Back to Journals » Veterinary Medicine: Research and Reports » Volume 13
Public Awareness, Prevalence and Potential Determinants of Bovine Tuberculosis in Selected Districts of Gamo Zone, Southern Ethiopia
Authors Tora E , Getachew M, Seyoum W , Abayneh E
Received 5 May 2022
Accepted for publication 27 July 2022
Published 4 August 2022 Volume 2022:13 Pages 163—172
DOI https://doi.org/10.2147/VMRR.S370733
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Ephrem Tora, Minale Getachew, Wasihun Seyoum, Edget Abayneh
Department of Animal Science, College of Agricultural Sciences, Arba Minch University, Arba Minch, Ethiopia
Correspondence: Ephrem Tora, Email [email protected]
Purpose: Bovine tuberculosis (bTB) is infectious chronic disease of animals mainly caused by bacillus Mycobacterium bovis. It is known endemic disease of cattle in Ethiopia. In the current study sites, there is little information on bovine tuberculosis. Thus, this study was aimed to assess public awareness; and estimate prevalence and potential a risk factors of bovine tuberculosis.
Methods: A cross-sectional study was conducted, November 2018 to May 2019, on dairy cattle randomly selected from smallholder farms of Arba Minch Zuria and Chencha districts. Comparative intradermal tuberculin test (CIDT) was used in the diagnosis of bTB in dairy animals. CIDT was administered to 221 dairy cattle. Questionnaire survey was performed on 110 smallholders to assess risk factors accountable for the event of bTB in human population.
Results: 8.2% animal level prevalence of bTB was documented in the study sites. Dairy cattle with age of 4 to 8 eight years were 34% more likely to be affected by bTB less than 4 years age cattle (OR=1.34). However, factors like housing, body condition, herd size and parity were not significantly related with the event of bTB. The assessment of bTB awareness was shown 29.7% participants knew that cattle could be infected, and 13.4% of the respondents believed that it can spread from animal to human and vice versa. Besides, 66.1% of participants had habit for consumption of raw milk and soured milk products. Moreover, respondent’s attitude about zoonoses and consequences of bTB was risky.
Conclusion: Present study discloses that bTB is animal health problem affecting dairy cattle in Gamo zone, Southern Ethiopia. Based on the current findings, public awareness creation, bovine tuberculosis test and segregation strategy should be applied to minimize the public health hazards and risk factors for bovine origin tuberculosis.
Keywords: awareness, bovine tuberculosis, comparative intradermal tuberculin test, public
Introduction
Ethiopia is a country in Africa having a huge livestock population with estimated 64 million cattle heads,1 of which dairy cows estimated to be 6.3 million. The dairy cattle produce an estimated total 2.6 billion litres per year national milk production, of which 2% production is accounted for urban and peri-urban dairy farmers.2 In countries such as Ethiopia, livestock are indispensable for people’s livelihood creating 60–70% of the population job opportunity, animal diseases are real threat to animal productivity and public health, thus it negatively impacts the agricultural segment and economic development.3
The threats to human health from animals might be aggravated from existing and emerging zoonotic diseases.4,5 These diseases cause public health hazard to human beings, among which bovine tuberculosis is predominant disease in developing world including Ethiopia. Bovine tuberculosis is chronic communicable bacterial disease of both animals and human, belongs to members of mycobacterial tuberculosis complex (MTBC).6,7 Although, studies disclosed currently that Mycobacterium tuberculosis has been diagnosed from cattle6 and Mycobacterium bovis from humans diseased with bovine tuberculosis (bTB).6,8 Whereas M. tuberculosis is explicitly adapted to humans, M. bovis is to cattle which identified frequently from farmed cattle.6,9 Despite of its difference in host specificity, the belongings of MTBC are characterized by 99.9% or higher similarity at nucleotide sequence level and are essentially identical at sequence of 16s rRNA.10
Bovine tuberculosis is disastrous disease due to its chronicity, contagious nature and zoonosis to domestic animals, wild animals, and humans.11 Besides, tuberculosis affects a wide range of mammalian species.12 In those species the infection is characterized by granulomatous tissue formation in the lungs, lymph nodes, liver, intestines and kidney.13 Tuberculosis is a major health hazard, with an estimated 8–9 million new cases and 3 million deaths per annum worldwide.14 In Sub-Saharan Africa, roughly 2 million human tuberculosis cases occur every year; yet it has not completely known what role bTB plays in the mounting epidemic of tuberculosis adopted by acquired Immune deficiency syndrome.10
Cattle as a potential reservoir host, from which many other livestock become infected and transmission to humans claimed as a public health concern.15–17 Thus the agent, referred to bovine tubercle bacillus, is the necessary cause of bovine tuberculosis and the transmission of the microorganism could be through aerosol and/ or droplets of exudates having the bacilli. Moreover, it can transmitted by ingestion of contaminated feed, and water contaminated with urine, faecal material, or droplets from diseased animals.13,18,19 bTB is transmitted mainly in human population through consumption of fresh milk and manifested as an extra-pulmonary tuberculosis, particularly as cervical lymphadenitis.10 Consequently, it has been stated20 that M. bovis is responsible for tuberculous lymphadenitis in 17.1%, out of 29 human tuberculosis cases in Ethiopia.
