Back to Journals » Psychology Research and Behavior Management » Volume 14
Psychometric Properties of the Beck Depression Inventory (BDI-II) in Cancer Patients: Cancer Patients from Butaro Ambulatory Cancer Center, Rwanda
Authors Biracyaza E , Habimana S, Rusengamihigo D
Received 24 February 2021
Accepted for publication 14 May 2021
Published 2 June 2021 Volume 2021:14 Pages 665—674
DOI https://doi.org/10.2147/PRBM.S306530
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Igor Elman
Emmanuel Biracyaza,1– 3 Samuel Habimana,1,2 Donat Rusengamihigo4
1Department of Community Health, School of Public Health, University of Rwanda, Kigali, Rwanda; 2Rwanda Resilience and Grounding Organization (RRGO), Kigali, Rwanda; 3Programme of Sociotherapy, Prison Fellowship Rwanda (PFR), Kigali, Rwanda; 4Department of Clinical Psychology, University of Picardy Jules Verne, Amiens, France
Correspondence: Emmanuel Biracyaza Tel +250 785686886
Email [email protected]
Background: The prevalence of depressive disorders remains high in patients with cancer and their diagnosis and treatment remain an epidemiologic concern. Without proper screening and diagnosis, the necessary care and follow-up would not be possible for these patients who need potential support to increase their quality of mental health. Hence, the screening tools for depression are prominent in diagnosing this mental health disorder; however, there are few studies conducted for assessing psychometric properties of Beck Depression Inventory (BDI-II) amongst the cancer patients from developing countries. We, therefore, determined psychometric properties of the BDI-II among cancer patients from Butaro Ambulatory Cancer Center (BACC).
Methods: Cross-sectional study design was conducted among 425 cancer patients from the BACC, Rwanda. Confirmatory and exploratory factor (CFA) analyses were performed to compare the fit indices of three-factor and two-factor models of the BDI-II. The eligible participants were administered the BDI-II instrument.
Results: Average scores of depression was 16.3 (SD=9.8). Results showed an adequate consistency (Cronbach’s α=0.904) and high correlations with the subscales of this instrument. Our findings showed that the area under the curve of the receiver operating characteristics analysis of BDI-II was 0.805. Our CFA results revealed that three-factor model (χ2=1699.921, p< 0.001) represented a better model fit than the two-factor model of BDI-II (χ2=2115.397, p< 0.001). In addition, the goodness of fit indices were tested and showed that the Beck’s three-factor model had a better performance than the two-factor model. Kaiser–Meyer–Olin (KMO) measure of 0.916 demonstrated that the factor structure or sampling was adequate for analysis and the Bartlett’s test of sphericity was highly significant (χ2=3780, p< 0.001) and this revealed that the items of BDI-II were significantly correlated and sufficiently large.
Conclusion: BDI-II presented a good reliability and validity that represent adequate psychometric properties. Its sensitivity and specificity were suitable. This psychometric measure is important in diagnosing and treating depression in cancer patients.
Keywords: depression, cancer patient, psychometric, validity, consistency
Introduction
Depressive disorder is mental disorder that highly occurs among the cancer patients worldwide.1,2 Prior studies established that the prevalence of depression is high and varies between 4% and 58%.3 The research documented that low sociodemographic characteristics, utilization of substances or medical drugs, history of depression, community and family perceptions towards the cancer patients, family characteristics, cancer stigma, and satisfaction of the services from health-care providers are the factors contributing to depressive disorders among cancer patients.4,5 Major depressive disorder (MDD) is one of the common mental disorders characterized by depressed mood or loss, loss of interest, loss of sexual interest, sleeping disturbance, hopelessness, and suicidal ideation, lack of concentration, low self-esteem, and loss of appetite in their daily activities.6,7 The World Health Organization (WHO) stated that MDD is highly prevalent 4.4% (322 million people) and it remains one of important risk factors of morbidity and mortality particularly in developing countries where the screening instruments for psychiatric disorders such as depression remain less utilized.8,9 This psychiatric issue was documented as one of the global burden that leads to disabilities not only in cancer patients but also in general population.10
