Back to Journals » OncoTargets and Therapy » Volume 13
PTHLH Predicts the Prognosis of Patients with Oral Leukoplakia
Authors Lv Z, Cong R, Li J, Cao K, Bao Q, Li L, Yang F, Yuan J
Received 4 May 2020
Accepted for publication 21 September 2020
Published 8 October 2020 Volume 2020:13 Pages 10013—10023
DOI https://doi.org/10.2147/OTT.S261124
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjay Singh
Zhongjing Lv,1,2,* Rong Cong,1,* Jiafeng Li,1,2 Kun Cao,1,2 Qiang Bao,1,2 Linlin Li,1,3 Feng Yang,1,2 Jian Yuan1,2
1School of Stomatology, Xuzhou Medical University, Xuzhou City, Jiangsu Province, Mainland China; 2Department of Stomatology, Affiliated Hospital of Xuzhou Medical University, Xuzhou City, Jiangsu Province, Mainland China; 3Department of Pathology, Affiliated Hospital of Xuzhou Medical University, Xuzhou City, Jiangsu Province, Mainland China
*These authors contributed equally to this work
Correspondence: Jian Yuan; Feng Yang
Department of Stomatology, Affiliated Hospital of Xuzhou Medical University, Xuzhou City, Jiangsu Province 221000, Mainland China
Email [email protected] ; [email protected]
Background: Oral leukoplakia is the most common oral mucosal disease. A proportion of such cases can progress to oral squamous cell carcinoma (OSCC). The mechanism of oral leukoplakia malignant transformation is still unclear. In this study, we analyzed the expression of parathyroid hormone-like hormone (PTHLH) in oral leukoplakia and the effect on prognosis, so as to find reliable molecular markers that can predict oral leukoplakia malignant transformation.
Methods: We measured PTHrP which is coded by PTHLH in oral leukoplakia tissues of 79 cases (30 cases progressed to OSCC and 49 did not) and analyzed the clinical outcomes. Then, PTHLH expression was reduced using lentivirus-mediated small hairpin RNA (shRNA) interference to determine the biological role of PTHLH in DOK cells.
Results: PTHrP was found to be highly expressed in 38% of tissues of oral leukoplakia. There was weak or no PTHrP expression in 25 patients, moderate expression in 24 patients, and strong in 30 patients with oral leukoplakia. The expression level was associated with the degree of atypical hyperplasia and poor prognosis. The cell proliferation, invasion, migration, cell cloning, and cell cycle were affected after reducing PTHLH expression.
Conclusion: Our data suggest that either PTHLH or PTHrP plays a key role in the malignant transformation of oral leukoplakia and might be a reliable biomarker for predicting the carcinogenesis of oral leukoplakia.
Keywords: PTHLH, oral leukoplakia, malignant transformation
Introduction
Oral leukoplakia, also classified as an oral mucosal disease, is a precancerous lesion, which refers to a white or grayish-white keratotic disorder that cannot be characterized as any other specified disease, either clinically or histologically.1,2 Patients with oral leukoplakia do not generally have any symptoms, although some might experience an uncomfortable feeling of pain when eating a stimulating food.3 The pathogenesis of oral leukoplakia is still not clear. Long-term smoking, tooth friction, Candida albicans infection, alcohol consumption, etc. are considered high-risk factors for oral leukoplakia.4–7 As a result, nonspecific treatments are used to cure the disease.
The purpose of treating oral leukoplakia is to prevent transformation. According to literatures, 1~47% of oral leukoplakia cases have a malignant transformation potential due to different clinical features and medical history.8,9 Defining the degree of atypical hyperplasia of epithelial cells is still the current method to predict a malignant transformation;10 however, a morphological assessment of the atypical hyperplastic cells presents a certain amount of subjectivity. Therefore, there is no specific or objective method of monitoring to predict the prognosis of oral leukoplakia. Finding objective biomarkers that are better than histopathological observations to predict the prognosis of oral leukoplakia is our major challenge. To some extent, this can prevent the malignant transformation of oral leukoplakia and explain the mechanism of carcinogenesis more accurately.