According to14,21 major contributory factors to get infection by bTB in both urban and rural community are family ownership of cattle, previous livestock ownership, living together in one house with animals, drinking of non-pasteurised milk (fresh milk) or undercooked meat. Rural communities in Ethiopia, particularly in the study area practice daily these all causal habits notably. In particular, among societies where bTB is common and uncontrolled, milk borne infection is the main cause of non-pulmonary tuberculosis.10,15,20 Thus, the incidence of bTB in human is extensively distributed in parts of the country where such practices musk control measures or do not practice pasteurization of milk.22–24
With regard to zoonosis of bTB awareness and perception of the community is imperative, so that the prevention and control actions of the zoonotic bTB can be undertaken.13,25 Low awareness on how bovine tuberculosis could be transmitted by consumption habits and livestock management practices make community vulnerable to disease.11,13 Therefore, the socio-economic status and low living standard for humans are more contributory factor in bTB transmission amongst human to human and human to cattle or vice versa.6,26–28 Hence, there is strong association between bTB and public awareness, by implying high flesh meat and unpasteurized milk consumption, which is the foremost factor of morbidity and mortality, and is driving to the bovine originated tuberculosis prevalence.5,11,13,26
Bovine tuberculosis is reported as serious hazard for dairy farmers in most developing world including Ethiopia. In Ethiopia, the disease has been reported from corners of the country with apparent prevalence ranging from 0.8 to 50% depending on the geographical location, breed and the husbandry practices.29–33 However, there is scanty information in the current study area on the estimate, risk factors assessment and public health awareness of this disease since most of the stated reports were concerted around the central part of the country. Despite the actuality of potential predisposing factors in the study sites, the prevalence of bTB has yet not been estimated. This study was therefore intended to estimate prevalence, assess the risk factors of bTB and assess community awareness on its public health scenario in Chencha and Arba Minch Zuria districts of Gamo zone, southern Ethiopia.
Materials and Methods
Study Area Description
The study was executed in Arba Minch Zuria and Chencha districts, Gamo zone, southern Ethiopia from November 2018 to May 2019. Chencha is district in Gamo zone and located astronomically between 37° 29’ East to 37° 39’ West longitude and between 6° 8’ North and 6°25’ South latitude. The altitude of the district ranges from 1300 to 3250 masl. Chencha is defined by different agro-climatic distribution and vegetation cover due to its high altitudinal range. Two agro-ecological zones, namely high land and low land, account for about 82% and 18% of the total land coverage, respectively. The rainfall pattern is bimodal. Whereas first round of rain begins between March to April, the second round of rain occurs from June to August. The farming system is a mixed farming system where crop and the livestock production are equally important to each other.34
Arba Minch Zuria district is also a part of the Gamo Zone located in the great East Africa rift valley and surrounded by Abaya and Chamo lakes as well as the “Nech Sar” national park. Its astronomical location is 6° 5’ North latitude and 37° 38’ East longitude. The district has bimodal pattern of rainfall, having a short rain falls between March and April, and long rainy season from June and September. Average annual temperature of the district is 26.33◦C and rainfall ranges from 800 to 1200 mm. The district is has both midland and lowland agro-ecological zones, which include 45.55 and 55.5% of the total land cover, respectively. The livelihood of the society largely depends on crop and livestock mixed production system.1
Study Population
This study was executed on 221 dairy cattle belonged to seventy small holders dairy farm in the study district depending on the willingness of the cattle owners. Some of the farms contained both cross and zebu (local) breed, and some contain mixed zebu cattle in their farm and others even only cross breed cattle. The dairy cattle enrolled for this study were noted for the detailed information by recording the age, breed, sex, body condition score, reproductive status, and origin and size of herd. The dairy cattle eligible for this study (excluding calves aged below six months and late pregnancy (>7 month) cows) were exposed to CIDT test.