Moreover, prior studies indicated that in the cancer patients, multiple factors might influence the diagnostic process of depressive disorders. For instance; the cancer patients often represent various signs and symptomatology compromising loss of weight, fatigue, physical pain, loss of interest, lack of concentration, reduction of appetite, sleeping disturbance, and loss of interest in sexual activity that contribute to the severity of depression among these patients.3,11 Preceding studies indicated that physical pain and symptoms of depression may partly share common pathways of psychological distress which make it difficult to differentiate cause and effects. The physical symptoms mostly increase the risk of depressed feelings among cancer patients and in contrast depressed feelings significantly result in more physical complaints among cancer patients.12 Earlier studies established that it is so difficult for both cancer patients and health-care providers or physician that they could develop emotional disturbances that need to be addressed for promoting the quality of life for cancer patients and emotional state for the health-care provider of the cancer patient. These researchers have recently published that only minority (17%) of cancer patients explicitly expresses their emotional distress to their health-care provider or physician. This indicated that they do not often address their emotional and behavioral problems in their communication with cancer patients, possibly because they do not feel well trained in communicating with cancer patients especially those who are provided health-care interventions in the palliative care.13,14
BDI-II is one the psychometric instrument widely used to screen depression with the purpose to diagnose and treat depression effectively.15,16 This psychometric tool from western countries, BDI-II, was previously translated into many languages worldwide. In Rwanda, prior researchers validated the BDI-II and documented that it has good psychometric properties to be used in the Rwanda sample for assessing the severity of depression. Although this screening tool was applied less in medical settings specifically in cancer patients, preceding researchers translated it into the mother tongue of Rwandans, Kinyarwanda version.17,18 In a preceding comprehensive review of psychometric properties of BDI-II using more than 118 studies conducted in more than 60,126 study participants worldwide, we found that BDI-II that has been used for a long time as a cost-effective psychometric instrument used to assess severity of depressive disorders. Thus, this screening tool is widely applicable not only in the research domain, but also in clinical settings.19 Recent research has found that screening depression remains health concern; however, many of prior studies have documented different findings of depression and its treatment.20,21 Previous studies indicated that a short screening method is appealing because the costs for screening such disorders would be considerably lower than other methods for diagnosing and treating depressive disorders in the clinical settings; therefore, the utilization of short screening is very important and it is time efficient and many people can be screened for depression in a quick and simple way with efficiently and effectively.22,23 Erstwhile studies conveyed that BDI-II was the gold standard to diagnose depression due its better sensitivity and specificity.24–27 The literature described that BDI-II has several items on somatic symptoms of depression (such as items about loss of energy, fatigue, and loss of appetite), which may cause an overestimation of positive cases in patient groups with somatic illnesses.2,28
Although the BDI-II was initially standardized for reflecting and monitoring the severity of depressive disorder over the course of diseases and arioso therapies for promoting health of the patient,7 it was agreed that BDI-II is an effective psychometric instrument that has standard cutoff scores to categorize depressive disorders. In different studies, the cutoff of depression was documented and indicated the range of variability in different populations.29,30 The research gap is that there is no study about psychometric properties in cancer patients using BDI-II in Rwanda. Although we found that the BDI-II was previously validated in the general population and genocide survivors of Rwanda especially in the aftermath of genocide,31,32 very little is known about this screening tool among cancer patients, as the utilization psychometric tools remain a concern in diagnosing and treating psychiatric disorders in developing countries and in Rwanda.33 It is our interest to conduct this study for investigating the usefulness of BDI-II as a severity assessment instrument. We also assessed the psychometric properties mainly reliability, validity and factor structures and appropriateness of BDI-II among cancer patients from Butaro Cancer Centre of Excellence (BCCOE), Rwanda.
Methods
Study Design and Participants
An observation cross-sectional study design based on quantitative approach was carried out among 425 cancer patients aged 18 years and above. All participants were consecutively selected among those who were seeking medical and psychological interventions as well as the participants from the palliative care department. All participants who took part in this study were recruited from the center of cancer patients located in Burera District Hospital. The authors of this study were blinded to medical charts of cancer patients who represented psychiatric conditions. Then, they conducted the diagnostic interviews using sociodemographic and psychometric instruments. The study included the patients who were fluent in the mother tongue, Kinyarwanda, patients who were able to respond to the research questions and those who agreed to take part in this study.
