Back to Journals » Psoriasis: Targets and Therapy » Volume 6
Psoriasis in children
Authors Pinson R, Sotoodian B, Fiorillo L
Received 30 December 2015
Accepted for publication 9 March 2016
Published 20 October 2016 Volume 2016:6 Pages 121—129
DOI https://doi.org/10.2147/PTT.S87650
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Uwe Wollina
Roxanne Pinson,1 Bahman Sotoodian,2 Loretta Fiorillo2,3
1School of Medicine, 2Division of Dermatology and Cutaneous Sciences, Department of Medicine, 3Division of Pediatric Dermatology, Department of Pediatrics, University of Alberta, Edmonton, AB, Canada
Abstract: The clinical presentation, disease associations, and diverse treatment modalities in overcoming the challenges of managing pediatric psoriasis have been extensively summarized in this article. An extensive literature review revealed the differences in presentation of psoriasis during infancy, childhood, and adolescence. We also summarized the latest topical, systemic, and biological modalities in treating recalcitrant psoriasis. The association of psoriasis with juvenile arthritis and obesity and the significant influence of the disease on the children's quality of life were explored. The clinical presentation of psoriasis can evolve during the child's lifespan. While many treatment modalities already exist for treating pediatric psoriasis, some of the new biologics that are approved for adult patients have not been investigated in the pediatric population and no algorithm exists for their use in this population. Large clinical studies in the future will enhance our understanding with regards to their safety and potential implications in pediatric populations.
Keywords: pediatric, epidemiology, juvenile arthritis, topical treatment, systemic treatment, phototherapy, biologics
Introduction
Psoriasis is a common, chronic inflammatory disorder that affects the skin, nails, and joints of ~ 2.0%–3.5% of the general population.1,2 Psoriasis begins in childhood in approximately one-third of the cases.1,3–5 When psoriasis starts in childhood, it has more adverse implications. Extensive research has focused on the comorbidities associated with psoriasis and its effects on the quality of life (QoL) of the child and the adult caretaker. Children suffering from psoriasis have a higher prevalence of obesity, diabetes mellitus, hypertension, juvenile arthritis, Crohn’s disease (CD), and psychiatric disorders.1,3,5,6
Objectives
The goal of this article is to review the common and unique manifestations of pediatric psoriasis, its specific treatment approaches, and the challenges that this disease presents in children.
Materials and methods
A literature search was conducted using the PubMed database. The search terms were pediatric (all fields) or pediatrics (all fields) AND psoriasis (MeSH terms) OR psoriasis (all fields) AND therapy (subheading) OR therapy (all fields) OR treatment (all fields) OR therapeutics (MeSH terms) OR therapeutics (all fields). Only the articles published in English within the last 5 years were selected. Relevant older references were also assessed for the comprehensiveness of our review.
Epidemiology
Although pediatric psoriasis is not uncommon, there are limited epidemiological data available to date.7 Prevalence rates vary according to the following: age, sex, geographical location, study design, and case definition.8 One-third of patients develop psoriasis in childhood, and the incidence of childhood psoriasis increases with age.1,3,4 A study by Gelfand et al9 found that the prevalence of psoriasis during childhood in the UK was ~0.55% in children aged 0–9 years and 1.37% in children aged 10–19 years. Augustin et al1 demonstrated that the prevalence of psoriasis increases linearly with age during childhood as opposed to having a particular peak range.8
Similar prevalence rates have been reported in both Germany (age 0–9 years, 0.18%; age 10–19 years, 0.83%)1 and Dutch populations (age 0–10 years, 0.4%; age 11–19 years, 1.0%).10 The variation among countries perhaps suggests that the difference may be due to both genetic susceptibilities and environmental trigger factors, including sun exposure.11 A recent systematic review suggested that countries further from the equator tend to have a higher prevalence of psoriasis among their populations.8