Oral leukoplakia can gradually progress into oral squamous cell carcinoma (OSCC), which is considered to be a multistep and complex process. In recent years, more and more genes have been involved in the development of OSCC, which might be a molecular target for cancer therapy.11–13 Due to close relation of gene imbalance to oral cancer, salivary biomarkers become more important in the diagnosis and prognosis of oral cancer.14 For example, salivary IL-8, IL-6 and TNF-α were recognized as potential diagnostic biomarkers for oral cancer.15 Parathyroid hormone-like hormone (PTHLH), that encodes for parathyroid hormone-related protein (PTHrP) secreted by few normal tissues and many tumor cells in an autocrine or paracrine manner, is found to be upregulated in many tumors, including OSCC.16–18 In our previous study,19 PTHLH mRNA and PTHrP protein were found to be upregulated in OSCC with an effect on the biological functions of OSCC cells, suggesting that PTHLH plays an important role and might be an oncogene in OSCC carcinogenesis. In this study, the PTHLH gene was further analyzed to discover genes critical to malignant transformation of oral leukoplakia and to provide an objective basis for predicting its prognosis.
Patients and Methods
Patients and Specimens
In this study, samples were obtained from 79 patients who were diagnosed with oral leukoplakia and had not undergone any treatment between 2010 and 2018. All samples, obtained by surgical incisional biopsy, with complete follow-up information were available from the Department of Stomatology, Affiliated Hospital of Xuzhou Medical University. Then, patients who underwent any previous treatments such as drugs, laser or traditional Chinese medicine and so on were ruled out. Full data of 79 patients including basic information and clinicopathological data were obtained to analyze the correlation between PTHrP levels and clinicopathological parameters. Samples were embedded and then stained with hematoxylin and eosin to analyze the pathological diagnosis, the degree of keratosis and atypical hyperplasia. The pathological diagnosis and atypical hyperplasia were confirmed by two experienced pathologists. Each of the patients involved signed a written informed consent form, and the work was approved by the Medical Ethics Committee of the Affiliated Hospital at Xuzhou Medical University.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue was cut into 4 μm sections. The procedure was performed according to the instructions in the ABC kit; the tissue sections were deparaffinized in xylene and rehydrated in graded ethanol, and then were boiled and treated with citrate buffer for 15 minutes for antigen retrieval. Tissue sections were incubated with rabbit polyclonal PTHrP antibody (1:150; Bioworld, USA) at 4°C overnight, followed by incubation with a biotinylated secondary antibody for half an hour at room temperature, and finally stained with diaminobenzidine (DAB) (Dako, Denmark). The staining index was divided into scores of 0, 1, 2, and 3 according to the staining intensity. In this study, the percentage of positive epithelial cells expressed as 0–100% was analyzed by two experienced pathologists. The total score was calculated by multiplying the staining intensity index by the percentage of positive epithelial cells. Then, the scores were ranked from low to high and divided into three groups to determine the weak, moderate, and strong expression of PTHrP.
Cell Culture
Two oral leukoplakia cell lines, including DOK and Leuk-1 cells (purchased from Shanghai Baili Biotechnology Co., Ltd), were cultured in KSF medium (GIBCO-BRL) with 25 µg/mL BPE and 0.2 ng/mL recombinant epidermal growth factor (rEGF) (Invitrogen, USA). All cells were cultured in a 5% CO2, 37°C cell incubator.
ShRNA Knockdown
Three PTHLH-targeted shRNAs and negative control shRNA CON077 (named psc4504-1) were purchased from Shanghai Genechem Co., Ltd. The PTHLH- targeted shRNA sequences were 5′-ACGATTCTTCCTTCACCAT-3′ for (psc15995-1), 5′- AGATACCTAACTCAGGAAA-3′ for (psc15996-1), and 5′- AAGATTTACGGCGACGATT-3′ for (psc15997-1). The negative control shRNA sequence was 5′-TTCTCCGAACGTGTCACGT-3′ which shows no homologous sequencing to human genes. For more convenience, we marked them as KD1, KD2, and KD3, respectively; also, the negative control psc4504-1 was named as NC group. All transfection experiments were performed according to the manufacturer’s protocols. The protocols were as follows: the two abovementioned cells were inoculated into a six-well plate containing 3 mL culture solution. Subculture was carried out when the cell density reached 80% in order to maintain a good growth state. 6~10×104 cells were cultured in a six-well plate containing 2 mL culture solution, exchanged 1mL of culture solution and then an appropriate amount of virus (MOI values were 10 and 20, respectively, for DOK and Leuk-1 cells) was added when the cell density reached 20%. Then, conventional culture medium replaced the above culture medium when the cells were infected for 16 hours and the next experiments were carried out 72 hours after cell infection.