Study Design and Sampling Method
A cross-sectional study was used to operate comparative intradermal tuberculin (CIDT) test on 221 dairy cattle. It is performed to estimate the prevalence of bTB and assess potential risk factors related with bTB prevalence in dairy cattle. Moreover, a questionnaire survey was conducted by using structured format to assess the attitudes, awareness and practices of cattle holders on bTB and its zoonotic impacts as well as the courses of transmission of the disease.
A list of 5 kebeles and head of household and their dairy cows was obtained from the district office of livestock and fisheries development. All the kebeles were selected deliberately based on accessibility to the aforementioned districts. Whereas, simple random sampling was used to collect the study herds, purposive sampling was applied to female animals above one year. Accordingly, a total 221 dairy cattle were tested by an average herd size of 43 animals in each kebele (Supplementary material).
Sample Size Determination
Meta-analysis of the different studies reported in Ethiopia has shown a pooled estimate of the bovine tuberculosis about 5.8%.17 Thus, the required sample size for the validation of the test was estimated by considering the expected prevalence of 5.8% (95% CI: 4.5–7.5). Accordingly, a pooled prevalence report, 5.8% and desired precision, 5% with the confidence level of 95% was computed for sample size using formula described by Thrusfield et al35 as follows:
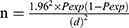 Where: n = sample size; Pexp = expected prevalence; d=desired absolute precision; Z
Where: n = sample size; Pexp = expected prevalence; d=desired absolute precision; Z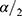 = 1.96 (at 95% confidence level)
= 1.96 (at 95% confidence level)
Which gives 83 cattle to be tested, though, for requisite sample size was three approximately folded and 221 cattle were tested to maximize the sample size for precision.
Study Methodology
Comparative Intradermal Tuberculin Test
CIDT was executed to discriminate between animals infected with Mycobacterium bovis and those reactive to tuberculin due to experience to other mycobacterium or related genera. At the middle of the neck two sites were shaved and cleaned 12 cm apart on the same side of the neck, then the shaved and cleansed areas were examined for the presence of any gross lesions. The skin fold thickness at the two sites were measured by caliper and recorded. Then, each animal was injected 0.1 mL (25,000 IU) avian PPD (Avituber, symbiotic corporation, France) and 0.1 mL (25,000 IU) bovine PPD (Bovituber, symbiotic corporation, France) intradermal using insulin syringe at the anterior and posterior parts respectively.36 Type four hypersensitivity reaction at each site was computed as the change of the skin thickness after 72 hours minus before injection. The interpretation of result was made using diagnostic cut-off points used to define positive test results: an animal was measured positive if the bovine minus the avian tuberculin reaction was > 2 mm based on Ameni et al37 recommendation to Ethiopian zebu breeds and their crosses due to breed susceptibility and environmental effects, rather than OIE who recommend > 4 mm, result of increased sensitivity and without specificity the > 2 mm cut points used to detect bovine tuberculosis by the CIDT. A herd (farm) was considered as positive if it had at least one bovine tuberculin reactor animal.
Questionnaire Survey
A questionnaire survey was conducted to collect information on attitude and awareness of farmers on zoonotic impacts and risk factors linked with the occurrence of bTB. Accordingly, 110 households (28 herdsmen in each kebele) engaged in dairy farming were interviewed with the aid of a structured questionnaire. The questionnaire also comprised herdsmen’s habit and practices of consuming milk or meat and recent history of bovine tuberculosis upon them or in family members.
Data Analysis
A cattle level skin test result was entered into Microsoft® Excel for the Windows 2010. Data analysis was conducted using STATA 16 Statistical Software (STATA Corporation, College Station, TX). Data obtained from the questionnaire were computed by descriptive statistics. A cattle level prevalence was defined as the number of positive reactors per 100 cattle which tested during study period. Moreover, analytic inference was computed using multivariate logistic regression to examine the association of potential risk factors with the prevalence of bTB; strength of association was calculated by odds ratio (OR) and 95% confidence interval (CI). A P-value ≤ 0.05 was revealed to be statistically significant.