Settings
The study setting was Butaro Cancer Centre of Excellence (BCCOE) which is an ambitious collaboration between the Ministry of Health, Rwanda (MoH) and PIH. BCCOE is a national center of oncology located in Butaro District Hospital built in the Northern Province, Rwanda. This national referral cancer center has various specialists and health-care providers to offer a spectrum of diagnostic oncology and treatments services, including chemotherapy, surgery, a pathology laboratory, counseling, and palliative care.34,35 The center is designed to facilitate patient and staff flows, and comfortably accommodate patients and their attendants during extensive treatment regimens. This center also helps the government of Rwanda through the MoH to develop cancer centers in other referral hospitals in rural areas. It is also the primordial center that partners with the MoH to develop clinical protocols and standard operating procedures for cancer care that are applicable to the low-income settings of the country and contributes to storing electronic medical records systems (OpenMRS) for increasing clinical workflow, quality of care and ability to conduct research. Due to its capacity to provide health interventions to cancer patients, this BCCOE is the national cancer referral center from 2012. In addition, the current cancer center provides home and psychosocial support for patients and families traveling long distances for treatment at the BCCOE.36
Procedures
This study was conducted in accordance with the Declaration of Helsinki.37 Concerning the ethics statement, this study used prior ethical approval obtained from the Institutional Review Board, College of Medicine and Health Sciences (IRB/CMHS) at the University of Rwanda with the reference number (ref. no:136/UR-CMHS/SPH/2028). Confidentiality was ensured and all data were anonymously collected from the participants. Confidentiality and voluntariness of the patients were ensured. The researchers of this study were the investigators of this approved study. To conduct this study, they were provided with the authorization to access the dataset by their colleagues.
Sample Size
Prevalence of depression among the population of Rwanda having one or more chronic diseases is 40%.37 The formula (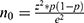 ) for precision of proportion with 95%CIs (1.96) is expected to be about 0.4 and margin error of 5% (0.05) was used to find the sample size.
) for precision of proportion with 95%CIs (1.96) is expected to be about 0.4 and margin error of 5% (0.05) was used to find the sample size. 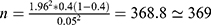 . The parameters such as
. The parameters such as 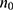 stands for the minimal sample size, “n” for final sample size. Based on the above calculation, the sample size was found to be 369, but because of the nonavailability of some participants during conduction of the study, the extra 20% was added to the sample size to increase the sample size and the final sample size was 442.
stands for the minimal sample size, “n” for final sample size. Based on the above calculation, the sample size was found to be 369, but because of the nonavailability of some participants during conduction of the study, the extra 20% was added to the sample size to increase the sample size and the final sample size was 442.
Materials
Beck Depression Inventory (BDI-II)
BDI-II is the psychometric instrument that has 21 items classified into the four major domains namely, emotional, cognitive, motivational, and physiological domains that are used to assess severity of depression symptomatology.38,39 Each of the items of BDI-II is scored using 4-point Likert scale ranging from 0–3. In this screening tool the total score varies between 0 and 63. Using the total scores of BDI-II, prior studies classified that individuals with 0–13 scores represent minimal depression, 14–19 scores represent mild depression, 20–28 scores indicate moderate depression and 29–63 scores for severe depression or MDD.15,27 Prior studies indicated that that consistent with the BDI-II the cancer patients selected one of four statements for each item that best described how they felt during the last two weeks before the research. The cancer patients with higher scores indicated depressive symptoms are more severe.40 In addition to that, the items of the BDI-II relate to different symptoms of depression such as sadness, hopelessness, self-blame, guilt, fatigue, and loss of appetite. On each item, patients are asked to choose the statement that best describes their attitude towards the item.
Statistical Analysis
Descriptive and inferential statistical analyses were conducted. Descriptive analysis focused on the statistical parameters such as mean, standard deviation, sensitivity, and specificity. A receiver operating characteristics (ROC) analysis was performed to determine the optimal cut score for the BDI-II as a screen for depression in this population. We applied analytical analyses that indicated correlational analysis, and ROC cure analysis. Confirmatory factor analysis (CFA) was computed. In this study, we used the absolute model fit indices such as the model chi-squared (χ2) to indicate which model represented goodness over another. In order to evaluate model fit, we employed model fit indices such as the Tucker–Lewis Index (TLI), comparative fit index (CFI), absolute fit indices like the model chi-squared (χ2), normed fit index (NFI), nonlinear index (NLI), root mean square error of approximation (RMSEA), standardized room mean-square residuals (SRMR), and standardized root mean squared residual (SRMR), and information criteria such as Akaike’s information criterion (AIC), Bayesian information criteria (BIC), and sample-size-adjusted BIC (aBIC). In the interpretations of the results, we employed the standard criteria or cutoffs for each of the indices including NFI (>0.9), NLI (>0.9 or closer to 1), CFI and TLI whose score must be more than 0.95, RMSEA (≤0.08) with 90% for confidence intervals, SRMR (≤0.08). The indices that respect the abovementioned values indicate good model fit. Finally, the lower the information criteria values, the better the model fit.41–43 We interpreted this model using the 0.05 of the statistical significance level.44,45 Indeed, to assess the factorial validity, an exploratory common factor analysis (principal axis factoring) with promax rotation was applied. The number of factors was determined by the size of eigenvalues (>1) and the variance explained by each factor, as well as the coherence and interpretability of the factors. Items allocated to a specific factor were based on a loading of more than 0.30 on the corresponding factor, and items were excluded when the difference of factor loadings was less than 0.1. The level of significance was set at <0.05 and 95% confidences intervals were used. All of the analyses were performed with Statistical Package for the Social Sciences (SPSS version 25) for Windows (IBM Inc., Armonk, NY, USA).