Western Blot
Cells were washed with PBS buffer and harvested in an SDS lysis buffer (Beyotime, China); then, the proteins were boiled and the concentrations were analyzed to ensure consistent protein quality, and the total protein lysate was separated by SDS-PAGE. The target protein was then transferred to a PVDF membrane and blocked with 1% non-fat milk for an hour at room temperature, then incubated with primary antibodies against PTHrP (rabbit, Proteintech) and GAPDH (mouse, Santa Cruz) for two hours at room temperature or 4°C overnight, and secondary antibodies labeled with IRDyeTM 800. In this study, PVDF membrane was washed using phosphate-buffered saline (PBS) buffer after each step. Finally, an Odyssey Infrared Imaging System (Rockland, USA) was used to detect specific immunoreactivity.
RNA Extraction
Cells were washed using PBS buffer after 72 hours infection, the total RNA was extracted from cells using Trizol reagent (Invitrogen, USA), and the major procedures included cell lysis, iodoform extraction, ethanol purification and RNA dissolution. The specific steps were as follows: about 5×106 cells were harvested and lysed for ten minutes by 1 mL cold TRIzol reagent, and then 200 μL iodoform reagent was added upon to react for 10 minutes. The supernatant was collected after centrifuge for 15 minutes, and then an equal volume of isopropanol was added to precipitate RNA and centrifuge for 15 minutes, followed by 1 mL of 75% ethanol to purify RNA and centrifuge for 5 minutes. Finally, RNA was precipitated in the centrifuge tube after discarding the supernatant and it was then dissolved by 30 μL DEPC water. Then, finally RNA converted to cDNA using the SuperScript reverse transcriptase reagent kit (Takara, Japan).
Real-Time PCR
Real-time PCR amplification systems include cDNA 1 μL, SYBR Premix E x Tap 20 μL, ROX Reference Dye 0.4 μL, forward primers 0.4 μL, reverse primers 0.4 μL, and sterile distilled water 7.8 μL. Real-time PCR was performed according to the SYBR Premix E×Tap DNA polymerase kit instructions (Takara, Japan). And then the ABI 7300 real-time PCR system (ABI, USA) was used to complete the PCR amplification. The results of PCR were analyzed using the 2−ΔΔCt method, where ΔCt indicated the difference between the target gene Ct values and GAPDH Ct values, and ΔΔCt indicated the difference between the ΔCt of the experimental and control groups.
Primer Sequences for PTHLH
The forward primer was 5′-AAGTCCATCCAAGATTTACGG-3′, and the reverse primer was 5′-TCCACCTTGTTAGTTTCCTGAG-3′.
Primer Sequences for GAPDH
The forward primer was 5′-TGACTTCAACAGCGACACCCA-3′, and the reverse primer was 5′-CACCCTGTTGCTGTAGCCAAA-3′.
Cell Proliferation Assay
The cell viability of the transfected cells was measured every 24 h for five days using a Cell-Counting Kit-8 (CCK-8) (Dojindo, Japan). 1 × 103 cells/well cultured in 150 μL culture medium were transferred to triplicate wells of a 96-well cell culture plate after transfection with shRNA for 72 hours. The cells were cultured in a 5% CO2, 37°C cell incubator, and then the growth curve was measured from the second day and for five consecutive days. The specific methods were as follows: 10 mL CCK-8 reagent was added to each dish and continued to culture the above cells for 2 hours. Two hours later, the optical densities (ODs) value was read at 450 nm according to the CCK-8 kit instruction and the OD values were analyzed between the experimental and control groups. In this study, each experiment was independently repeated three times.
Plate Colony Formation Assay
1 × 103 transfected cells were cultured in a 2.4 cm dish containing 6 mL medium for two weeks. The cell colonies which included more than 50 cells were washed with PBS buffer and fixed with formalin for 30 minutes, then stained with crystal violet solution, and washed with double distilled water. The number was counted according to the size and density of the colonies. Each experiment was independently repeated three times. In this study, all cells were cultured in a 5% CO2, 37°C cell incubator.