Tollefson et al12 suggested that among pediatric patients with psoriasis, the female-to-male ratio is ~1.10. In contrast, a recent US-based multicenter study among 181 children with plaque psoriasis showed a female-to-male ratio of 1.48.8,13 The incidence of psoriasis in children seemed to have doubled from 1970 to 2000’s from 29.6/100,000 to 62.7/100,000.12 Perhaps an increase in triggers for psoriasis such as psychosocial stress, infectious diseases, and an increasing trend in obesity could potentially explain this development.12 It is also important to note that 51.4% of patients had a family history of psoriasis.13,14
Clinical presentation
Diagnosis of psoriasis in the pediatric population is more challenging when compared to the well-delineated adult psoriasis. Although the clinical subtypes may be similar, the distribution, morphology, and clinical symptoms at presentation vary according to the age group.13 Additionally, skin biopsy may not often be performed on younger patients, thus making the diagnosis more difficult. According to a recent study of 887 patients <18 years of age, the most common subtypes of psoriasis are as follows: plaque psoriasis (73.7%), followed by guttate psoriasis (13.7%), scalp psoriasis (7.6%), and pustular psoriasis (1.1%).12
Infancy
Infants usually present with rash in the diaper area that is refractive to standard diaper dermatitis treatment.7,13 Psoriatic diaper rash is characterized by sharply demarcated, minimally elevated erythematous plaques in the diaper area, involving the inguinal folds.14 Figure 1 depicts plaque psoriasis over the genitalia area. The lesions are often macerated, not scaly, and not associated with satellite pustules, differentiating them from candidiasis. The scalp is also frequently involved with thick scaly plaques, the so-called sebopsoriasis. Furthermore, young children may present with an admixture of eczematous and psoriatic lesions.15
Childhood
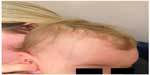
In younger children, typical erythematous plaques with overlying white scale are often thinner and smaller than in adults and tend to develop more often on the scalp, face, and flexural areas. Despite these predilection sites, psoriasis papules and plaques can develop on any skin area and are usually symmetrically distributed.7 The scalp is the most frequently involved area and often the first site of presentation in children.7,14,16,17 Figure 2 depicts plaque psoriasis affecting the scalp area. A 2013 multicenter study evaluated the frequency of involved sites.13 Scalp involvement was noted in 79.0% of participants. Scalp psoriasis around the frontal hairline can cause significant impairment in QoL due to its visibility.16
In school-aged children, psoriasis often involves the ear canals and can be misdiagnosed as otitis externa or swimmers ears. Figure 3 depicts plaque psoriasis affecting the external ear. Another specific site of involvement is on the upper eyelids, especially the medial side, and is characterized by erythema with fine white scales, often confused with allergic or contact dermatitis. The psoriasis lesions are well demarcated and often limited to the medial side of eyelids, while contact dermatitis is more diffuse all over the upper eyelids and often involves the lower eyelids too.
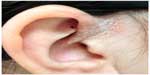  | Figure 3 Well-demarcated erythematous plaques with fine micaceous scale over periauricular area and crus of helix are noted. |
Nail disease is also commonly found in this age population.13 Nail psoriasis presents as pitting on the nail plate, oil spots, onycholysis, subungual hyperkeratosis, onychodystrophy, and splinter hemorrhages.7,16 Figure 4 demonstrates nail pitting in psoriasis. Nail changes can precede, coincide with, or develop after skin psoriasis. Nail pitting can be a useful sign to aid in the diagnosis of psoriasis when skin manifestations are equivocal.
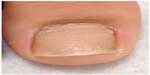  | Figure 4 Significant nail pitting and onycholysis are depicted on this image. |
Guttate psoriasis is the second most common type of psoriasis in children, being present in 14%–30% of patients.7,13 Guttate psoriasis is an acute form of psoriasis in which monomorphic scaly papules erupt on the trunk ~2 weeks after a β-hemolytic streptococcal or viral infection.7 Guttate psoriasis is often self-limiting, resolving within 3–4 months of onset.7 It has been reported that a proportion of individuals with guttate psoriasis eventually develop plaque psoriasis.7 Mercy et al13 suggested that the risk of developing severe psoriasis is much higher if it started as guttate psoriasis and persisted. The systemic review by Rachakonda et al18 demonstrated that tonsillectomy may be utilized as a treatment option in refractory psoriasis since improvement in the course of disease had been clearly documented in some patients post tonsillectomy.
