Back to Journals » Cancer Management and Research » Volume 13
Psychosocial Burden and Quality of Life of Lung Cancer Patients: Results of the EORTC QLQ-C30/QLQ-LC29 Questionnaire and Hornheide Screening Instrument
Authors Koch M, Gräfenstein L, Karnosky J, Schulz C, Koller M
Received 5 April 2021
Accepted for publication 3 July 2021
Published 7 August 2021 Volume 2021:13 Pages 6191—6197
DOI https://doi.org/10.2147/CMAR.S314310
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Myriam Koch,1 Laura Gräfenstein,2,3 Julia Karnosky,4 Christian Schulz,1 Michael Koller2
1Department of Internal Medicine 2, University Hospital Regensburg, Regensburg, Germany; 2Center for Clinical Studies University Hospital Regensburg, Regensburg, Germany; 3Hospital Wörth an der Donau, Germany; 4Department of Surgery, University Hospital Regensburg, Regensburg, Germany
Correspondence: Myriam Koch
Klinik und Poliklinik für Innere Medizin II, Universitätsklinikum Regensburg, Franz-Josef-Strauß Allee 11, Regensburg, D-93053, Germany
Tel +49 941 9440
Email [email protected]
Background: Overall survival is the ultimate criterion for the therapy of lung cancer, but psychosocial care, which helps the patient to cope with the disease, becomes a more and more important issue in the treatment of this life-threatening disease.
Methods: We report the satellite project within a prospective, international, cross-cultural, multicenter study to validate the EORTC QLQ-LC29, a new designed module to assess the quality of life of lung cancer patients. The participants filled in the EORTC QLQ-C30, the recently updated lung cancer module QLQ-LC29 and the Hornheide questionnaire (HSI).
Results: A total of 81 patients (32 female and 49 male, mean age 65.2 years, SD = 9.7) were enrolled in this study by completing the questionnaires. Fatigue (mean 55.4, SD = 26.3) and dyspnea (mean 46.3, SD = 36.2) were the most prominent symptoms. Thirty-nine patients (48.1%) according to the HSI needed psychosocial support. When using the EORTC questionnaires as screening instrument with 50 as cut-off in contrast only 29.5% of our patients needed psychosocial support. The need for psychosocial support according to the HSI correlated most with the EORTC scales “fatigue” (38.3% overlap between the two questionnaires), “existential fear” (38.3% overlap between the two questionnaires) and worse “global quality of life” (27.2% overlap between the two questionnaires).
Conclusion: If psychosocial distress is at the core, the HSI is a suitable instrument for quick screening. The EORTC measures help to specify impaired quality of life areas and also cover somatic symptoms that are specific for cancer patients. Once psychosocial distress has been ascertained, clinicians should be particularly aware of specific problems regarding “fatigue”, “existential fear” and diminished “global quality of life”.
Trial Registration: clinicaltrials.gov, reference number NCT02745691. Registered 20 April 2016.
Keywords: lung cancer, quality of life, psychosocial burden, EORTC QLQ-C30/QLQ-LC29 questionnaire, Hornheide screening instrument
Introduction
Lung cancer, one of the most common cancers worldwide, is the leading cause of cancer-associated death.1–4 Although overall survival is the ultimate criterion for an oncological therapy, psychosocial care, which helps the patient to cope with the disease, becomes a more and more important issue in the treatment of this life threatening disease.5–7
Approximately 25% of all cancer patients suffer from psychosocial distress.8 Distress is defined as “a multifactorial, unpleasant experience of a psychologic, social, spiritual, and/or physical nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment” by the NCCN Guidelines for Distress Management.9 A large epidemiological study found that lung cancer patients report more depression and anxiety than other cancer patients.10 In the course of a cancer disease, many burdensome events may occur, such as, initial diagnosis or detection of tumor progress.8,11 Psychosocial intervention, if done early enough, can help to reduce this distress.7,8 Therefore, early detection is very important.8,12 Due to high workload of physicians treating critically ill patients, issues of psychosocial distress are often not sufficiently addressed,6,8,12,13 which may be detrimental to their quality of life.7,11,12,14,15
The Hornheide Screening Instrument (HSI) is a standardized tool to measure psychosocial burden very quickly in daily routine.16 To assess broader areas of quality of life the EORTC (European Organization for Research and Treatment of Cancer) QLQ-C3017–19 together with a specific module for lung cancer patients is a suitable instrument.20,21
The goal of the present project was, to administer the Hornheide Screening Instrument (HSI) and the EORTC QLQ-C30 plus QLQ-LC29 in a sample of lung cancer patients and determine their level of psychosocial distress and areas of impaired quality of life. Furthermore, we were interested to analyse consistencies and possible differences between the HSI and the EORTC measures.
