, and mild blood liquid ejected from the endotracheal tube. SpO2 96%, BP 110/70 mmHg, HR 58 bpm, EtCO2 48mmHg were immediately aspirated into the trachea, sucking out a large amount of pink foam liquid, with manual ventilation conducted, and the airway resistance was high (35cm/H2O). The tidal volume is about 60mL. Confirm the catheter and withdraw the tracheal catheter to 16cm, yet the airway resistance is not significantly improved. The fiberoptic bronchoscope showed that the left and right main bronchi were edematous, the lumens were narrowed, and there was fluid exudation inside. After anti heart failure treatment, they retained the tracheal catheter and sent it to the intensive care unit for further symptomatic support treatment. They provides patients with diuretic, hormonal, anti infective, and invasive ventilator treatments. Fiber bronchoscopy examination showed that edema of left and right main bronchi basically disappeared. Eight hours later, they pulled out the trachea catheter and separated from the ventilator. The symptoms were relieved on the fifth day after surgery and they were discharged from the hospital. Three months after discharge, they recovered well after follow-up.
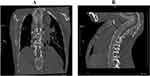 |
Figure 1 Plain CT scan of the patient’s chest and abdomen ((A): Spinal AP and lateral position (B): Spinal lateral position). |
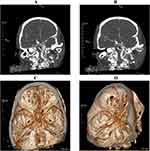 |
Figure 2 CT head enhanced scan of patients ((A and B): CT head (C and D): CT head vascular 3D reconstruction enhanced scan). |
Discussion
Intracranial Aneurysm
Intracranial Aneurysms are divided into ruptured intracranial aneurysms and unruptured intracranial aneurysms. The incidence of subarachnoid hemorrhage caused by ruptured intracranial aneurysms in most Western populations is 6–8/100,0004 and the mortality rate of conservative treatment in the first few months is 50–60%. The incidence of complications after intracranial aneurysm rupture is high, such as vasospasm, hydrocephalus, delayed ischemic defect and other complications further affect the prognosis. Unruptured aneurysms can also be divided into asymptomatic incidental aneurysms, symptomatic aneurysms, and symptomatic unruptured aneurysms usually cause cerebral palsy, yet rarely cause arterial embolism. Risk factors include smoking, excessive drinking, hypertension, race, sex, etc.5–7 When deciding the treatment of unruptured aneurysms, not only the maximum diameter of the aneurysm, but also the age of the patient, the location of the aneurysm History of hypertension should be considered. At present, treatment includes craniotomy aneurysm clipping and vascular interventional embolization. Finally, smoking history, aneurysm size, posterior circulation aneurysm, old age and previous ischemic cerebrovascular disease can independently predict the poor prognosis of open surgery.8,9 Patients with symptomatic aneurysms, regardless of age, should be treated with aneurysm occlusion. Finally, smoking cessation and active treatment of hypertension are important for patients with unruptured aneurysms, regardless of the treatment of these lesions.9
Scoliosis and Anesthesia Management
Scoliosis is the most common three-dimensional deformation abnormality of the spine, which directly affects the thorax. It is reported that the prevalence rate in the general population ranges from 0.3% to 15.3%.10–12 It may be congenital, secondary to various systemic or neuromuscular diseases due to vertebral or rib deformities, or idiopathic, which is the result of the pathological process leading to scoliosis. Most researchers who study the pulmonary function damage of scoliosis generally believe that the angle of curvature of the spine is greater than 90 degrees, which is prone to cardiopulmonary failure. When it is greater than 50~60 degrees, abnormal pulmonary function can be detected. The influence on lung function is mainly reflected in the reduction of lung volume, and the main influencing factors include the reduction of chest wall compliance, the reduction of respiratory driving force and muscle strength. Hypoxemia in severe scoliosis may be caused by diffusion limitation and/or alveolar hypoventilation, and may be accompanied by carbon dioxide retention. Congenital spinal malformation not only has an impact on pulmonary function, but also has a significant impact on cardiac function. Previous studies have shown that patients with scoliosis have a high incidence of elevated pulmonary artery pressure, and there is a medium correlation between Cobb angle and pulmonary artery pressure.13 It has been reported that the reduction of right heart function in patients with scoliosis may be related to long-term direct thoracic compression, rather than necessarily secondary to decreased pulmonary function. Therefore, the effect of scoliosis on cardiac function is due to the increase of pulmonary artery pressure secondary to long-term hypoxemia caused by pulmonary function damage, and the direct compression of the heart caused by thoracic malformation. The synergistic effect of the two will eventually lead to the impairment of diastolic function in patients with severe scoliosis. The patient is more sensitive to the increase of preload, so the accumulation of carbon dioxide should be avoided in anesthesia management, which aggravates the patient’s pulmonary artery pressure, leading to the decrease of right heart function; fluid infusion should not be overloaded and the spine bends and compresses the pulmonary artery, reducing the blood flow into the pulmonary artery, and the left ventricle is not fully filled. Long term preload reduction leads to “disuse dysfunction” of the left ventricle (characterized by ventricular remodeling, reduced compliance, and further damage to ventricular filling, which ultimately leads to a decline in cardiac function), a decline in cardiac diastolic function, a decline in the heart’s ability to compensate for preload, and an increase in the amount of fluid infusion leads to an increase in left atrial pressure, which ultimately leads to cardiogenic pulmonary edema and failure to maintain oxygenation. For high-risk patients with pulmonary edema, we can combine TEE to monitor the patient’s heart function and blood volume in real time during operation with fluid infusion guide, and check blood gas frequently, which will help us find pulmonary diffusion dysfunction early.