Results
Participant Demographics
Based on the response of participants, 56.4% of respondents had urban background while the remaining 43.6% live in rural areas. The majority percentage of participants had no formal education (43.8%). Sixty eight percent (68.2%) of the participants were dairy farmers with a minimum and maximum age of 21 and 70 years. Table 1 is shown the demographics of the participants who were questioned being part of the study.
 |
Table 1 Socio Demographics of Respondents in the Dairy Farms in the Study Districts |
Assessment of Owners’ Awareness on Bovine Tuberculosis
Cattle owners’ knowledge was assessed on the role of various risk factors responsible for the occurrence and spread of bTB amongst cattle and human population using a questionnaire. A total of 110 farm households and their response were shown in Table 2. As it is shown below, 29.7% (35 of 110) of the participants knew that cattle may have bTB, and 13.4% (15 of 110) replied that bTB could be transmitted from animal to human and vice versa. The awareness of the participants relating to cattle infection by mycobacterium bovis and transmission of bTB from cattle to human is described in Table 2. Also, respondents disclosed their view that education can update knowledge on zoonotic bTB.
 |
Table 2 Knowledge of Cattle Owners on bTB and Transmission to Humans in the Study Area |
We discovered that 66.1% of the participants had practice of raw milk consumption, however 33.9% of the participants practice boiling of milk before consumption. Participants also disclosed that they consume soured milk and products without pasteurization or other heat treatment. Consumption habit of respondents is depicted under Tables 3 and 4. Vis-à-vis meat consumption, 74.2% of respondents consumes flesh meat. This study disclosed that habit of fresh milk drinking is considerably inclined by livelihood and literacy of respondents (Tables 3 and 4). However, the habit of consuming fresh meat seemed to be not impacted by the status of education (Table 4).
 |
Table 3 Knowledge and Awareness of Participants on Zoonotic Nature of Bovine Tuberculosis |
 |
Table 4 The Statistical Association Between Practices and Education Level of the Respondents |
Awareness of Participants on Public Health Importance of bTB
A 110 cattle owners or members of households were questioned to assess awareness of participants on public health impact of bTB (Table 3). 29 (26.3%) of the respondents stated that they have knowledge or they have heard about bTB, but only 15 (13.4%) participants became attentive to the zoonotic impacts of bTB (Table 3). Likewise, few respondents 18.2% (20/110) knew the transmission route of bTB to human. However, 39.1% (43/110) respondents knew that tuberculosis can affect cattle. Moreover, of the 110, 31 and 32 believed that raw milk and meat could be vehicle for the transmission of tuberculosis from animal to human, respectively. Nonetheless, they did not know clinical sign of tuberculosis in human.
Association Between Practices and Education Level of the Participants
The practice of participants in association with education status for the transmission of bTB is depicted (Table 4). As indicated in the Table the habit of raw milk drinking was significantly different between education status for bTB transmission (χ2 = 5.03; P = 0.025), but raw meat consumption habit was not significant (χ2 = 3.16; P = 0.075). However, type of cattle to be sold were not affected by the education status of respondents (χ 2 = 3.69; P = 0.158) and by the information source of respondents about bovine tuberculosis (χ 2 = 2.57; P = 0.588).
Association Between Putative Factors and Bovine Tuberculosis Infection
Strength of association between different risk factors and prevalence of bovine tuberculosis was computed using univariable logistic regression (Table 5). Accordingly, bTB occurrence in dairy cattle managed in Chencha were 42% more likely to be diseased than in Arba Minch zuria, Shelle (OR=1.42; 95% CI=0.42–3.48) yet statistically insignificant. The comparative tuberculin skin test in this study has presented that animals become more probable to be infected by bTB as age of the cattle increases. Dairy cattle with age of 4 to 8 eight years were 34% more possible to be diseased with bTB than younger dairy cattle of age less than 4 years (OR=1.34; 95% CI=0.49–3.64). The odds of bovine tuberculosis were higher in crossbred cattle than in indigenous/ local cattle. Thus, even though the association is not statistically significant cross breed dairy cows were 51% more likely to be diseased with M. bovis than indigenous breeds (OR=1.51; 95% CI = 0.56–3.96). Animals with good body condition score were 2.4 times higher tuberculin positive than those with poor body condition (OR=2.40; 95% CI=0.75–7.74), however insignificant.