Pustular psoriasis is seen in only 1.0%–5.4% of children with psoriasis. Pustular psoriasis is characterized by localized or generalized superficial sterile pustules and can be accompanied by fever, malaise, and arthralgia in the case of classical von Zumbusch type. Although pustular psoriasis is more common in adults, von Zumbusch pustular psoriasis and pustular psoriasis with an annular configuration occur more frequently in childhood.7,16 Erythrodermic psoriasis is characterized by diffuse erythema affecting >90% of the total body surface area. The condition is extremely rare in children and can lead to life-threatening hypothermia, hypoalbuminemia, and cardiac failure.16
Adolescents
Up to 75% of older children have chronic plaque psoriasis.7 The lesions are sharply demarcated, round/oval plaques with adherent silvery white scales. Psoriasis plaques can appear at any part of the body but are generally distributed symmetrically with elbows, knees, and scalp being the most common site of involvement.16 New lesions may appear following direct cutaneous trauma, which is known as the Koebner phenomenon.
Complications of juvenile arthritis and its prevalence
Juvenile psoriatic arthritis is the most common comorbidity of psoriasis. Because of difficulties in diagnosis and classification of psoriatic arthritis in children, prevalence data range from 1% to 10% of children with psoriasis.13 The peak of onset in childhood is between ages 9 and 12, and skin psoriasis often precedes psoriatic arthritis. A relationship between nail involvement and psoriatic arthritis in adults has been suggested, and a recent study in children supports this correlation.19
Obesity among pediatric patients with psoriasis
A multicenter, cross-sectional study of 409 children assessed the prevalence of obesity among children with psoriasis and control group. The odds ratio of obesity in the group of children with psoriasis was 4.29; unfortunately adiposity does not appear to decrease when psoriasis improves according to another study.20 A retrospective pilot study of 27 obese children demonstrates how being overweight or obese preceded psoriasis by at least 2 years in 93% of children.5
Diagnostic tools
Psoriasis is usually diagnosed clinically and occasionally a skin biopsy may be necessary. Histological features of psoriasis are epidermal acanthosis with parakeratosis, indicative of chronicity of the disease, loss of the granular cell layer, elongation of the rete ridges, and neutrophilic aggregates within the parakeratosis (microabscesses of Munro). The neutrophils and parakeratosis alternate in stratum corneum forming the so-called sandwich sign. The neutrophils are also grouped within the epidermis forming spongiform pustules of Kogoj. Tortuous and dilated blood vessels are present in the dermis, and there is a perivascular lymphocytic infiltrate.16 Dermoscopy is a new diagnostic tool that features psoriatic plaques as having dotted vessels regularly distributed over a light red background and diffuse superficial white scales.21 Further research is warranted to determine the added value of dermoscopy in diagnosing psoriasis. If a patient complains of joint pain and swelling, X-ray or magnetic resonance imaging of involved joint may help differentiate features of psoriatic arthritis from other forms of arthritis.
Differential diagnosis
Table 1 summarizes the most common differential diagnosis of psoriasis in the pediatric population.
  | Table 1 Differential diagnosis for pediatric psoriasis17 |
QoL of children with psoriasis
According to a study by Manzoni et al,22 psoriasis was one of the skin diseases that had the most negative influence on one’s QoL. Using the Infant Dermatitis Quality of Life Index score, a regression analysis was performed, which concluded that patients with psoriasis had 2.7 times more impaired QoL as compared to the general pediatric population. Compared to atopic dermatitis, psoriasis had a higher impact on patients within the following categories: having a symptomatic cutaneous state and suffering from sleep disturbance secondary to their underlying disease.22 A recent Swedish study including children as young as 4 years old used the Infant Dermatitis Quality of Life and the Dermatitis Family Impact and showed that younger children (aged 5–8 years) and those with joint pain had a greater impairment to their QoL than those aged 9–16 years or without joint pain.23
A retrospective study done by Kimball et al3 evaluated the relationship between the age of onset of psoriasis and the comorbidities associated with it. In this study, patients were given a questionnaire regarding their current disabilities, relationships, education, finances, and health status. The study demonstrated that those diagnosed at a younger age were more likely to have a higher lifetime disease life quality index (P<0.001), be more depressed (P 0.003), believe that psoriasis had caused their depression (P<0.001), experience lifetime sleep problems (P 0.004), use recreational drugs (P<0.001), and are more likely to experience lifetime discrimination in social settings.3
Management
This section summarizes the most current literature on the treatment options available for pediatric psoriasis.