Cell Cycle Analysis
5× 105 cells were cultured in 6 cm dish overnight in KSF medium without rEGF and BPE after transfection with shRNA for 72 hours, and were then cultured in KSF medium with rEGF and BPE for 24 hours and the cell density reached about 70~80%, the cells were washed with PBS buffer and collected in centrifuge after digestion with the cell number not less than 106 cell/tube, then fixed in 70% ethanol overnight at 4°C. The cells were then washed with PBS and stained with PI/RNase Staining Buffer (BD Pharmingen) for 30 minutes. Cell cycle analysis was performed using flow cytometry. In this study, all cells were cultured in a 5% CO2, 37°C cell incubator.
Cell Migration Array in vitro
A 24-well Millicell Hanging Cell Culture Insert (Millipore Corporation, USA) was used. 1 × 105 cells contained in 500 μL of KSF media without rEGF and BPE were cultured in the upper chambers, and 750 μL of KSF media with rEGF and BPE was placed in the lower chambers. After 24 hours of incubation in a 5% CO2, 37°C cell incubator. The cells were fixed with 4% formaldehyde and stained with Giemsa solution. The cells that did not migrate in the upper chamber were wiped off, and then migratory cells from each field were photographed, and at least five random microscopic fields (200×) were counted. All experiments were repeated three times.
Cell Invasion Array in vitro
Matrigel (BD Biosciences) and KSF were mixed, and the mixture was plated into the 24-well Millicell Hanging Cell Culture Insert. After rehydration, 1 × 105 transfected cells were cultured in 500 μL KSF media without rEGF and BPE in the upper chambers, and 750 μL KSF media with rEGF and BPE was placed in the lower chambers. After 24 hours of culture in a 5% CO2, 37°C cell incubator, the upper cells were removed with a cotton swab and fixed with 4% formaldehyde stained with Giemsa solution. Invasion cells from each field were photographed, and at least five random microscopic fields (200×) were counted. All experiments were repeated three times.
Statistical Analysis
A Student’s t-test was used to compare the difference between the two groups; one-way analysis of variance was used in the comparison between more groups. Event-free survival (EFS) was the outcome variable. The Log rank test was used to analyze the EFS of patients with weak, moderate, or strong PTHrP expression. Univariate and multivariate Cox proportional hazards regression model analyses were used to analyze various parameters associated with the prognosis of patients with oral leukoplakia. In this study, all analyses were conducted using SPSS 13.0 software and all analyses were two-tailed tests, where P < 0.05 was considered statistically significant.
Results
Patients with Oral Leukoplakia and PTHrP Expression
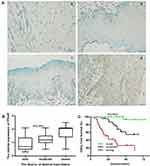
In this study, 79 patients with oral leukoplakia were recruited, and the follow-up time ranged from 5 to 75 months after diagnosis. Of these patients, 30 (38%) later developed OSCC within five years, whereas 49 (62%) remained OSCC-free during the follow-up interval. PTHrP is mainly expressed in epithelial cell nuclei. Among the 79 patients, PTHrP expression in oral leukoplakia tissues ranged from negative to strongly positive, and it was mainly expressed in about 68.4% (moderate and strong) of patients. It was found that 30 samples showed strong PTHrP staining, 24 showed moderate PTHrP staining, and 25 revealed weak PTHrP staining. PTHrP expression was strongly associated with the degree of dysplasia (Figure 1A and B); meanwhile, the expression was independent of dysplasia after analyzing the correlation between PTHrP expression and clinical parameters such as medical history, pathology, location, smoking, alcohol, etc. (Table 1).
 |
Table 1 Univariate and Multivariate Cox Proportional Hazards Regression Models for Estimating the Prognosis of Oral Leukoplakia |
PTHrP Expression and OSCC Development
To determine the role of PTHrP expression in oral leukoplakia malignant transformation, we further analyzed the relationship between PTHrP levels and the prognosis of oral leukoplakia. We found that the PTHrP expression level was strongly associated with oral leukoplakia that had progressed to OSCC (Figure 1C). From a total of 30 patients developed OSCC during the follow-up period, 66.7% (20/30) of patients had a high PTHrP expression, 30% (9/30) of patients had a moderate PTHrP expression, and only 3.3% (1/30) of patients showed a weak PTHrP expression. In the univariate analysis, in addition to dysplasia, PTHrP expression was also significantly associated with OSCC development. In the multivariate analysis, PTHrP expression and dysplasia presented higher risks for oral leukoplakia malignant transformation (Table 1).