Method
Study Design
The current report is based on a satellite project within a prospective, international, cross-cultural, multicenter study to validate the EORTC QLQ-LC29, a new designed module to assess the quality of life of lung cancer patients.22
Patients were stratified according to their primary therapy (surgery, radiochemotherapy or targeted therapy) and time frame (during or shortly after therapy) in order to pick up side effects related to the therapy when assessing HRQL. The study recruitment for this satellite project took place from April 2016 to April 2017 in the University Hospital Regensburg and the hospital Barmherzige Brüder in Regensburg.
This study was conducted in accordance with the Declaration of Helsinki. The Phase 4 study protocol was registered with clinicaltrials.gov (reference number NCT02745691). Approval from the Ethical Committee of the University Regensburg was obtained (reference number 16-101-0059).
Patients
The following eligibility criteria applied: histologically proven non-small cell lung cancer (NCSLC) or small cell lung cancer (SCLC), 18 years of age or older, no previous other or recurrent tumor, ability to fill in a questionnaire and written informed consent. Patients were excluded from the study if any of the above criteria was not fulfilled.
Procedure
Upon being informed about the study and providing written consent, patients filled in the paper-and-pencil version of the EORTC QLQ-C30, the recently updated lung cancer module QLQ-LC29 and the Hornheide questionnaire.
Questionnaires
The EORTC QLQ-C30 (version 3.0) is a core questionnaire designed for the use in international clinical trials and addresses issues relevant for cancer patients of any tumor type. As its name suggests, the questionnaire consists of 30 individual items that are aggregated into five multi-item function scales (social, role, physical, cognitive and emotional functioning), three multi-item symptom scales (nausea, pain, fatigue), five single items (diarrhea, constipation, dyspnea, appetite loss, insomnia) and one two-item scale to assess global quality of life. Items are accompanied by four-item Likert scale with the response options labeled (1) “not at all”, (2) “a little”, (3) “quite a bit” and (4) “very much”. The two global quality of life items are to be completed using a seven-item Likert scale (1=very bad to 7=very good). According to the EORTC scoring manual, all scores are subjected to linear transformation and are presented on scales ranging from 0 to 100. In the case of functional scores 0 denotes lowest and 100 highest functioning, in the case of symptom scales 0 denotes lowest and 100 highest symptom burden.23
The updated EORTC lung cancer module consists of 29 items.20 According to the calling results of the Phase 3 study, it consists of five multi-item symptom scales (coughing, shortness of breath, side effects, fear of progression, surgery-related symptoms) and five single item scales (coughing blood, pain in the chest, pain in the shoulder, bodily pain, problems with weight loss).20
To the discern between acceptable and impaired quality of life for any of the EORTC scales, we first scored all scales in uniform manner so that 0 represents worst and 100 represents best quality of life. We then used the 50-score point criterion to dichotomise each scale: 0 to 49 (impaired) versus 50 to 100 (acceptable).24,25 The category “surgical symptoms” was excluded from analyses due to the small number of patients who completed this subscale.