 |
Table 5 Univariable Logistic Regression Analysis of Tuberculin Reactivity and Risk Factors Among Dairy Cattle in the Farms in Study Area |
Discussion
The present study revealed that bTB is an important disease of dairy cattle in selected districts of Gamo Zone, Southern Ethiopia, with an overall prevalence of 8.2%. This result was comparatively lower than the findings of Alemu et al.6 Firdessa et al.29 Bekele et al38 and Endalew et al,39 who reported an overall prevalence bTB as 13.5%, 30%, 17.5%, and 16.5% respectively in the central part of Ethiopia. A meta-analytic study report by Sibhat et al17 were in agreement with current finding. Likewise, similar studies done by Belete et al16 and Nega et al40 in the Northwest part of Ethiopia showed an overall prevalence of 9.1% and 7.1% respectively. Generally, an overall prevalence of bTB in this study was lower compared to the previous reports done by different authors6,29,38,39 from the central part of the country, where dairy cattle were mainly kept under intensive production system. Such variation between reports might be due to the differences in management system, in which in the present study dairy cattle were kept under extensive and semi-intensive management system which might decreases the risk of transmission between individual animal. Overcrowding and intensification of dairy cattle causes stress and make more suitable condition for the transmissions of M. bovis between animals. This is also related to transmission route, mainly respiratory route, and variation in husbandry practices of the animals that could affect the tuberculin skin test prevalence in a given study.5
The prevalence ratio of bTB found was to be higher in cross than zebu breed dairy cattle both kept under similar management system. Similarly, Ameni et al26 in central parts Ethiopia reported a lower prevalence ratio of bTB infection in grazing local zebu than either exotic or exotic crosses breeds. Based on dairy cattle body condition score (BCS), the prevalence of bTB was found to be significantly lower in dairy cattle with good body condition than poor body conditioned score. However, it is not statistically significant and it contradicts to the previous studies done in different parts of the country5,29,37,41 in which they vindicated that intradermal tuberculin test is highly dependent on animal immune-competence and which in turn also associated with the body condition score of animals such that animals with good body condition, in good plane of nutrition in terms of energy, protein and micronutrients, are immune-competent and thus give a better reaction to CIDT.37 Hence, poor body conditioned animals could be immune-compromised, and may not react to CIDT even if they could have been infected by M. bovis.
The prevalence ratio of bTB was found to be significantly linked with different age groups of study animals. The prevalence was higher in adult and older dairy cattle (34%) than the heifer dairy cattle; even if this is not statistically significant. In close agreement to this, reports done by Ameni et al26 and Rebuma et al29 from central Ethiopia showed increased occurrences of bovine TB with increased age group of grazing dairy cattle. The possible reason for this was noted by Ayele et al15 as due to the influence of gamma delta T cells (γδ T cells), which are abundantly found in the blood stream of young calves shown to have its anti-mycobacterial effect. It has been also suggested that increased occurrence of bTB in older age group animals could be explained by a waning of protective capability in aging animals as confirmed experimentally in the murine system.12,42 Furthermore, it might be also associated with increase probability of encountering M. bovis infection with a longer period of life.29 Such variation in results between different age groups of dairy cattle could also be due to the latent infection of disease to a detectable level. Dissimilarly, very young and older animals infrequently react to tuberculin inoculation regardless of the status of infection of animals; and the level of tuberculin reaction is directly associated with the maturation and wasting of organs of the immune system.16,43
This study revealed that the prevalence ratio of bTB was significantly influenced by the reproductive stage female animals (OR = 1.57, P = 0.004). This result might be because of some of the reproductive stages of dairy cattle especially in late pregnancy and early lactation stages are challenged by stress and which in turn reduces the response of dairy cattle to intradermal tuberculin test. Similar result was reported by Ameni et al5 from central part of Ethiopia. Although it was not statistically significant, the prevalence bTB was 34% more likely in dairy animals with age greater than four years compared to lower aged cattle in this study. Likewise, previous studies in Ethiopia showed 6.2 times higher occurrence of bovine tuberculosis with increased age.39 This could be related as the age group of dairy cattle increases, they become more susceptible to be infected with M. bovis.