PTHLH Affects DOK Cell Function
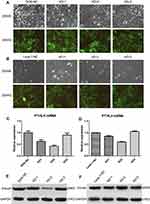
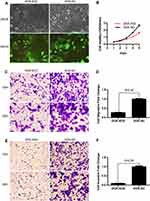
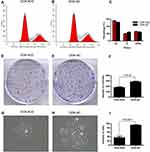
To further explore the potential role of PTHLH in oral leukoplakia, lentivirus-mediated shRNA interference technique was targeted to downregulate PTHLH expression in DOK and Leuk-1 cells, which are premalignant cells of oral mucosa. In this study, three types of PTHLH-shRNA, named KD1, KD2, and KD3, were analyzed, and PTHLH expression was downregulated (Figure 2A and B). We found that PTHLH levels could be reduced by 50% using shRNA-KD2 in DOK cell (Figure 2C and E, Supplementary Figure S1), and we found the interference efficiency of DOK cells was better than that of Leuk-1 cells (Figure 2D and F, Supplementary Figure S1). Then, we used shRNA-KD2 (recorded as KD2) to analyze the potential PTHLH function in DOK cells. Cell proliferation (Figure 3A and B), cell migration (Figure 3C and D), and invasion (Figure 3E and F) were decreased with the downregulation of PTHLH. Cell cycle (Figure 4A–C) and cell colonies (Figure 4D–I) were also significantly decreased after reducing PTHLH expression in DOK cells. These data suggest that PTHLH may play a role in promoting the malignant transformation of oral leukoplakia.
Discussion
Oral leukoplakia is considered a serious risk to human health as it can progress into OSCC. Clinically, it has been found that some lesions disappear after surgical treatment or medical therapy whereas others progress to OSCC within several years of treatment. Certain risk factors such as long-term smoking, age and sex (young women being more at risk), or long-term friction can make oral leukoplakia more susceptible to malignant transformation. Although smoking induces changes in salivary PH and inflammatory biomarker levels associated with oral diseases and it was found that sodium bicarbonate mouth rinse is effective in decreasing IL-1β and increasing salivary PH favorable for prevention of oral diseases among smokers.20 And the occurrence of oral leukoplakia is closely related to smoking according to the literature. In this study, we did not find that smoking was associated with the malignant transformation of oral leukoplakia. The mechanism of oral leukoplakia malignant transformation has long been a focus of investigation for oral oncologists. In recent years, major breakthroughs have been made in the molecular research of oral leukoplakia, and many genes have been found to be dysregulated. For example, CCND1, EZH2, Ki67, IL-6, Snail, and Axin2 have been found to be upregulated in oral leukoplakia.4,8,21–24 Furthermore, a copy number variation frequently occurs at 3p, 9p, and 13q loci during progressive dysplasia of oral leukoplakia,25 as oral leukoplakia represents an uncontrollable inflammatory lesion. To some extent, molecular mechanism research can reveal the uncontrolled inflammation mechanism of OSCC. This will play an important guiding role in the treatment of oral leukoplakia. It can also help to prevent malignant transformation of oral leukoplakia.
PTHLH and its encoded protein PTHrP were reported to be upregulated in many cancers, including lung cancer,26 breast cancer,27–29 prostate cancer,30–32 and gastroesophageal carcinoma,33 with their expression level closely related to the prognosis of these tumors. In addition, our previous study revealed that either PTHLH or PTHrP was elevated in OSCC,19 which means that the PTHLH gene plays a stimulating role in carcinogenesis and is a potential oncogene. PTHrP is an autocrine or paracrine protein, which can be secreted by many tumor cells. Tang et al found that PTHrP secretion was upregulated upon the reintroduction of PTHLH in the intrahepatic cholangiocarcinoma cell lines which down regulated PTHLH expression,34 so we thought that downregulation of PTHLH can lead to the reduction of intracellular PTHrP as well as secreted PTHrP. In the current study, we found that PTHrP was upregulated in the majority of oral leukoplakia tissues, of which 54 showed moderate to high expression. We also found that oral leukoplakia with high PTHrP expression was more likely to progress into OSCC when compared with tissues with low PTHrP expression. This means that oral leukoplakia with higher PTHrP expression has a poor prognosis. Combined with our previous research revealing PTHrP high expression in most OSCC,19 it can be concluded that PTHLH or PTHrP expression may gradually increase from normal oral mucosa, precancerous lesions, to malignancy in order. This largely explains the molecular mechanisms involved in carcinogenic uncontrolled inflammation. In univariate and multivariate analyses, we found that, in addition to the atypical hyperplasia, PTHLH or PTHrP expression can be used as an independent risk factor for assessing the prognosis of oral leukoplakia.