Limitation
Fluid replacement is more accurate in patients with scoliosis and intracranial aneurysm, pulmonary edema is prevented by monitoring blood volume throughout the perioperative period. Transthoracic echocardiography or transesophageal Echocardiography can monitor blood volume, pulmonary edema, cardiac systolic and diastolic function at any moment and direct liquid management. Unfortunately, this patient was not monitored.
Conclusion
Intracranial aneurysm is a kind of cerebrovascular accident disease with high morbidity and mortality. During the operation, mannitol and other diuretics are required. At the same time, in order to maintain circulation, a large amount of fluid replacement is required. When the patient is complicated with scoliosis, restrictive ventilation leads to decreased pulmonary function and decreased cardiac function. Therefore, ultrasound and other equipment are required to evaluate and guide fluid replacement at all times, maintain effective circulating blood volume, and prevent aggravation of cardiac insufficiency and pulmonary edema.
Patient Consent
Patient provided written informed consent for the case details and accompanying images to be published. No institutional approval was required to publish this case report.
Author Contributions
All authors made a significant contribution to the work reported; took part in drafting, revising or critically reviewing the article, gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest.
References
1. Chu D, Zhang GM, Li QQ, et al. Research progress of inflammatory response and signaling pathway in the development of intracranial aneurysms. J Med J PLA. 2022;2022:254.
2. Qi K, Fu H, Yang Z, et al. Effects of core stabilization training on the cobb angle and pulmonary function in adolescent patients with idiopathic scoliosis. J Environ Public Health. 2022;2022:4263393. doi:10.1155/2022/4263393
3. Wick JM, Konze J, Alexander K, Sweeney C. Infantile and juvenile scoliosis: the crooked path to diagnosis and treatment. AORN J. 2009;90(3):347–376. doi:10.1016/j.aorn.2009.06.019
4. Linn FH, Rinkel GJ, Algra A, van Gijn J. Incidence of subarachnoid hemorrhage: role of region, year, and rate of computed tomography: a meta-analysis. Stroke. 1996;27(4):625–629. doi:10.1161/01.str.27.4.625
5. Feigin VL, Rinkel GJ, Lawes CM, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke. 2005;36(12):2773–2780. doi:10.1161/01.STR.0000190838.02954.e8
6. Kissela BM, Sauerbeck L, Woo D, et al. Subarachnoid hemorrhage: a preventable disease with a heritable component. Stroke. 2002;33(5):1321–1326. doi:10.1161/01.str.0000014773.57733.3e
7. Juvela S, Hillbom M, Numminen H, Koskinen P. Cigarette smoking and alcohol consumption as risk factors for aneurysmal subarachnoid hemorrhage. Stroke. 1993;24(5):639–646. doi:10.1161/01.str.24.5.639
8. Wiebers DO, Whisnant JP, Huston J, et al; International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103–110. doi:10.1016/s0140-6736(03)13860-3
9. Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. J Neurosurg. 2000;93(3):379–387. doi:10.3171/jns.2000.93.3.0379
10. Lonstein JE. Adolescent idiopathic scoliosis. Lancet. 1994;344(8934):1407–1412. doi:10.1016/s0140-6736(94)90572-x
11. Koukourakis I, Giaourakis G, Kouvidis G, et al. Screening school children for scoliosis on the island of Crete. J Spinal Disord. 1997;10(6):527–531. doi:10.1097/00002517-199712000-00013
12. Trobisch P, Suess O, Schwab F. Idiopathic scoliosis. Dtsch Arztebl Int. 2010;107(49):
13. Li XY, Li Z, Feng F, et al. Correlation between severity of adolescent idiopathic scoliosis and pulmonary artery systolic pressure: a study of 338 patients. Eur Spine J. 2016;25(10):3180–3185. doi:10.1007/s00586-016-4471-y
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