Topical treatments
Topical steroid preparations
Topical treatments are considered the first-line treatment for psoriasis in both the pediatric and the adult population.21 The potency of steroid selected is based on the age and the location of the lesions. A recently published systematic review highlighted two studies that focused on the efficacy of corticosteroids, in particular, halobetasol cream 0.05% and clobetasol propionate emulsion 0.05%. Both of these studies showed efficacy after a 2-week treatment with local side effects including skin atrophy and hypopigmentation.24 Due to the risks of cutaneous atrophy, striae, and telangiectasia, it is best to limit the application of these high-potency steroids to a short period; low-potency topical corticosteroids can be used on the face and genital areas with lower risk of causing skin atrophy.21
Vitamin D analogs
There have been several case studies and clinical trials suggesting that vitamin D analogs are safe and effective for pediatric psoriasis.21 The two vitamin D analogs, calcipotriol and calcitriol, inhibit keratinocyte proliferation and thus can work synergistically with corticosteroids.21 A systematic review in 2010 evaluated ten studies in this category.24 Both calcitriol and calcipotriol were proven to be effective. However, calcitriol was less irritating than calcipotriol.21 Further, Oranje et al,25 performed a randomized double-blind study in 77 children aged 2–14 years. Calcipotriol was applied twice daily to lesions on all body areas except the face, scalp, genital region, and areas covered with occlusive clothing (eg, diapers) for 8 weeks. The investigators reported a decrease in Psoriasis Area and Severity Index (PASI) score of 52% in the calcipotriol (n=43) and 37.1% in the placebo group (n=34). Although theoretically serum calcium levels may be increased by this treatment, this was not observed in the study of Oranje et al.25
Three studies used the compounded formulation of calcipotriol and betamethasone dipropionate.26–28 In a prospective cohort study, 73 children (aged 3–18 years) with plaque-type psoriasis were treated with calcipotriol/betamethasone dipropionate ointment once daily for 4 weeks and 4 days a week thereafter, with a median treatment duration of 35 weeks. An improvement of the PASI score was noticeable after 1 week and progressed at weeks 12 and 24, with maintenance of improvement thereafter to week 35. Five children reported an adverse event, most commonly, the development of striae.26 A multicenter, open-label study by Gooderham et al27 found calcipotriol/betamethasone dipropionate gel applied once daily for 8 weeks in adolescents (aged 12–17 years) with moderate to severe plaque psoriasis to be well tolerated and effective. Oostveen et al28 demonstrated that calcipotriene/betamethasone dipropionate gel can be used as a treatment in pediatric scalp psoriasis (patients aged 4–17 years). Striae of the scalp skin were described as an adverse effect (AE) in three patients.27 Currently, vitamin D analogs are not approved by the US Food and Drug Administration for individuals <18 years of age.