Results
Based on the total scores of BDI-II the results indicated that the total mean score for the research participants was 16.3 (SD=9.8). Our results found that 365 (84%) of the cancer patients enrolled in this study scored 14 and more for the BDI-II. This means that 84% of cancer patients had depression. Basing on the type of depression, the results stated that only 192 cancer patients (45.3%) presented minimal depression, 90 patients (21.2%) mild depression, 88 patients (20.8%) moderate depression, and 54 patients (12.7%) MDD. The mean, item-total correlations, standard deviations for each item of BDI, and total scores are presented. Item-total correlations ranged from 0.342 to 0.699, which also indicates a good internal consistency.
Alpha of Cronbach coefficient for the internal consistency was 0.904 which indicates a high level of internal reliability for the BDI-II. Our results found that the coefficients of Cronbach’s alpha ranged from 0.895 to 0.895 if the individuals items were deleted, suggesting that there is no significant advantage from excluding any item of this psychometric tool. Table 1. indicates the means, standard deviations, item-total correlations and alpha of Cronbach for the items. The results from the item-total correlations varied from 0.342 to 0.699, which also indicates good internal consistency because the values of corrected-total correlation of items is greater acceptable since it is greater than the threshold documented in the previous studies.46,47 Concerning the convergent validity of the BDI-II total scores and its coefficients, the results indicated a good validity for the two-factor model between somatic affective factor and BDI-II total (r=0.962, p<0.001), cognitive factor and BDI-II (r=0.904, p<0.001), cognitive and somatic affective factor (r=0.76, p<0.001). Regarding the three-factor model, there was a good internal validity between somatic affective factor and BDI-II (r=0.836, p<0.001), somatic affective and performance factor (r=0.63, p<0.001), somatic affective and negative attitude factor (r=0.606, p<0.001), performance difficulty factor and negative attitude factor (r=0.831, p<0.001) (Table 2).
 |
Table 1 Mean, Standard Deviations, and Item-Total Correlations of the Rwandan BDI-II |
 |
Table 2 Correlation Coefficients of the BDI-II Total Score with BDI-II Sub-factors by on Its Models |
Factor Analysis
Confirmatory factor analysis (CFA) was performed to assess factor structure of BDI-II and indicate the model that performs better than another for BDI-II. The traditional model of BDI-II, two-model factor comprised the somatic affective factor whose items were 4, 10–13, and 15–21, and the second factor that was the cognitive factor for the items 1, 2, 3, 5, 6, 7, 8, 9, and 14.15 However, other researchers suggested a three-factor model that has two factors (such as negative attitude factor for items 1,2,3,5,6,7,8,9,10 and 14; performance difficulty factor that has the 4, 11, 12, 13, 17, and 19; and somatic factor of the items 15, 16, 18, 20, and 21). In the current study, we tested the two-factor model and three-factor model of BDI-II. Thus, our results found that the three-factor model represented a greater fit than the two-factor model.48 In our study, we tested both two-model and three-model factors to compare them by indicating a better model fit than the other. Thus, the results indicated that three-model factor (χ2 =1699.921, p<0.001) was a better model fit than the two-factor model (χ2=2115.397, p<0.001). Correlational coefficients between BDI-II total score and the three sub-factors for the three-factor model. Indeed, the correlational coefficients between BDI-II total scores and two subscales (two-factor model) were presented. These results indicated the significant correlations between 2-factor model and three-factor model and total scores of BDI-II at 0.001. Pearson’s correlation analyses showed that there were significant positive correlations among these two factors and three factors of BDI-II. In particular, there was the strongest positive correlation between somatic factor (SM) and BDI-II (r=0.962, p<0.001), CF and BDI-II (r=0.904, p<0.001), CF and SF (r=76, p<0.001) for the two-factor model. In addition to the two-factor model, the three-factor model showed strong significant correlations between BDI-II and its components. For instance; SM and BDI-II (r=0.836, p<0.001), PD and BDI (r=0.914, p<0.001), NA and BDI-II (r=0.92, p<0.001), PD and SM (r=0.63, p<0.001), NA and PD (r=0.8321, p<0.001), NA and SM (r=0.606, p<0.001) (Table 2).