Questionnaire survey result revealed the habit of raw meat and milk consumption as 30.3% (30/110) and 50.9% (54/110) respectively which is higher than the previous works done by Ameni et al.5 However, practices of raw milk consumption was lower when we compare with the reports done by Kelly et al11 in pastoralists and small-scale dairy farmers of Cameroon. Also, the raw milk consumption was statically significant (P = 0.025; χ2 = 5.03) among respondents who had different educational status. The habit of raw meat consumption was not statistically significantly associated among different dairy farm workers’ educational status (χ2 = 3.16; P = 0.075). Some of the respondents in this study were attended formal education and hence aware about tuberculosis, particularly bovine origin zoonotic tuberculosis. However, respondents’ awareness on animals to be sold due to disease, impaired productivity or vigour production was not influenced by education level. This was similar with the findings of Ameni et al5 in different districts of central Ethiopia. Generally, regardless of knowledge, raw meat consumption is a well know traditional and cultural practices in Ethiopia.
Conclusions and Recommendations
This study has clearly disclosed that bTB is 8.2% cow level prevalent in the study sites. The assessment of bTB awareness among participants in the study shown 26.3% of respondents knew that cattle could be infected by TB, and 13.4% responded that bTB could be transmitted from animal to human. Likewise, majority of the respondents had practice of consuming raw milk and soured milk products without heat treatment. Also, 91.8% of respondents consume raw meat. Likewise, practices such as habit of fresh milk and meat consumption; dairy herd replacement option; handling of cattle with tuberculosis cases and information source of bTB were significantly affected by literacy of respondents. Therefore, public awareness should be created by mobilization on means of transmission, its risk factors, and managing of tuberculin reactive animals and consumption of risk raw milk and meat by the public. Moreover, movement restriction of reactor animals should be experienced by a regional bureau and state ministry of agriculture to prevent the spread of the disease to new farm.
Abbreviations
bTB, Bovine tuberculosis; CIDT, Comparative Intradermal Tuberculin; M. bovis, Mycobacterium bovis.
Data Sharing Statement
The entire required datum are available and recorded in Excel format and are available in the Supplementary Material).
Ethical Consideration
The research was carried out with regard for animal welfare and ethical approval was obtained from Arba Minch University, Ethical Review Committee (Ref. No.: AMU/AREC/3/ 2014). Also, verbal consent was obtained from the dairy cattle owners for the involvement of their animals in the study. The obtained consent was approved by the Arba Minch University.
Acknowledgments
The authors would like to acknowledge Arba Minch University and the staff members of Chencha and Arba Minch Zuria districts livestock and fishery office for their technical and material support during the study period. The authors also would like to acknowledge cattle owners and animal health professionals in each study district for collaboration and technical support during sample collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This study was conducted without the support of any funding sources or institution.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
1. CSA. Central statistical authority of Ethiopia: report on livestock and livestock characteristics (Private Peasant Holdings). Stat Bulettin. 2020;II:45.
2. Girma S, Mammo A, Bogele K, Sori T, Tadesse F, Jibat T. Study on prevalence of bovine mastitis and its major causative agents in West Harerghe zone, Doba district, Ethiopia. J Vet Med Anim Heal. 2012;4(8):116–123. doi:10.5897/JVMAH12.016
3. Biadglegne F, Tesfaye W, Sack U, Rodloff AC. Tuberculous lymphadenitis in northern Ethiopia: in a public health and microbiological perspectives. PLoS One. 2013;8(12):1–8. doi:10.1371/journal.pone.0081918
4. Narayan B. Tuberculosis in India: a need of public awareness & education. Univ Mauritius Res J. 2015;21:1–27.
5. Ameni G, Tadesse K, Hailu E, et al. Transmission of Mycobacterium tuberculosis between Farmers and Cattle in Central Ethiopia. PLoS One. 2013;8(10):1–10. doi:10.1371/journal.pone.0076891
6. Alemu J, Mamo G, Ameni G, Pal M. Molecular epidemiology of bovine tuberculosis in cattle and its public health implications in gambella town and its surroundings, Gambella Regional State, Ethiopia. Glob J Med Res. 2015;15(5):34.
7. Tschopp RA. Jacobs Journal of Epidemiology and preventive medicine bovine tuberculosis and other Mycobacteria in animals in Ethiopia: a systematic. Jacobs J Epidemiol Prev Med. 2016;2(2):43.
