Back to Journals » Breast Cancer: Targets and Therapy » Volume 10
Psychological symptoms in adult Saudi Arabian cancer patients: prevalence and association with self-rated oral health
Authors Ahmed AE , Albalawi AN, Qureshey ET, Qureshey AT, Yenugadhati N , AL-Jahdali H, Jazieh AR
Received 14 March 2018
Accepted for publication 31 May 2018
Published 3 October 2018 Volume 2018:10 Pages 153—159
DOI https://doi.org/10.2147/BCTT.S168139
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Anwar E Ahmed,1,2 Alhanouf N Albalawi,3 Eiman T Qureshey,3 Aisha T Qureshey,3 Nagarajkumar Yenugadhati,2 Hamdan AL-Jahdali,4 Abdul Rahman Jazieh5
1King Abdullah International Medical Research Center (KAIMRC), Riyadh, Saudi Arabia; 2College of Public Health and Health Informatics, King Saud Bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia; 3Riyadh Elm University, Riyadh, Saudi Arabia; 4College of Medicine, King Saud Bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia; 5Department of Oncology, King Abdulaziz Medical City for National Guard, Ministry of National Guard, Riyadh, Saudi Arabia
Background: Although psychological symptoms and oral health status are associated with health management and outcomes among cancer patients, their association has not been assessed in Saudi Arabia. We aimed to assess the symptoms of depression, anxiety, and stress and their association with their oral health status, adjusting for sociodemographic and clinical factors.
Methods: A self-reported study included 375 adult cancer patients who received outpatient healthcare services in the Oncology Department, King Abdulaziz Medical City-Riyadh, Saudi Arabia, between April 1 and August 31, 2017. We used the Arabic version of the Depression Anxiety Stress Scale to dichotomize a binary outcome for each. Oral health was evaluated by self-rating from “very good” to “bad”.
Results: A high prevalence of subjective depression, anxiety, and stress was found (44.8%, 52.5%, and 42.7%, respectively). Of the sample, 17.9% self-reported “bad” oral health, which is associated with a high risk of anxiety and stress, and its association remains significant after controlling for other factors (adjusted odds ratio=6.48 and 4.73, respectively). Being <60 years old, high level of formal education, low income, breast cancer, and lung cancer were associated with increased psychological symptoms.
Conclusion: Every 6 in 10 cancer patients in this study reported at least one psychosocial symptom. The findings suggest that there exists an association between self-reported “bad” oral health and psychosocial symptoms. Being <60 years old, low income, high level of formal education, breast cancer, and lung cancer were associated with psychological symptoms. Routine psychological counseling and oral health screening in outpatient oncology clinics may improve psychological outcomes and cancer management.
Keywords: depression, anxiety, stress, dental health, cancer, Saudi
Introduction
Evidence has shown that psychological distress is increasingly recognized as a major health burden and could impair individual cancer patients and family caregivers.1,2 Similar attention to poor oral health was also documented among cancer patients.3,4 The psychological distress and poor oral health were negatively related with time, because of the diagnoses of cancer,3,5 treatment,4,6 and poor prognosis in cancer patients.7,8 In Saudi Arabia, due to a wide array of national screening programs and diagnosis of new cancer cases at an early stage,9 the survival rate among cancer patients continues to increase.10 This necessitates routine assessment and intervention among cancer survivors to maintain quality of life11,12 and better health management and outcomes.13
A few studies have documented symptoms of depression and anxiety among cancer patients in Saudi Arabia. The most recent estimated rate of depression14 was 57.1% among patients with colorectal cancer and 10.4% anxiety15 among patients with breast cancer. Yet, it is not documented in Saudi Arabia if prevalence of anxiety, depression, and stress differ between various cancer sites.
A single study has documented the oral health status among cancer patients in Saudi Arabia,16 which reported the prevalence of oral health problems. Lack of studies on oral health status among cancer patients in Saudi Arabia has directed the attention of the study authors to explore its relation with anxiety, depression, and stress.