Criterion Validity
To examine the criterion validity of BDI-II, we computed the ROC analyses for detecting depression among cancer patients. The area under the curve (AUC) to measure depression was 0.805 and for detecting the types of depression the AUC showed 0.781 (0.736–0.826) for mild depression, 0.754 (0.705–0.803) for moderate depression, and 0.745 (0.665–0.824) for MDD (Table 3). We also used the optimal cutoff points, scores of ≥29 for indicating the MDD. Using this cutoff, our results indicated 0.754 for sensitivity, 0.985 for specificity, 0.15 for positive predictive value (PPV), and 0.246 for negative predictive value (NPV). To measure the prevalence of depression among cancer patients we used the threshold of score of ≥17 for BDI-II and the found 0.805 for sensitivity, 0.764 for specificity, 0.402 for PPV and 0.953 for NPV.
 |
Table 3 Results of ROC Analysis for Depression in Cancer Patients |
Using the tradition cutoff of BDI-II, we examined sensitivity and specificity of the BDI-II and found 14 is the cutoff for mild depression, 20 for moderate depression and 29 for major depressive disorder. The results of ROC were applied and indicated that the BDI-II and its subscales had statistical significance at 0.001. They also represented a favorable sensitivity and specificity to measure depression, mild depression, moderate depression and MDD.
The results of this study indicated that the three-factor model and two-factor model of BDI-II represented appropriate sensitivity and specificity for diagnosing the depressive symptomatology among cancer patients. The results in the two-factor model showed the high sensitivity and specificity of both somatic affective and cognitive factors of BDI while the results for the three-factor model indicated that there was a favorable sensitivity and specificity of the three factors of BDI-II (somatic factor, performance difficult factor, and negative attitude factor). Thus, using the goodness-of-fit indices for CFA, the results reported that the three-factor model presented better fit indices than the two-factor model of BDI-II ().
The values of the model fit indices were employed in the analysis for assessing which model of BDI-II presents a better goodness of fit among the cancer patients in Rwanda. Therefore, the model fits indices such as RMSEA, TLI, NFI, NLI, RFI, CFI, AIC, BIC, TLI, and aBIC were assessed. All these indices reported that the three-factor model had a better goodness of fit than the two-factor model. All the models met most of the criteria for good fit with RMSEA≤0.06; CFI≥0.95; TLI≥0.95; SRMR≤0.08. NFI>0.9; TLI>0.9; CFI>0.9; lower value of AIC, BIC or aBIC for the three-factor model. Due to these values, the three-factor model presents lower values than in the two-factor model that indicates that the three-factor model had a more reasonable model fit than the two-factor model (Table 4).
 |
Table 4 Statistical Summary of Goodness-of-Fit Indices for CFA |
To conduct the factorial analysis we used the three-factor model and then the findings from our analyses showed that the eigenvalues were less than 1. The Kaiser–Meyer–Olkin (KMO) measure of 0.916 and Bartlett’s test of sphericity of 3780 (p<0.001) reported that the factor structure or sampling was adequate for analysis. Bartlett’s test of sphericity was highly significant (χ2=3780.1, p<0.001) indicating that correlations between items are sufficiently large to be administered among the cancer patients (Table 5).
 |
Table 5 Factor Structure and Loadings of 21 Items from BDI-II in Cancer Patients |
Discussion
The aim of this study was to assess the usefulness of the BDI in diagnosing depression and indicating the psychometric properties of BDI-II among cancer patients. We also investigated if the BDI-II is an appropriate measure based on its sensitivity and specificity. The BDI-II screening tool revealed a satisfactory consistency of the BDI-II among cancer patients and indicated the homogeneity and convergent validity for BDI-II among the sample of cancer patients. The coefficient of alpha of Cronbach (0.916) was higher than the one documented in the prior studies which indicated that the internal consistency of BDI-II varies between 0.84 and 0.94.19 The items of the BDI-II indicated significant correlations. For the two-factor model, both subscales revealed significant correlations and for the three-factor model, significant correlations were found. These results are consistent with the prior studies.19,40 When we performed the individual items, the results indicated that all items of the original version designed by Beck were maintained and no item removed.