Calcineurin inhibitors
Topical tacrolimus (0.03% and 0.1% ointment) and topical pimecrolimus (1% cream) are the topical calcineurin inhibitors approved for atopic dermatitis. Some literature suggests their efficacy for treatment of psoriasis in the pediatric population. These agents act by decreasing the production of interleukin (IL)-2 via inhibition of calcineurin, reducing T-cell activation and proliferation. Their use is most commonly seen as corticosteroid-sparing agents in highly sensitive areas such as face, genitals, and flexures.21 In an open-label study, eleven patients with psoriasis from 6–15 years old applied tacrolimus 0.1% to the facial and intertriginous areas for up to 180 days. In total, 12% of patients reported complete clearing, while the other 88% reported 90%–99% improvement.29 Another study treated 12 patients with inverse psoriasis aged 22 months to 16 years of age with tacrolimus 0.1% ointment daily – all cleared within 2 weeks.30 Topical pimecrolimus has been used successfully in infantile psoriasis and childhood psoriasis in the facial and anogenital area in two case reports.31,32
Phototherapy
A systematic review published in 2010 showed that narrowband ultraviolet B light (UVB) is an effective treatment with mild side effects for psoriasis refractive to topical treatment.24 This conclusion was based on three randomized controlled studies and two open-label studies.24
There are also several case series within the last 5 years that attest to the efficacy of UVB phototherapy in children ranging from 2–18 years of age.33–35 Most of these cases resulted in almost or complete remission over an average of 25–34 treatments. The side effects were erythema, sunburn, pruritus, and burning sensations.33–35 There is also a risk of cumulative photo damage to the skin.36 Psoralen ultraviolet A (PUVA) can be particularly associated with development of lentigines, folliculitis, polymorphic light eruption, onycholysis, and induction of skin cancers. No study has investigated the long-term sequelae of light treatment in pediatric population and its association with future skin cancers.37 Tay et al38 studied the use of UVB treatment in children aged 14 months to 12 years and indicated that psoriasis cleared after an average of 34 sessions. Having the family involved in making the treatment decisions and allowing the children to become comfortable with the devices and the lamps enhance the children compliances with the treatments.36 Considering that the role of PUVA in developing skin cancer is well documented, it is generally recommended not to use it for children.36,39
Systemic treatments
Methotrexate
Methotrexate (MTX) is a systemic immunosuppressant used in moderate-to-severe plaque psoriasis. MTX is a folic acid antagonist that irreversibly binds and inhibits the dihydrofolate reductase and thus inhibits RNA and DNA synthesis resulting in cell cycle arrest. Furthermore, it has anti-inflammatory and immunosuppressive properties that inhibit the production of inflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-6, and IL-8. MTX is approved for adult psoriasis and in the pediatric population for inflammatory bowel disease (IBD) and juvenile arthritis. A recent analysis of a perspective registry known as CHILD-CAPTURE, in the Netherlands, demonstrated the safety and efficacy of oral MTX with folic acid 5–25 mg for patients with moderate-to-severe plaque psoriasis.40 PASI 75 was reached in 32% of patients after 12 weeks, and PASI 90 was achieved in 20%. The most common side effects reported were nausea and fatigue40; other side effects include vomiting, stomatitis, and abnormal liver function tests.41–43 Folic acid can be given in conjunction with MTX to minimize AEs.44
Cyclosporine
Cyclosporine (CyA) is an immunosuppressant drug approved for treatment of psoriasis in adults. The recommended initial dose is 5 mg/kg/d for adults; once remission is achieved, the dose is tapered down every 2 weeks.45 CyA has been indicated for generalized pustular psoriasis or severe refractive psoriasis. The onset of action for CyA is relatively rapid with improvement reported as early as 2 weeks from the beginning of therapy.43 AEs can include nephrotoxicity and hypertension, both of which are of particular concern in long-term use.46 These side effects are dose dependent and, in almost all cases, reversible after discontinuation of CyA. Provided the patient is monitored for side effects, treatment can be continued for up to 2 years. To date, the efficacy of CyA for pediatric psoriasis remains unclear. A previous systematic review published in the JAAD in 2010 reviewed four studies (n=9) to conclude that there are limited data on its efficacy and safety.24
Retinoids
Retinoids are vitamin A analogs that work systematically by altering cellular metabolic pathways, cellular differentiation, and apoptosis. The two documented retinoids used for treatment of psoriasis in the literature are etretinate and acitretin (a metabolite of etretinate).47 Acitretin replaced etretinate as of 1998.47 Acitretin is considered as a first-line therapy for generalized pustular psoriasis.43 It is also used as a maintenance treatment for severe guttate psoriasis, palmoplantar psoriasis, erythrodermic psoriasis, or plaque psoriasis.47 Side effects include pruritus, cheilitis, skin fragility, hair loss, musculoskeletal pain, focal osteoporosis, elevated serum transaminase levels, dyslipidemia, glomerulonephritis, and paronychia.48 Due to the teratogenicity of oral retinoids and specific concerns about the delayed clearance of acitretin, oral isotretinoin may be a more appropriate choice for use in adolescent girls of childbearing potential due to the significantly more rapid clearance.49 The washout period for isotretinoin is 1 month; however, due to re-esterification of acitretin, contraceptive measures has to be continued for at least 3 years post stoppage date of acitretin.50 Regarding the application of topical retinoids in the treatment of psoriasis, Diluvio et al51 demonstrated in an isolated case report that off-label use of tazarotene 0.05% gel once daily for 8 weeks provided considerable improvement in the treatment of pitting associated with nail psoriasis.