We, therefore, aimed to estimate the prevalence of anxiety, depression, and stress and their contributing factors among cancer survivors in Saudi Arabia. Particularly, we tested the hypothesis of whether perceived oral health and patients with cancer factors independently predict anxiety, depression, and stress in a sample of cancer patients who attended outpatient clinics between April 1 and August 31, 2017, in the Oncology Department, King Abdulaziz Medical City in Riyadh, Saudi Arabia.
Methods
This is a cross-sectional study of adult cancer patients (age ≥18 years) receiving routine outpatient healthcare services in the Oncology Department, King Abdulaziz Medical City in Riyadh, Saudi Arabia. The study was conducted between April 1 and August 31, 2017, and recruitment was performed in various outpatient oncology clinics. We did not include seriously ill cancer patients in palliative care services. The study received ethical approval from the Institutional Review Board at the Ministry of National Guard-Health Affairs (MNG-HA), Riyadh, Saudi Arabia (Clearance No RC16/213/R). Written informed consent were obtained to participate and to publish the study findings from all patients who were recruited in the study. We collected data on sociodemographic factors, including gender, age, married (Yes/No), university degree (Yes/No), low income (less than SR 5,000 ≈ $1,333), and being a smoker (Yes/No). Cancer characteristics included type of cancer (breast, lung, colon, and other), cancer stage (I, II, III, IV), number of tumors (single or multiple), and whether patient was newly diagnosed for cancer (<12 months after cancer diagnosis). Cancer treatment included surgical therapy (Yes/No), chemotherapy (Yes/No), radiation therapy (Yes/No), and immunotherapy (Yes/No). We assessed patients’ clinical conditions, and the survey included chronic diseases other than cancer (Yes/No). We assessed patients’ activity, and the survey included physical exercise (Yes/No). We evaluated whether patients received full health insurance coverage (Yes/No). We asked them where they received oral health information (Yes/No) while attending oncology outpatient clinics. The study’s cancer patients self-rated their oral health status: “How do you rate your oral or dental health related to gums, teeth, and tongue?” and possible answers included “very good”, “good”, “acceptable”, and “bad”.
Outcome measures
Each patient’s perceived psychological distress was measured utilizing the Depression Anxiety Stress Scale (DASS-21).17 It is a 21-item self-report scale to measure subjective symptoms of depression (7 items), anxiety (7 items), and stress (7 items). The patients rated the degree to which they have encountered a particular symptom over the past week. This was measured on four points ranging from “0” where a symptom “did not apply to me at all – never” to “3” where a symptom “applied to me very much, or most of the time – almost always”. The original English DASS-21 version and its culturally adopted Arabic translation version18 were found to be reliable and valid. The Arabic DASS-21 version is commonly used among Arabic speakers in various outpatient clinics in Saudi Arabia.18–21 The Arabic version was assessed in our oncology outpatient clinics. Reliability analysis indicated that stress (Cronbach’s alpha=0.85), anxiety (Cronbach’s alpha=0.83), and depression (Cronbach’s alpha=0.81) subscales were reliable in screening for psychological symptoms in our population. The overall Arabic DASS-21 version was also found to be reliable (Cronbach’s alpha=0.93). We used the scoring system and cutoff points described in Lovibond and Lovibond17 to classify each patient according to stress, anxiety, or depression. The total of each psychological symptom scale (0–21) was classified into present or absent. A score >4 was used to classify depression, >3 was used to classify anxiety, and >7 was used to classify stress.17
Sample population
The study recruitment to complete the questionnaire was between April 1 and August 31, 2017, in various outpatient oncology clinics (breast cancer, colon cancer, lung cancer, etc.). Our population comprises adult cancer patients (age ≥18 years) receiving routine outpatient healthcare services in the Oncology Department, King Abdulaziz Medical City in Riyadh, Saudi Arabia. The minimum required estimated sample for this study was 341 cancer patients based on a proportion of 50%, with a precision of 5% adjusted for a finite population of size 3,000. A total of 500 cancer patients were approached to participate in the study, 375 consented to participate and completed the questionnaire with a response rate of 75%.