The results of the current study revealed that PTHrP level and degree of atypical hyperplasia are the two major prognostic factors of oral leukoplakia, and also the expression of PTHrP is closely related to the degree of atypical hyperplasia. But it has its own advantages. For example, 4/30 cases of mild dysplasia developed into OSCC, as did 12/27 cases of moderate dysplasia and 14/22 cases of severe dysplasia. However, during 45 months of follow-up, only 1/25 cases with weak PTHrP expression progressed into OSCC, as did 9/24 cases with moderate PTHrP expression and 20/30 cases with high PTHrP expression. Therefore, it is obvious that, relative to pathological atypical hyperplasia, it is preferable to predict the malignant transformation of oral leukoplakia based on PTHrP expression. In addition, as the pathology of atypical hyperplasia relates to the change in cytomorphology, it is feasible to objectively and quantitatively analyze the prognosis of oral leukoplakia based on gene expression. Therefore, in addition to the pathological features, PTHrP expression is also a good choice for us to determine the prognosis of leukoplakia.
In this study, DOK cells were used as a biological medium to further confirm the role of PTHLH in oral leukoplakia. We found that this can slow down the cell growth, affect the cell cycle, and participate in cell migration and invasion after downregulating PTHLH expression. In combination with our clinical studies, it is not difficult to speculate that the PTHLH gene plays a positive role in the transformation of oral leukoplakia into OSCC. As in our previous study,19 we found that PTHLH affects the cell cycle of OSCC cell lines because it can cause the down-regulation of Cyclin D1 and CDK4 as well as up-regulation of P21. Therefore, we also considered that PTHLH may also affect the expression of cyclin associated proteins and thus affecting cell function in oral leukoplakia in indirect or direct manners.
Limitation
This study was based on only a small sample analysis of the Chinese population; hence, it has certain limitations. However, the study opens up new ways of estimating the prognosis of leukoplakia.
Conclusion
In this study, we can conclude that either PTHLH or PTHrP is highly expressed in a portion of oral leukoplakia, indicating that PTHLH might be involved in the pathogenesis of oral leukoplakia. There is a correlation between PTHrP level and the atypical hyperplasia of oral leukoplakia. Furthermore, the leukoplakia with high expression of PTHLH or PTHrP is more prone to malignant transformation. It was also found that PTHLH or PTHrP is prior to judge the prognosis of oral leukoplakia compared with atypical hyperplasia according to the prognosis analysis.
Moreover, cell functions including the proliferation ability, migration ability, invasion ability, cell cycle and plate colony formation ability were obviously reduced after down-regulating the PTHLH expression in oral leukoplakia cell line, indicating PTHLH involvement in regulating the biological functions of oral leukoplakia cells. To some extent, this can explain the reason of high PTHLH or PTHrP expression in oral leukoplakia that was prone to malignant transformation. So it is not difficult for us to come to a conclusion that PTHLH or PTHrP can be used as an independent biomarker to predict the prognosis of oral leukoplakia.
Acknowledgment
This study was supported by the Natural Science Foundation of Jiangsu Province (Grant Number: BK20160225).
Author Details
Zhongjing Lv (Email: [email protected]). Rong Cong ([email protected]). Jiafeng Li ([email protected]). Kun Cao ([email protected]). Qiang Bao ([email protected]). Linlin Li ([email protected]). Feng Yang ([email protected]). Jian Yuan ([email protected]).
Disclosure
The authors declare no competing interests.