The Hornheide Screening Instrument (HSI) is a well-established screening instrument to revise the psychological support needs of tumor patients. It consists of 7 items: global health condition, global mental condition, burden, person of trust, burdened family member, temporary internal disturbance and information about the disease and treatment. The single items are aggregated into a summary score ranging from 0 to 14. The cut-off is set at 4 score points, with scores ≥4 points indicating need for psychosocial support.16
Statistical Analyses
Basic descriptive statistics included counts, percentages, median/interquartile range (IQR), means/standard deviations (SD). Crosstabs Chi2-tests were performed to detect overlapping between the EORTC scales and the HSI. Although some QoL scales are known to be skewed, the research field uses by convention means/standard deviations to report these data, as well as parametric Pearson correlations to express associations.26–28 In the present analyses, we follow this convention.
p < 0.5 was used as threshold of statistical significance. All calculations were done in an explanatory manner and, therefore, no adjustments for multiplicity were made.
All analyses were performed using the software packages SPSS Statistics 23.0 (IBM Corporation, Armonk, NY, USA) and SAS 9.4 (SAS Institute, Cary NC).
Results
Patient Characteristics
A total of 81 patients (32 female and 49 male) were enrolled (Table 1). Mean age was 65.2 years. Most of the patients had advanced disease (NSCLC stage IV n=32, 39.5%). Non-small cell lung cancer was the predominant histological type (NSCLC 74% vs SCLC 26%). Primary treatment at the time of questionnaire completion was either chemotherapy (n=34), surgery (n=12), targeted therapy (n=11), immune therapy (n=10), radio-chemotherapy (n=7) or sole radiotherapy (n=7).
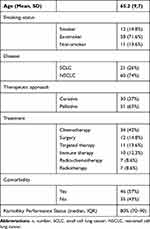 |
Table 1 Baseline Characteristics (n=81) |
QLQ-C30 and QLQ-LC29 Questionnaires
Table 2 depicts the results of the QLQ-C30. Fatigue (mean 55.4, SD= 26.3) and dyspnea (mean 46.3, SD= 36.2) were the most prominent symptoms. Table 2 shows also the results of the QLQ-LC29. Existential fear (mean 66.0, SD= 32.7) and shortness of breath (mean 39.0, SD= 26.9) were the most prominent symptoms.
 |
Table 2 EORTC QLQ-C30 and QLQ-LC29 Results (n=81 Patients) |
Hornheide Screening Instrument
Thirty nine patients (48.1%) have a total of 4 or more points in the HSI, indicating need for psychosocial support. HSI is marginally correlated with Karnofsky Performance Status (r = −0.210, p = 0.060). There are no correlations between HSI and comorbidity, chemotherapy, therapeutic approach (curative/palliative) or the application of chemotherapy, all p-values >0.050.
Associations Between EORTC Scales and Hornheide Screening Instrument
Table 3 depicts the correlation between the EORTC QLQ-C30 and the Hornheide screening instrument. The most prominent correlation in terms of psychosocial support is found in “emotional function” (−0.591) and “global quality of life” (−0.495).
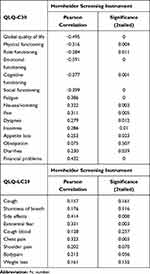 |
Table 3 Correlation Between EORTC QLQL-C30/QLQ-LC29 and the Hornheide Screening Instrument (n=81 Patients) |
Table 3 shows also the correlation between the EORTC QLQ-LC29 and the Hornheide screening instrument. The most prominent correlation between LC-29 and the HSI is found particularly in “side effects” (0.414) and “existential fear” (0.331).
To determine possible overlaps between the quality of life scales and the Hornheide screening instrument, cross tabulations were performed.
Figure 1 compares the need for psychosocial help from the HSI with the need for psychosocial help from the EORTC C30/LC-29 subscales. When using the EORTC questionnaires as screening instrument with 50 as cut-off in contrast only 29.5% of our patients needed psychosocial support. Patients in the present study, who needed psychosocial help according to the HSI, felt distinctive “existential fear” and “fatigue” (overlap 38.3% for both). The category “quality of life” showed an overlap of 27.2%.