Statistical analysis
Data were analyzed using the SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Univariate analyses were used to summarize the overall sample characteristics (Table 1). Subgroup analyses or Chi-square tests were used to assess association between sample characteristics and psychological symptoms (Table 1). We evaluated the association between self-rated oral health and psychological symptoms among Saudi patients with cancer. Multivariate Logistic Regression analyses were used to assess the association between self-rated oral health and each psychological symptom (depression, stress, and anxiety) after adjustment for the confounding effects of the factors considered in the univariate analyses (Table 2). An association was considered significant when the P-value (P) was ≤0.05. We evaluated the goodness-to-fit model for each model using the Hosmer–Lemeshow test.
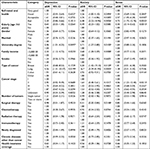
  | Table 1 Characteristics of study subjects by psychological symptoms Notes: aP-values for differences based on chi-squared test for proportions. bDifferences significant at P≤0.05. |
Results
We analyzed 375 cancer patients in which 125 (33.3%) were diagnosed with breast cancer, 170 (45.3%) with colon cancer, 35 (9.3%) with lung cancer, and 35 (9.3%) with other types of cancer, while 10 (2.7%) chose not to report their cancer type. The mean age of our sample was 51.79±14.71 years with sample ages ranging between 18 and 87 years. More descriptions of the patients’ sociodemography and their clinical characteristics are reported in Table 1. Depression symptoms were present in 168 (44.8%; 95% CI, 39.7%–50.0%), anxiety symptoms in 197 (52.5%; 95% CI, 47.3%–57.7%), and stress symptoms in 160 patients (42.7%; 95% CI, 37.6%–47.9%).
According to the subgroup analysis in Table 1, anxiety and stress were negatively associated with “bad” oral health (P=0.0146, P=0.0070, respectively). Anxiety and stress were significantly less frequent among the elderly (≥60 years; P=0.0053, P=0.0007, respectively) and were more frequent among the female gender (P=0.0025, P=0.0339, respectively). Depression, anxiety, and stress were highly prevalent in cancer patients with low income (less than SR 5,000 ≈ $1,333; P=0.0010, P=0.0001, P=0.0038, respectively). Depression and anxiety were significantly more prevalent in lung cancer patients (P=0.0063, P=0.0001, respectively) and less frequent in cancer patients with health insurance coverage (P=0.0445, P=0.0024, respectively). Depression was highly prevalent in cancer patients who also had other chronic diseases (P=0.0184).
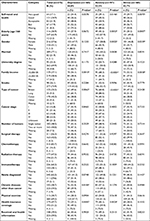
Findings of the multivariable logistic models are presented in Table 2. Determinants of depression in cancer patients included university degree (adjusted odds ratio [aOR], 2.06; 95% CI, 1.01–4.25), low income (aOR, 2.3; 95% CI, 1.12–4.72), and lung cancer (aOR, 5.56; 95% CI, 1.31–23.57). Determinants of anxiety in cancer patients included bad oral health (aOR, 6.48; 95% CI, 2.22–18.96), university degree (aOR, 2.71; 95% CI, 1.27–5.80), low income (aOR, 2.68; 95% CI, 1.28–5.59), breast cancer (aOR, 5.20; 95% CI, 1.42–19.10), and lung cancer (aOR, 16.7; 95% CI, 3.39–81.94). Determinants of stress in cancer patients included acceptable oral health (aOR, 2.57; 95% CI, 1.05–6.28) and bad oral health (aOR, 4.73; 95% CI, 1.75–12.76). Elderly patients were less likely to develop stress symptoms (aOR, 0.30; 95% CI, 0.14–0.62). Cancer patients who underwent surgery were less likely to develop anxiety symptoms (aOR, 0.41; 95% CI, 0.20–0.84). The goodness-of-fit test indicates that the three models fit the data well for depression (P=0.6274), anxiety (P=0.4940), and stress (P=0.8114).