In a similar vein with preceding studies conducted on the BDI-II for detecting its psychometric properties,49 based on the criterion-related validity our findings reported a good specificity and sensitivity for assessing depressive symptomatology among the cancer patients in comparison to the gold standard of the BDI-II. Our results revealed that the two-model factor and three-model factor were adequate and represented that all the items were considered reliable to be administered among cancer patients. Our results are in the same vein with the prior studies from Western countries that documented that the BDI-II has two versions including the two-factor model and three-factor model that are all appropriate to assess depression based on the factors for each instrument.15,19,40,50 In concurrence with the prior studies that documented that the somatic affective factor of the two-factor model was divided into performance difficulty (PD) and somatic factor (SM). Comparing the model and factors, our results revealed that the three-factor model of BDI-II presented a better fit than the two-factor model among the cancer patients of this study because the cancer patients of this study presented various symptoms such as agitation, difficulties in concentration, irritability that occurred when experiencing excessive pressure for achievement. Our results are in sameness with the preceding studies conducted in the East Asians that indicated the three-factor model might have a better fit than two-factor model because the three-factor model might have a better fit for individuals in East Asia.40 Indeed, comparing to the original criteria suggested that 23-point cutoff score showed better performance to detect the MDD than the moderate 9-point score of 20) our results revealed that the 17-point cutoff score showed the best result in our study. So, we found that the threshold of 14-point score has better performance than the original middle level criterion (score of 14). Our results are in line with the prior studies.15,19,26 In accordance with earlier studies,24,30,51 our results revealed that BDI-II has a good sensitivity and specificity as well as validity and reliability to diagnose depression among cancer patients.
Strengths and Limitations
The study encountered several strengths. First, the psychometric instrument was standardized and the sample size of the cancer patients was large. The research materials did not affect the life conditions of the patients. Second, our findings are of merit because the CFA was performed in a standard manner using a large sample size and with a broad variety of indices to demonstrate the goodness fit of three- and two-factor models of BDI-II. However, some limitations were found. First, the study was limited to the methods since other depressive screening measures of depression and anxiety disorders were not used for assessing external validity. Although our study was cross-sectional and based on only one population, our findings have merit because we performed the CFA in a standard manner, using a large sample size and with a broad variety of indices to judge the fitness of hierarchical and dimensional models to the data.
Conclusion
In conclusion, this study reported a meaningful factor solution using CFA of the BDI-II among cancer patients. The three-factor model was the better model to demonstrate the factors of depression. This measurement was valid and reliable screening tool with better sensitivity and specificity that are sufficient in screening depression among cancer patients in medical settings. In the future, specialized pharmacological or psychological treatments should be tailored for each symptom of depression and the health-care providers are recommended to use the BDI-II while diagnosing depression in cancer patients. Based on the findings from the current study conducted among cancer patients that differs from the general population because it is made up of a homogeneous group of individuals enrolled from oncology settings, therefore, further studies in the general population and other clinical settings are recommended for exploring the psychometric properties of the BDI-II.
Data Sharing Statement
Dataset for this study is available from the authors of this study. The dataset maybe shared on request.
Ethics Statement
As recommended by the National Institute of Health (NIH) and World Medical Association (WMA) that developed the Declaration of Helsinki as a statement of ethical principles for protecting the participants who are involved in the research, this study was carried out in accordance with the Declaration of Helsinki.37,52 The ethics approval of this study was sought from the IRB/CMHS at the University of Rwanda with the reference number (ref. no: 136/UR-CMHS/SPH/2028). Confidentiality was maintained. Data were gathered anonymously. Consent forms were received from the participants who took part in this study and their guardians. Patients from palliative care services provided the consent forms while their guardians or parents provided the consent forms before they participated in the research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funds were provided to the research authors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Khalil A, Faheem M, Fahim A, et al. Prevalence of depression and anxiety amongst cancer patients in a hospital setting: a Cross-Sectional Study. 2016;2016. doi:10.1155/2016/3964806
2. Warmenhoven F, Van RE, Engels Y, et al. The Beck Depression Inventory (BDI-II) and a single screening question as screening tools for depressive disorder in Dutch advanced cancer patients. Support Care Cancer. 2012;20(2):319–324. doi:10.1007/s00520-010-1082-8
3. Massie M. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;32(32):57–71. doi:10.1093/jncimonographs/lgh014
4. Stiefel R, Die T, Berney A, Olarte J, Razavi A. Depression in palliative care: a pragmatic report from the expert working group of the european association for palliative care. Support Care Cancer. 2001;9(7):477–488. doi:10.1007/s005200100244
5. Kelly BJ, Turner J. Depression in advanced physical illness: diagnostic and treatment issues. Med J Aust. 2009;190(S7):S90–S93. doi:10.5694/j.1326-5377.2009.tb02478.x
6. APA. Diagnostic and Statistical Manual of Mental Disorders (DSM).
7. Beck A, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561–571. doi:10.1001/archpsyc.1961.01710120031004
8. World Health Organisation [WHO]. Depression and Other Common Mental Disorders: Global Health Estimates; 2017.
9. Sang Won Jeon MA. Biopsychosocial risk factors for depression. 2017:1–20.
10. Kirk CM, Dedeken P. Validity, reliability, and diagnostic cut-off of the kinyarwandan version of the hamilton depression rating scale in Rwanda. Front Psychol. 2020;11(July):1343. doi:10.3389/fpsyg.2020.01343
11. Smith HR Depression in cancer patients: pathogenesis, implications and treatment (Review). 2015:1509–1514. doi:10.3892/ol.2015.2944.