Discussion
Health-related quality of life and psychosocial burden of tumor patients have been a subject in many analyses. Based on the notion that need for psychosocial support correlates with a worse quality of life of lung cancer patients, we started the present study.
One of the most notable findings of the present study was that almost half of our participants (48.1%) need psychosocial support according to the HSI. By and large, this is consistent with a number of other findings in the literature. In one large-scale study using the HSI in 455 patients with various types of cancer, 41.8% of these patients indicated need for psychosocial support.12 According to the guideline for psychooncological diagnosis, advice and treatment for adult cancer patients of the German Society of Cancer in contrast high distress is found in 59% of all cancer patients.29 In a study on 98 newly diagnosed lung cancer patients, 51% of the patients reported considerable distress, using the Distress Thermometer and the Edmonton Symptom Assessment Scale as screening tools.30
When using the EORTC questionnaires as screening instrument with 50 as cut-off across a variety of different quality of life areas, on average only 29.5% of our patients showed impaired quality of life. One explanation for this finding could be that the HSI is solely focused on psychosocial distress whereas the EORTC questionnaires cover wider areas of quality of life. Another issue is the cut-off points chosen that may discriminate between different groups of patients within the study sample.
Another notable finding of the present study was that need for psychosocial support according to the HSI correlated most with the EORTC scales “fatigue”, “existential fear” and worse “global quality of life”. In a study on 208 tumor patients undergoing radiooncological treatment, the need for psychosocial support was significantly associated with higher fatigue scores.7 This is an important finding, since cancer-related fatigue, the most frequent reported symptom of lung cancer patients, is increasingly being recognized as an unmet need of cancer patients.31,32 Cancer-related fatigue can occur at any time, at diagnosis, during treatment or even after treatment and reduces quality of life itself.33,34
A life-threatening disease results in the feeling of “existential fear” in many of these cancer patients. And patients who feel “existential fear” need more social support to cope with their disease.29 According to this assumption, our study shows that need for social support according to the HSI correlated most not only with the EORTC scales “fatigue” but also with “existential fear”. But it has to be mentioned that the statistical correlation showed only a mild association.
It is very important to put attention on psychosocial impairment. In a study on 450 patients with cerebral tumors, who were admitted for elective cranial neurosurgical procedure, psychooncological distress resulted in a reduced quality of life.35 Thus, psychosocial support is an important part of tumor treatment.6 But patients are not always willing to report about their psychological distress themselves, so questionnaires, like the Hornheide Screening instrument, are a good instrument to measure it.13 In an investigation on 333 lung cancer patients 61.6% of the patients reported significantly high distress, but only 22.5% of the patients requested help.36
One limitation of the study relates to the sampling procedure that, according to ethical requirements, relied on the voluntary participation of patients. Experience suggests that patients with advanced disease stages are less likely to be willing to complete questionnaires. Furthermore, these patients are also more difficult to approach because of their intense treatment schedules. Hence, the present sample may be biased toward a better performance status. Another limitation of the study is the small sample size.
Conclusion
If psychological distress is at the core, the HSI is a suitable instrument for quick screening in a clinical setting. The EORTC instruments help to specify impaired quality of life areas and also cover somatic symptoms that are specific for cancer patients. Once psychosocial distress has been ascertained, clinicians should be particularly aware of specific problems regarding “fatigue”, “existential fear” and diminished “global quality of life”.
Abbreviations
EORTC, European Organization for Research and Treatment of Cancer; HSI, Hornheide Screening Instrument; N, number; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; SD, standard deviation.
Data Sharing Statement
Authors will respond to data sharing requests under the premise that an adequate research question is formulated. Original anonymized data will be made available up to one year after the publication of the paper. Readers may contact the corresponding author [email protected].
Disclosure
Prof. Dr. Michael Koller reports a grant from EORTC to conduct the study (Reference number: Koller Lung 03/2016). The authors report no other conflicts of interest in this work.
References
1. Ulas A, Tokluoglu S, Kos M, et al. Lung cancer in women, a different disease: survival differences by sex in Turkey. Asian Pacific J Cancer Preven. 2015;16(2):815–822.