Discussion
This cross-sectional study assessed the psychological symptoms and subjective oral health status of cancer patients who attended outpatient oncology clinics at King Abdulaziz Medical City in Riyadh, Saudi Arabia. We found a significant proportion of cancer patients met the criteria for depression (44.8%), anxiety (52.5%), and stress (42.7%) in our sample. Of the sample, 62.4% reported at least one psychological symptom. This finding supports the result of an earlier Saudi Arabian study that reported the rate of depression at 57.1% among patients with colorectal cancer.14 According to our knowledge, there is lack of research on self-reported psychological symptoms among cancer patients in Saudi Arabia. The high prevalence of psychological symptoms could alert oncologists to consider psychological needs during cancer treatment and management.
This is the first investigation for subjective oral health status among cancer patients in Saudi Arabia. The study has shown that 17.9% of the cancer patients analyzed perceived their oral health as “bad”. This could be explained by cancer treatment as it may contribute to greater risk of oral health complications.22 In our sample, “bad” self-rated oral health was a risk indicator for anxiety and stress among cancer patients. This association remains highly significant after adjusting for several factors. For instance, the odds of anxiety were 6.48 times higher among cancer patients with “bad” self-rated oral health. This also has been documented in an earlier study.23 In our sample, “bad” self-rated oral health was not related to depression.
Graner et al found that older patients tend to show negative expectations concerning the cancer diagnosis.24 In this study, the results suggest that age was negatively associated with stress among cancer patients, and the odds of stress tended to reduce 70% among elderly patients (age ≥60 years) as opposed to patients <60 years old. A prospective study is needed to monitor psychosocial needs of cancer patients <60 years old.
As the level of formal education rises, the odds of depression and anxiety increase. This finding contradicts a study conducted on breast cancer outpatients in Turkey, where the educational level is inversely related to depression.25 In our study, the level of formal education may be translated into a high level of awareness toward cancer and its burden.26
In these cancer patients, the odds of depression and anxiety were 2.3 and 2.68 times higher among low-income cancer survivors (<SR 5,000 ≈ $1,333) than in cancer survivors with income ≥SR 5,000 ≈ $1,333. Several earlier reports documented the positive association between low income and psychosocial symptoms.27–32 The high risk of psychosocial symptoms among low-income cancer survivors could be attributed to economic barriers to cancer treatment and care33 as well as lack of mental health services.29
In this sample, cancer type was a predictor for a high risk of depression and anxiety. Significant increase in psychosocial symptoms was observed in lung and breast cancer patients. Patients who were diagnosed with these cancer sites may need more mentoring and attention or intervention to reduce the burden of psychosocial symptoms.34
We noted a number of limitations that should be considered in future studies. The study was based on the self-report of subjective oral health status and psychosocial symptoms and not on clinical examination. Due to the nature of the study design, the results indicate an association rather than causation. Despite the mentioned limitations, the study provides a considerable contribution to the area of oral health and psychosocial symptoms among cancer patients in Saudi Arabia as no such data exist in this population.
Conclusion
Every six in ten of the cancer patients in this study had at least one psychosocial symptom. The findings suggest that association exists between self-reported “bad” oral health, anxiety, and stress. Being <60 years old, low income, high level of formal education, breast cancer, and lung cancer were associated with psychological symptoms. Routine psychological counseling and oral health screening in outpatient oncology clinics may improve psychological outcomes and cancer management.
Availability of data and materials
The dataset is available from the corresponding author who is also the principal investigator of the study.
Acknowledgments
We would like to thank King Abdullah International Medical Research Center for approving the study. Funding was obtained for open access from King Abdullah International Medical Research Center.