8. Jemal AM. Review on zoonotic importance of bovine tuberculosis and its control. Open Access Libr J. 2016;1–13. doi:10.4236/oalib.1102504
9. Lakew M, Tolosa T, Tigre W. Prevalence and major bacterial causes of bovine mastitis in Asella, South Eastern Ethiopia. Trop Animal Health Prod. 2009;41:1525–1530. doi:10.1007/s11250-009-9343-6
10. Shitaye JE, Tsegaye W, Pavlik I. Bovine tuberculosis infection in animal and human populations in Ethiopia: a review. Vet Med. 2007;52(8):317–332. doi:10.17221/1872-VETMED
11. Kelly RF, Hamman SM, Morgan KL, et al. Knowledge of bovine tuberculosis, cattle husbandry and dairy practices amongst pastoralists and small-scale dairy farmers in Cameroon. PLoS One. 2016:1–20. doi:10.1371/journal.pone.0146538
12. O’Reilly LM, Daborn CJ. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tuber Lung Dis. 1995;76 Suppl 1:1–46. doi:10.1016/0962-8479(95)90591-x
13. Saidu AS, Okolocha EC, Dzikwi AA, et al. Public health implications and risk factors assessment of Mycobacterium bovis infections among abattoir personnel in Bauchi State, Nigeria. J Vet Med. 2015;2015. doi:10.1155/2015/718193
14. Baddeley A, Dean A, Monica Dias H, Falzon D, Figueroa C, Floyd K. Global tuberculosis control: WHO report. France; 2011.
15. Ayele WY, Neill SD, Zinsstag J, Weiss MG, Pavlik I. Bovine tuberculosis: an old disease but a new threat to Africa. Int J Tuberc Lung Dis. 2004;8(8):924–937.
16. Belete A, Tilahun S, Haile B, et al. Prevalence of bovine tuberculosis and distribution of tuberculous lesions in cattle slaughtered at Gondar, Northwest Ethiopia. Infect Ecol Epidemiol. 2021;11(1). doi:10.1080/20008686.2021.1986919
17. Sibhat B, Asmare K, Demissie K, Ayelet G, Mamo G, Ameni G. Bovine tuberculosis in Ethiopia: a systematic review and meta-analysis. Prev Vet Med. 2017;147(September):149–157. doi:10.1016/j.prevetmed.2017.09.006
18. Thoen CO, Lobue PA, Enarson DA, Kaneene JB, de Kantor IN. Tuberculosis: a re-emerging disease in animals and humans. Vet Ital. 2009;45(1):135–181.
19. Chambers MA. Review of the diagnosis and study of tuberculosis in non-bovine wildlife species using immunological methods. Transbound Emerg Dis. 2009;56(6–7):215–227. doi:10.1111/j.1865-1682.2009.01076.x
20. Kidane D, Olobo JO, Habte A, et al. Identification of the causative organism of tuberculous lymphadenitis in Ethiopia by PCR. J Clin Microbiol. 2002;40(11):4230–4234. doi:10.1128/JCM.40.11.4230-4234.2002
21. World Health Organization. Zoonotic tuberculosis (Mycobacterium bovis): memorandum from a WHO meeting (with the participation of FAO)*. Bul World Heal Organ. 1994;72(6):851–857.
22. Elemo KK, Bedada BA, Kebeda T. Prevalence, risk factors and major bacterial causes of bovine. Glob J Sci Front Res. 2018;18(4):34.
23. Getabalew M, Dairy H, Alemneh T. Status of artificial insemination; Its constraints and estrous synchronization in Ethiopia. J Reprod Health Infert. 2020;4:e34.
24. Mulshet Y, Derso S, Nigus A. Prevalence of bovine subclinical mastitis and associated risk factors In Addis Ababa, Central Ethiopia. Online J Anim Feed Res. 2017;7(5):124–133.