12. Periyakoil VS, Hallenbeck J. Identifying and managing preparatory grief and depression at the end of life. Am Fam Physician. 2002;65(5):883–890.
13. Anderson WG, Alexander SC, Rodriguez KL. “What concerns me is … ” Expression of emotion by advanced cancer patients during outpatient visits. Support Care Cancer. 2010;16(7):803–811. doi:10.1007/s00520-007-0350-8
14. Niedzwiedz CL, Knifton L, Robb KA, Katikireddi SV, Smith DJ. Depression and anxiety among people living with and beyond cancer: a growing clinical and research priority. BMC Cancer. 2019;2019:1–8.
15. Beck A, Steer R, Brown G. BDI-II: Beck Depression Inventory Manual.
16. McDowell I. Measuring Health: A Guide to Rating Scales and Questionnaires. Oxford University Press; 2006.
17. Mutabaruka J, Séjourné N, Bui E, Birmes P, Chabrol H. Traumatic grief and traumatic stress in survivors 12 years after the genocide in Rwanda. Stress Heal. 2012;28(4):289–296. doi:10.1002/smi.1429
18. Biracyaza E, Mutabaruka J, Habimana S. Validation of anxiety sensitivity index (asi-16) on a nonclinical sample of Rwandans: a Cross-sectional Study. Int J Behav Sci. 2019;12(4):176–182.
19. Wang Y, Gorenstein C. Assessment of depression in medical patients: a systematic review of the utility of the Beck Depression Inventory-II. Clinics. 2013;68(11):1274–1287. doi:10.6061/clinics/2013(09)15
20. Mitchell AJ, Kaar S, Coggan C, Herdman J. Acceptability of common screening methods used to detect distress and related mood disorders-preferences of cancer specialists and non-specialists. Psychooncology. 2008;17(3):226–236. doi:10.1002/pon.1228
21. Arroll B, Goodyear-Smith F, Kerse N, Fishman T, Gunn J. Effect of the addition of a “help” question to two screening questions on specificity for diagnosis of depression in general practice: diagnostic validity study. BMJ. 2005;331(7521):2–5. doi:10.1136/bmj.38607.464537.7C
22. Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: a systematic review of assessment instruments. J Natl Cancer Inst. 2009;101(21):1464–1488. doi:10.1093/jnci/djp336
23. Mystakidou K, Tsilika E, Parpa E, Smyrniotis V, Galanos A, Vlahos L. Beck depression inventory: exploring its psychometric properties in a palliative care population of advanced cancer patients. Eur J Cancer Care. 2007;16(3):244–250. doi:10.1111/j.1365-2354.2006.00728.x
24. Park K, Jaekal E, Yoon S, Lee S, Choi K. Diagnostic utility and psychometric properties of the Beck depression inventory-II among Korean Adults. Front Psychol. 2020;10(January):1–10. doi:10.3389/fpsyg.2019.02934
25. Basker M, Moses PD, Russell S, Russell PSS. The psychometric properties of Beck depression Inventory for adolescent depression in a primary-care paediatric setting in India. Child Adolesc Psychiatry Ment Health. 2007;1(1):1–7. doi:10.1186/1753-2000-1-8