Author contributions
AEA designed the study and wrote the manuscript. The data analysis was carried out by NY. ANA, ATQ, and ETQ collected the data. ARJ and HALJ reviewed and edited the manuscript. All authors read and approved the final manuscript. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Badger TA, Braden CJ, Mishel MH, Longman A. Depression burden, psychological adjustment, and quality of life in women with breast cancer: patterns over time. Res Nurs Health. 2004;27(1):19–28. | ||
Ullrich A, Ascherfeld L, Marx G, Bokemeyer C, Bergelt C, Oechsle K. Quality of life, psychological burden, needs, and satisfaction during specialized inpatient palliative care in family caregivers of advanced cancer patients. BMC Palliat Care. 2017;16(1):31. | ||
Holmes JD, Dierks EJ, Homer LD, Potter BE. Is detection of oral and oropharyngeal squamous cancer by a dental health care provider associated with a lower stage at diagnosis? J Oral Maxillofac Surg. 2003;61(3):285–291. | ||
Amódio J, Palioto DB, Carrara HH, Tiezzi DG, Andrade JM, Reis FJ. Oral health after breast cancer treatment in postmenopausal women. Clinics (Sao Paulo). 2014;69(10):706–708. | ||
Pérez-Fortis A, Schroevers MJ, Fleer J, et al. Psychological burden at the time of diagnosis among Mexican breast cancer patients. Psychooncology. 2017;26(1):133–136. | ||
Dzebo S, Mahmutovic J, Erkocevic H, Foco F. Frequency of depression and its correlation with quality of life of patients with oral cavity cancer. Mater Sociomed. 2017;29(2):97–100. | ||
Hamer M, Chida Y, Molloy GJ. Psychological distress and cancer mortality. J Psychosom Res. 2009;66(3):255–258. | ||
Farquhar DR, Divaris K, Mazul AL, Weissler MC, Zevallos JP, Olshan AF. Poor oral health affects survival in head and neck cancer. Oral Oncol. 2017;73:111–117. | ||
Abulkhair OA, Al Tahan FM, Young SE, Musaad SM, Jazieh AR. The first national public breast cancer screening program in Saudi Arabia. Ann Saudi Med. 2010;30(5):350. | ||
Hamdan NA, Ravichandran K, Dyab AR. Breast cancer survival in Riyadh, Saudi Arabia, 1994–1996. IARC Sci Publ. 2011;(162):179–181. | ||
Ahmed AE, Almuzaini AS, Alsadhan MA, et al. Health-related predictors of quality of life in cancer patients in Saudi Arabia. J Cancer Educ. 2017;7:1–9. | ||
Ahmed AE, Alharbi AG, Alsadhan MA, et al. The predictors of poor quality of life in a sample of Saudi women with breast cancer. Breast Cancer Target Ther. 2017;9:51–58. | ||
Maurice A, Evans DG, Shenton A, et al. Screening younger women with a family history of breast cancer--does early detection improve outcome? Eur J Cancer. 2006;42(10):1385–1390. | ||
Shaheen Al Ahwal M, Al Zaben F, Khalifa DA, Sehlo MG, Ahmad RG, Koenig HG. Depression in patients with colorectal cancer in Saudi Arabia. Psychooncology. 2015;24(9):1043–1050. | ||
Al-Zaben FN, Sehlo MG, Koenig HG. A cross-sectional study of anxiety and marital quality among women with breast cancer at a university clinic in western Saudi Arabia. Saudi Med J. 2015;36(10):1168–1175. | ||
Yenugadhati N, Albalawi AN, Qureshey AT, et al. Associated factors for oral health problems in a sample of Saudi cancer patients. Cancer Manag Res. 2018;10:1285–1293. | ||
Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33(3):335–343. | ||
Taouk M, Lovibond PF, Laube R. Psychometric properties of an Arabic version of the Depression Anxiety Stress Scales (DASS21). Report for New South Wales Transcultural Mental Health Centre, Cumberland Hospital, Sydney. 2001. | ||