References
1. Lee JJ, Hong WK, Hittelman WN, et al. Predicting cancer development in oral leukoplakia: ten years of translational research. Clin Cancer Res. 2000;6(5):1702–1710.
2. Silverman S
3. Ha PK, Pilkington TA, Westra WH, Sciubba J, Sidransky D, Califano JA. Progression of microsatellite instability from premalignant lesions to tumors of the head and neck. Int J Cancer. 2002;102(6):615–617. doi:10.1002/ijc.10748
4. Kujan O, Huang G, Ravindran A, Vijayan M, Farah CS. CDK4, CDK6, cyclin D1 and Notch1 immunocytochemical expression of oral brush liquid-based cytology for the diagnosis of oral leukoplakia and oral cancer. J Oral Pathol Med. 2019;48(7):566–573. doi:10.1111/jop.12902
5. Gonzalez Segura I, Secchi D, Carrica A, et al. Exfoliative cytology as a tool for monitoring pre-malignant and malignant lesions based on combined stains and morphometry a) techniques. J Oral Pathol Med. 2015;44(3):178–184. doi:10.1111/jop.12219
6. Silva AD, Maraschin BJ, Laureano NK, et al. Expression of E-cadherin and involucrin in leukoplakia and oral cancer: an immunocytochemical and immunohistochemical study. Braz Oral Res. 2017;31:e19.
7. Acha A, Ruesga MT, Rodríguez MJ, Martínez de Pancorbo MA, Aguirre JM. Applications of the oral scraped (exfoliative) cytology in oral cancer and precancer. Med Oral Patol Oral Cir Bucal. 2005;10(2):95–102.
8. Cao W, Younis RH, Li J, et al. EZH2 promotes malignant phenotypes and is a predictor of oral cancer development in patients with oral leukoplakia. Cancer Prev Res (Phila). 2011;4(11):1816–1824. doi:10.1158/1940-6207.CAPR-11-0130
9. Lima CF, Crastechini E, Issa JS, Balducci I, Cabral LA, Almeida JD. Evaluation of apoptotic pathway in oral mucosa by smoking in a Brazilian outpatient smoking cessation program. Int J Cardiol. 2015;184:514–516. doi:10.1016/j.ijcard.2015.02.103
10. Schaaij-Visser TB, Bremmer JF, Braakhuis BJ, et al. Evaluation of cornulin, keratin 4, keratin 13 expression and grade of dysplasia for predicting malignant progression of oral leukoplakia. Oral Oncol. 2010;46(2):123–127. doi:10.1016/j.oraloncology.2009.11.012
11. Fan HX, Li HX, Chen D, Gao ZX, Zheng JH. Changes in the expression of MMP2, MMP9, and ColIV in stromal cells in oral squamous tongue cell carcinoma: relationships and prognostic implications. J Exp Clin Cancer Res. 2012;31:90. doi:10.1186/1756-9966-31-90
12. Wu X, Cao W, Wang X, et al. TGM3, a candidate tumor suppressor gene, contributes to human head and neck cancer. Mol Cancer. 2013;12(1):151. doi:10.1186/1476-4598-12-151
13. Yan B, Li H, Yang X, et al. Unraveling regulatory programs for NF-kappaB, p53 and microRNAs in head and neck squamous cell carcinoma. PLoS One. 2013;8(9):e73656. doi:10.1371/journal.pone.0073656
14. Khurshid Z, Zafar MS, Khan RS, Najeeb S, Slowey PD, Rehman IU. Role of salivary biomarkers in oral cancer detection. Adv Clin Chem. 2018;86:23–70.
15. Sahibzada HA, Khurshid Z, Khan RS, et al. Salivary IL-8,IL-6 and TNF-α as potential diagnostic biomarkers for oral cancer. Diagnostics (Bases). 2017;7(2):21.