2. Kim HR, Kim SY, Kim CH, et al. Sex-specific incidence of EGFR mutation and its association with age and obesity in lung adenocarcinomas: a retrospective analysis. J Cancer Res Clin Oncol. 2017;143(11):2283–2290. doi:10.1007/s00432-017-2473-8
3. Rauma V, Salo J, Sintonen H, Rasanen J, Ilonen I. Patient features predicting long-term survival and health-related quality of life after radical surgery for non-small cell lung cancer. Thoracic Cancer. 2016;7(3):333–339. doi:10.1111/1759-7714.12333
4. van Montfort E, de Vries J, Arts R, Aerts JG, Kloover JS, Traa MJ. The relation between psychological profiles and quality of life in patients with lung cancer. Support Care Cancer. 2019;28(3):1359–1367.
5. Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22(4):714–724. doi:10.1200/JCO.2004.06.078
6. Strittmatter G, Tilkorn M, Mawick R. How to identify patients in need of psychological intervention. Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 2002;160:353–361.
7. Brix C, Schleussner C, Fuller J, Roehrig B, Wendt TG, Strauss B. The need for psychosocial support and its determinants in a sample of patients undergoing radiooncological treatment of cancer. J Psychosom Res. 2008;65(6):541–548. doi:10.1016/j.jpsychores.2008.05.010
8. Keller M, Sommerfeldt S, Fischer C, et al. Recognition of distress and psychiatric morbidity in cancer patients: a multi-method approach. Ann Oncol. 2004;15(8):1243–1249. doi:10.1093/annonc/mdh318
9. Riba MB, Donovan KA, Andersen B, et al. Distress management, version 3.2019, NCCN clinical practice guidelines in oncology. JNCCN. 2019;17(10):1229–1249.
10. Vodermaier A, Linden W, MacKenzie R, Greig D, Marshall C. Disease stage predicts post-diagnosis anxiety and depression only in some types of cancer. Br J Cancer. 2011;105(12):1814–1817. doi:10.1038/bjc.2011.503
11. Riedl D, Gastl R, Gamper E, et al. Cancer patients’ wish for psychological support during outpatient radiation therapy: findings from a psychooncological monitoring program in clinical routine. Strahlentherapie und Onkologie. 2018;194(7):655–663.
12. de Zwaan M, Mosch P, Sinzinger H, et al. [The association between the need for psychosocial support, patients’ desire for psychosocial support and received psychosocial interventions in cancer patients]. Neuropsychiatrie. 2012;26(4):152–158. German.
13. Ford S, Lewis S, Fallowfield L. Psychological morbidity in newly referred patients with cancer. J Psychosom Res. 1995;39(2):193–202. doi:10.1016/0022-3999(94)00103-C
14. Larsson M, Ljung L, Johansson BB. Health-related quality of life in advanced non-small cell lung cancer: correlates and comparisons to normative data. Eur J Cancer Care (Engl). 2012;21(5):642–649.
15. Wang B, Hao N, Zhang X. Factors influencing the psychology and quality of life in lung cancer patients. Saudi Med J. 2017;38(9):948–951. doi:10.15537/smj.2017.9.18532
16. Herschbach JW. Screeningverfahren in der Psychoonkologie, Testinstumente zur Identifikation betreuungsbedürftiger Krebspatienten. Eine Empfehlung der PSO für die psychoonkologische Behandlungspraxis. 2010.
17. Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur J Cancer (Oxford, England: 1990). 2001;37(11):1345–1351. doi:10.1016/S0959-8049(00)00447-0
18. Waldmann A, Schubert D, Katalinic A, Janda M. Normative data of the EORTC QLQ-C30 for the German population: a population-based survey. PLoS One. 2013;8(9):e74149. doi:10.1371/journal.pone.0074149
19. Damm K, Roeske N, Jacob C. Health-related quality of life questionnaires in lung cancer trials: a systematic literature review. Health Econ Rev. 2013;3(1):15. doi:10.1186/2191-1991-3-15
20. Koller M, Hjermstad MJ, Tomaszewski KA, et al. An international study to revise the EORTC questionnaire for assessing quality of life in lung cancer patients. Ann Oncol. 2017;28(11):2874–2881.