Al-Zahrani R, Bashihab R, Ahmed AE, Alkhodair R, Al-Khateeb S. The prevalence of psychological impact on caregivers of hospitalized patients: the forgotten part of the equation. Qatar Med J. 2015;2015(1):3. | ||
Ahmed AE, Al-Dahmash AM, Al-Boqami QT, Al-Tebainawi YF. Depression, anxiety and stress among Saudi Arabian dermatology patients: cross-sectional study. Sultan Qaboos Univ Med J. 2016;16(2): e217–e223. | ||
Ali AM, Ahmed A, Sharaf A, Kawakami N, Abdeldayem SM, Green J. The Arabic Version of The Depression Anxiety Stress Scale-21: Cumulative scaling and discriminant-validation testing. Asian Journal of Psychiatry. 2017;1;(30):56–58. | ||
Chambers MS, Toth BB, Martin JW, Fleming TJ, Lemon JC. Oral and dental management of the cancer patient: prevention and treatment of complications. Support Care Cancer. 1995;3(3):168–175. | ||
Hassel AJ, Danner D, Freier K, Hofele C, Becker-Bikowski K, Engel M. Oral health-related quality of life and depression/anxiety in long-term recurrence-free patients after treatment for advanced oral squamous cell cancer. J Craniomaxillofac Surg. 2012;40(4):e99–e102. | ||
Graner KM, Rolim GS, Moraes ABA, et al. Feelings, perceptions, and expectations of patients during the process of oral cancer diagnosis. Support Care Cancer. 2016;24(5):2323–2332. | ||
Alcalar N, Ozkan S, Kucucuk S, Aslay I, Ozkan M. Association of coping style, cognitive errors and cancer-related variables with depression in women treated for breast cancer. Jpn J Clin Oncol. 2012;42(10):940–947. | ||
Olajide TO, Ugburo AO, Habeebu MO, Lawal AO, Afolayan MO, Mofikoya MO. Awareness and practice of breast screening and its impact on early detection and presentation among breast cancer patients attending a clinic in Lagos, Nigeria. Niger J Clin Pract. 2014;17(6):802–807. | ||
Sullivan DR, Forsberg CW, Ganzini L, et al. Depression symptom trends and health domains among lung cancer patients in the CanCORS study. Lung Cancer. 2016;100:102–109. | ||
Chen X, Zheng Y, Zheng W, et al. Prevalence of depression and its related factors among Chinese women with breast cancer. Acta Oncol. 2009;48(8):1128–1136. | ||
Ell K, Sanchez K, Vourlekis B, et al. Depression, correlates of depression, and receipt of depression care among low-income women with breast or gynecologic cancer. J Clin Oncol. 2005;23(13):3052–3060. | ||
Hengrasmee P, Padungsutt P, Boriboonhirunsarn D. Depression among gynecologic cancer patients at Siriraj Hospital: prevalence and associated factors. J Med Assoc Thai. 2004;87(Suppl 3):S74–S79. | ||
Fatiregun OA, Olagunju AT, Erinfolami AR, Fatiregun OA, Arogunmati OA, Adeyemi JD. Anxiety disorders in breast cancer: prevalence, types, and determinants. J Psychosoc Oncol. 2016;34(5):432–447. | ||
Dastan NB, Buzlu S. Depression and anxiety levels in early stage Turkish breast cancer patients and related factors. Asian Pac J Cancer Prev. 2011;12(1):137–141. | ||
Guidry JJ, Aday LA, Zhang D, Winn RJ. Cost considerations as potential barriers to cancer treatment. Cancer Pract. 1998;6(3):182–187. | ||
Stephenson NL, Weinrich SP, Tavakoli AS. The effects of foot reflexology on anxiety and pain in patients with breast and lung cancer. Oncol Nurs Forum. 2000;27(1): 67–72. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.