26. Farinde A. The Beck depression inventory. Pharma Inov J. 2013;2(1):56–63.
27. Beck A, Ward CH MM-A of general, 1961 U. An inventory for measuring depression. JamanetworkCom; 1960.
28. Hopko D, Bell J, Armento M, et al. The phenomenology and screening of clinical depression in cancer patients. J Psychosoc Oncol. 2008;26(1):31–51. doi:10.1300/j077v26n01_03
29. Williams J, Hirsch E, Anderson K, et al. A comparison of nine scales to detect depression in Parkinson disease: which scale to use? Neurology. 2012;78(13):998–1006. doi:10.1212/WNL.0b013e31824d587f
30. Lee K, Kim D, Cho Y. Exploratory factor analysis of the Beck anxiety inventory and the Beck depression inventory-II in a psychiatric outpatient population. J Korean Med Sc. 2018;33(16):1–11. doi:10.3346/jkms.2018.33.e128
31. Titov N, Dear B, McMillan D, Anderson T, Zou J, Sunderland M. Psychometric Comparison of the PHQ-9 and BDI-II for Measuring Response during Treatment of Depression. Cogn Behav Ther. 2011;40(2):126–136. doi:10.1080/16506073.2010.550059
32. Cameron IM, Cardy A, Crawford JR, et al. Measuring depression severity in general practice. Br J Gen Pract. 2011;61(588):e419–e426. doi:10.3399/bjgp11X583209.e419
33. Zich J, Attkisson C, Greenfield T. Screening for depression in primary care clinics: the CES-D and the BDI. Int J Psychiatry Med. 1990;20(3):259–277. doi:10.2190/LYKR-7VHP-YJEM-MKM2
34. Marie J, Dusengimana V, Hategekimana V, et al. Pregnancy-associated breast cancer in rural Rwanda: the experience of the Butaro cancer center of excellence. BMC Cancer. 2018;18(634):1–8. doi:10.1186/s12885-018-4535-y
35. Pace LE, Dusengimana J-M, Hategekimana V, et al. Benign and malignant breast disease at rwanda’s first public cancer referral center. Oncologist. 2016;21(5):571–575. doi:10.1634/theoncologist.2015-0388
36. Mpunga T, Tapela N, Hedt-Gauthier BL, et al. Diagnosis of cancer in rural Rwanda: early outcomes of a phased approach to implement anatomic pathology services in resource-limited settings. Am J Clin Pathol. 2014;142(4):541–545. doi:10.1309/AJCPYPDES6Z8ELEY
37. Carlson RV, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57(6):695–713. doi:10.1111/j.1365-2125.2004.02103.x
38. Mukeshimana M, Mchunu G. The co-morbidity of depression and other chronic non-communicable diseases: a review of literature on the epidemiology, diagnosis and health effects. Rwanda J. 2016;3(1):44–50. doi:10.4314/rj.v3i1.8F
39. Upton J. Beck Depression Inventory (BDI). In: Gellman M, Turner J editors. Encyclopedia of Behavioral Medicine. Springer; 2013. doi:10.1007/978-1-4419-1005-9_441
40. Lee E, Lee S, Hwang S, Hong S, Kim J. Reliability and validity of the Beck depression inventory-II among Korean adolescents. Psychiatry. 2017;14:30–36.
41. Bentler P. Comparative fit indexes in structural models. Psychol Bull. 1990;107(2):238–246. doi:10.1037/0033-2909.107.2.238
42. Akaike H. Factor Analysis and AIC. In: Parzen E, Tanabe K, Kitagawa G, editors. Selected Papers of Hirotugu Akaike. Springer Series in Statistics (Perspectives in Statistics). NY: Springer; 1987. doi:10.1007/978-1-4612-1694-0_29.
43. Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model a Multidiscip J. 1999;6(1):1–55. doi:10.1080/10705519909540118
44. Hu L, Bentler P. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model. 2009;6(1):1–55. doi:10.1080/10705519909540118
45. Akaike H. Factor Analysis and AIC. Psychometrika. 1987;371–386.
46. Cristobal E, Flavia C, Guinaliu M. Perceived e-service quality (PeSQ) Measurement validation and effects on. Manag Serv Qual. 2007;17(3):0960–4529. doi:10.1108/09604520710744326
47. Nunnally J. Psychometric Theory.
48. Osman A, Kopper BA, Barrios F, Gutierrez PM, Bagge CL. Reliability and validity of the Beck depression inventory–II with adolescent psychiatric inpatients. Psychol Assess. 2004;16(2):120–132. doi:10.1037/1040-3590.16.2.120
49. Wang Y-P, Gorenstein C. Psychometric properties of the Beck Depression Inventory-II: a comprehensive review. Brazilian J Psychiatry. 2013;35(4):416–431. doi:10.1590/1516-4446-2012-1048
50. Wu P, Huang T. Gender-related invariance of the Beck depression inventory II for Taiwanese adolescent samples. Assessment. 2014;21(2):218–226. doi:10.1177/1073191112441243
51. Brouwer D, Meijer R, Zevalkink J. Measuring individual significant change on the Beck depression inventory-II through IRT-based statistics. Psychother Res. 2013;23(5):489–501. doi:10.1080/10503307.2013.794400
52. World Medical Association. WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects.; 2011. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