25. Temesgen B, Shigut MM, Hailemariam TB, Chali E. Assessment of community awareness towards zoonotic tuberculosis in West Shoa, Ethiopia. Clin Med Res. 2017;6(2):37–42. doi:10.11648/j.cmr.20170602.12
26. Ameni G, Vordermeier M, Firdessa R, et al. Mycobacterium tuberculosis infection in grazing cattle in central Ethiopia. Vet J. 2011;188(3):359–361. doi:10.1016/j.tvjl.2010.05.005
27. Romha G, Gebre G, Ameni G. Assessment of bovine tuberculosis and its risk factors in cattle and humans, at and around Dilla town, southern Ethiopia. Anim Vet Sci. 2014;2(4):94–100. doi:10.11648/j.avs.20140204.12
28. Nuru A, Mamo G, Zewude A, Mulat Y, Yitayew G, Admasu A. Preliminary investigation of the transmission of tuberculosis between farmers and their cattle in smallholder farms in northwestern Ethiopia: a cross ‑ sectional study. BMC Res Notes. 2017;1–7. doi:10.1186/s13104-016-2349-z
29. Firdessa R, Tschopp R, Wubete A, et al. High prevalence of bovine tuberculosis in dairy cattle in central Ethiopia: implications for the dairy industry and public health. PLoS One. 2012;7(12):e52851. doi:10.1371/journal.pone.0052851
30. Tschopp R, Schelling E, Hattendorf J, Aseffa A, Zinsstag J. Risk factors of bovine tuberculosis in cattle in rural livestock production systems of Ethiopia. Prev Vet Med. 2009;89:205–211. doi:10.1016/j.prevetmed.2009.02.006
31. Berg S, Firdessa R, Habtamu M, et al. The burden of mycobacterial disease in Ethiopian cattle: implications for public health. PLoS One. 2009;4(4). doi:10.1371/journal.pone.0005068
32. Regassa A, Tassew A, Amenu K, et al. A cross-sectional study on bovine tuberculosis in Hawassa town and its surroundings, Southern Ethiopia. Trop Anim Health Prod. 2010;42(5):915–920. doi:10.1007/s11250-009-9507-4
33. Biffa D, Skjerve E, Oloya J, et al. Research article Molecular characterization of Mycobacterium bovis isolates from Ethiopian cattle. BMC Vet Res. 2010;6(1):2–11. doi:10.1186/1746-6148-6-28
34. Tora E, June T. Epidemiological Investigation of Calf Morbidity and Mortality and Associated Risk Factors in Milk-Shed Districts of Gamo Zone, Southern Ethiopia [Msc Thesis].
35. Thrusfield M, William Saville WT. Veterinary Epidemiology. University. (Blackwell Science Ltd, 9600 Garsington Road, Oxford OX4 2DQ U, ed.). Oxford OX4 2DQ. Blackwell; 2546.
36. OIE. Bovine tuberculosis. In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. OIE Terrestrial Manual; 2018.
37. Ameni G, Hewinson G, Aseffa A, Young D, Vordermeier,M. Appraisal of interpretation criteria for the comparative intradermal tuberculin test for diagnosis of tuberculosis in cattle in central Ethiopia appraisal of interpretation criteria for the comparative intradermal tuberculin test for diagnosis of tuberculosis. Clin Vaccine Immunol. 2008;15(8):1272–1276. doi:10.1128/CVI.00114-08
38. Meseret B, Gezahegn M, Samueal Mulat GA. Epidemiology of bovine tuberculosis and its public health significance in debre- zeit intensive dairy farms, Ethiopia. Biomed Nurs. 2016;2(2):8–18. doi:10.7537/marsbnj02021603
39. Endalew MA, Chimdi BD. Bovine tuberculosis prevalence, potential risk factors and its public health implication in selected state dairy farms, Central Ethiopia. World’s Vet J. 2017;6(1):21–29. doi:10.5455/wvj.20170290
40. Nega M, Mazengia H, Mekonen G. Prevalence and zoonotic implications of bovine tuberculosis in Northwest Ethiopia. Int J Med Med Sci. 2012;2(9):188–192.
41. Tigre W, Alemayehu G, Abetu T, Ameni G. Preliminary study on the epidemiology of bovine tuberculosis in Jimma town and its surroundings, South-western Ethiopia. Afr J Microbiol Res. 2012;6(11):2591–2597. doi:10.5897/AJMR11.553
42. McDaniel CJ, Cardwell DM, Moeller RB, Gray GC. Humans and cattle: a review of bovine zoonoses. Vector-Borne Zoonotic Dis. 2014;14(1):1–19. doi:10.1089/vbz.2012.1164
43. Yilma Z, Guernbableich E, Sebsibe A. A review of the Ethiopian dairy sector. Addis Ababa; 2011.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