16. Park SI, McCauley LK. Nuclear localization of parathyroid hormone-related peptide confers resistance to anoikis in prostate cancer cells. Endocr Relat Cancer. 2012;19(3):243–254. doi:10.1530/ERC-11-0278
17. Ono N, Nakashima K, Schipani E, et al. Constitutively active PTH/PTHrP receptor specifically expressed in osteoblasts enhances bone formation induced by bone marrow ablation. J Cell Physiol. 2012;227(2):408–415. doi:10.1002/jcp.22986
18. Hoshi S, Morimoto T, Saito H, et al. PTHrP and PTH/PTHrP receptor expressions in human endometrium. Endocr J. 2001;48(2):219–225. doi:10.1507/endocrj.48.219
19. Lv Z, Wu X, Cao W, et al. Parathyroid hormone-related protein serves as a prognostic indicator in oral squamous cell carcinoma. J Exp Clin Cancer Res. 2014;33:100. doi:10.1186/s13046-014-0100-y
20. Hamza SA, Wahid A, Afzal N, et al. Effect of sodium bicarbonate mouth wash on salivary ph and interleukin-1β levels among smokers. Eur J Dent. 2020;14(2):260–267. doi:10.1055/s-0040-1709896
21. Brennan M, Migliorati CA, Lockhart PB, et al. Management of oral epithelial dysplasia: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(Suppl):
22. Nasser W, Flechtenmacher C, Holzinger D, Hofele C, Bosch FX. Aberrant expression of p53, p16INK4a and Ki-67 as basic biomarker for malignant progression of oral leukoplakias. J Oral Pathol Med. 2011;40(8):629–635. doi:10.1111/j.1600-0714.2011.01026.x
23. Panneer Selvam N, Sadsksharam J. Salivary interleukin-6 in the detection of oral cancer and precancer. Asia Pac J Clin Oncol. 2015;11(3):236–241. doi:10.1111/ajco.12330
24. Zhang X, Kim KY, Zheng Z, Kim HS, Cha IH, Yook J. Snail and axin2 expression predict the malignant transformation of oral leukoplakia. Oral Oncol. 2017;73:48–55. doi:10.1016/j.oraloncology.2017.08.004
25. Kil TJ, Kim HS, Kim HJ, Nam W, Cha I-H. Genetic abnormalities in oral leukoplakia and oral cancer progression. Asian Pac J Cancer Prev. 2016;17(6):3001–3006.
26. Moseley JM, Kubota M, Diefenbach-Jagger H, et al. Parathyroid hormone-related protein purified from a human lung cancer cell line. Proc Natl Acad Sci U S A. 1987;84:5048–5052. doi:10.1073/pnas.84.14.5048
27. Ghoussaini M, Fletcher O, Michailidou K, et al. Genome-wide association analysis identifies three new breast cancer susceptibility loci. Nat Genet. 2012;44(3):312–318.
28. Linforth R, Anderson N, Hoey R, et al. Coexpression of parathyroid hormone related protein and its receptor in early breast cancer predicts poor patient survival. Clin Cancer Res. 2002;8:3172–3177.
29. Lindemann RK, Ballschmieter P, Nordheim A, Dittmer J. Transforming growth factor beta regulates parathyroid hormone-related protein expression in MDA-MB-231 breast cancer cells through a novel Smad/Ets synergism. J Biol Chem. 2001;276:46661–46670. doi:10.1074/jbc.M105816200
30. Bryden AA, Hoyland JA, Freemont AJ, Clarke NW, George NJ. Parathyroid hormone related peptide and receptor expression in paired primary prostate cancer and bone metastases. Br J Cancer. 2002;86(3):322–325. doi:10.1038/sj.bjc.6600115
31. Dougherty KM, Blomme EA, Koh AJ, et al. Parathyroid hormone-related protein as a growth regulator of prostate carcinoma. Cancer Res. 1999;59:6015–6022.
32. Iddon J, Bundred NJ, Hoyland J, et al. Expression of parathyroid hormone-related protein and its receptor in bone metastases from prostate cancer. J Pathol. 2000;191:170–174. doi:10.1002/(SICI)1096-9896(200006)191:2<170::AID-PATH620>3.0.CO;2-H
33. Deans C, Wiqmore S, Paterson-Brown S, Black J, Ross J, Fearon KC. Serum parathyroid hormone-related peptide is associated with systemic inflammation and adverse prognosis in gastroesophageal carcinoma. Cancer. 2005;103:1810–1818. doi:10.1002/cncr.20972
34. Tang J, Liao Y, He S, et al. Autocrine parathyroid hormone-like hormone promotes intrahepatic cholangiocarcinoma cell proliferation via increased ERK/JNK-ATF2-cyclinD1 signaling. J Transl Med. 2017;15:238.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.