21. Koller M, Warncke S, Hjermstad MJ, et al. Use of the lung cancer-specific Quality of Life Questionnaire EORTC QLQ-LC13 in clinical trials: a systematic review of the literature 20 years after its development. Cancer. 2015;121(24):4300–4323.
22. Koller M, Shamieh O, Hjermstad MJ, et al. Psychometric properties of the updated EORTC module for assessing quality of life in patients with lung cancer (QLQ-LC29): an international, observational field study. Lancet Oncol. 2020;21:723–732. doi:10.1016/S1470-2045(20)30093-0
23. Fayers PM, Bjordal K, Groenvold M, Curran D, Bottomley A; on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 Scoring Manual.
24. Koller M, Lorenz W. Quality of life: a deconstruction for clinicians. J R Soc Med. 2002;95(10):481–488. doi:10.1177/014107680209501002
25. Klinkhammer-Schalke M, Koller M, Steinger B, et al. Direct improvement of quality of life using a tailored quality of life diagnosis and therapy pathway: randomised trial in 200 women with breast cancer. Br J Cancer. 2012;106(5):826–838. doi:10.1038/bjc.2012.4
26. Johnson C, Aaronson N, Blazeby JM, et al. Guidelines for Developing Questionnaire Modules. Brussels: EORTC; 2011.
27. Fayers PM, Machin D. Quality of Life: The Assessment, Analysis and Reporting of Patient-Reported Outcomes.
28. Scott NW, Aaronson NK, Bottomley A, et al. Sprangers MAG on behalf of the EORTC Quality of Life Group. EORTC QLQ-C30 Reference Values. Brussels: EORTC; 2008.
29. Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF). Psychoonkologische Diagnostik, Beratung und Behandlung von erwachsenen Krebspatienten, Langversion 1.1, 2014, AWMF-Registernummer: 032/051OL, http://leitlinienprogramm-onkologie.de/Leitlinien.7.0.html.
30. Steinberg T, Roseman M, Kasymjanova G, et al. Prevalence of emotional distress in newly diagnosed lung cancer patients. Support Care Cancer. 2009;17(12):1493–1497. doi:10.1007/s00520-009-0614-6
31. Madsen UR, Groenvold M, Petersen MA, Johnsen AT. Comparing three different approaches to the measurement of needs concerning fatigue in patients with advanced cancer. Qual Life Res. 2015;24(9):2231–2238. doi:10.1007/s11136-015-0962-2
32. Carnio S, Di Stefano RF, Novello S. Fatigue in lung cancer patients: symptom burden and management of challenges. Lung Cancer (Auckland, NZ). 2016;7:73–82.
33. Horneber MFI, Dimeo F, Rüffer JU, Weis J. Cancer-related fatique: epidemiology, pathogensis, diagnosis, and treatment. Deutsches Ärzteblatt. 2012;109(9):161–172.
34. Fischer I, Riedner C, Bojko P, et al. Consultation program for patients with cancer-related fatigue: a systematic evaluation of the experiences of the Bavarian Cancer Society. Oncol Res Treat. 2016;39(10):646–651. doi:10.1159/000448907
35. Hoffmann K, Kamp M, Steiger HJ, Sabel M, Rapp M. Correlation of psychooncological distress-screening and quality of life assessment in neurosurgical patients. Oncotarget. 2017;8(67):111396–111404. doi:10.18632/oncotarget.22802
36. Graves KD, Arnold SM, Love CL, Kirsh KL, Moore PG, Passik SD. Distress screening in a multidisciplinary lung cancer clinic: prevalence and predictors of clinically significant distress. Lung Cancer (Amsterdam, Netherlands). 2007;55(2):215–224. doi:10.1016/j.lungcan.2006.10.001
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