Biologics
Biologics are pharmacologic agents developed to target specific mediators of inflammation and thus used against diseases such as psoriasis, IBD, and arthritis.24
Etanercept
Etanercept is a recombinant protein that binds to TNF-α and inhibits binding of TNF-α to its target receptor.21 It is approved for use in Europe for the treatment of plaque psoriasis in children aged 6 years and older who have not responded to conventional therapy and/or phototherapy. A case report of using etanercept in even much younger children (22 months of age) for the treatment of severe and recalcitrant psoriasis has been published.52
In 2008 a randomized, double-blind, Phase III clinical trial treated 211 patients from ages 4 to 17 years old with either etanercept or placebo for 12 weeks, followed by an open-label period of 24 weeks, and subsequent second randomization at 36 weeks to investigate the effects of withdrawal or treatment.53 At the end of the initial 12-week phase, PASI 75 was seen in 57% of the children versus 11% in the placebo arm.53 An open-label extension study enrolled 182 patients, of which 140 completed 96 weeks of therapy, demonstrated PASI 75 in 61% and PASI 90 in 30%.54 Of 181 patients, 145 (80.1%) reported one or more AEs, with the most common being upper respiratory tract infections (24.9%), nasopharyngitis (17.1%), streptococcal pharyngitis (12.7%), headache (11.6%), and sinusitis (10.5%).54
Adalimumab (Humira)
Adalimumab is a humanized monoclonal antibody to TNF-α. It is approved by the US Food and Drug Administration for use in adults with psoriasis and other disorders, and it is approved for the treatment of severe chronic plaque psoriasis in the European Union in children and adolescents from 4 years of age who have had an inadequate response to or are inappropriate candidates for topical therapy and phototherapy. In the US and Canada, it is approved for juvenile arthritis and CD from 4 years of age. A Phase III international multicenter randomized double-blind study showed superiority of adalimumab 0.8 mg/kg every other week to MTX. The parent company is presently seeking approval for the use of adalimumab in pediatric psoriasis in Canada and the US.55,56
Infliximab
Infliximab is a chimeric monoclonal antibody that recognizes soluble and membrane-bound human TNF-α, thus preventing it from binding to its receptor.57 It is approved in most countries for use in adults with psoriasis and other diseases. It is also approved for use in children aged 6 years and older with CD and ulcerative colitis.57 It is not approved in any country for the treatment of pediatric psoriasis. A recently published review of biologics in the pediatric population noted that infliximab had a consistently higher rate of reported malignancies (66/100,000 for all malignancies and 44/100,000 for lymphomas) than background rates in the general pediatric population (16.8/1,000,000 for all malignancies and 2.4/100,000 for lymphomas).57 Interestingly, psoriasis has been reported to be induced by treatment with infliximab for IBD in the pediatric population.58,59
Ustekinumab
Ustekinumab is a monoclonal antibody that attacks the p40 subunit of IL-12 and -23 in the inflammatory process. It is approved in most countries for the treatment of moderate-to-severe plaque psoriasis in adults. It is approved in Europe for the treatment of adolescent patients with moderate-to-severe psoriasis (ages 12–17 years) based on a positive Phase III of CADMUS study, a randomized, double-blind, placebo-controlled, parallel, multicenter trial. In this study, 80% and 61% of patients, respectively, achieved PASI 75 and PASI 90 at week 12. Dose regimen was two loading doses (weeks 0 and 4), followed by maintenance doses q12w. The dosing approved by the European Medicines Agency is as follows: <60 kg, 0.75 mg/kg; ≥60 to ≤100 kg, 45 mg; >100 kg, 90 mg. Because of its rapid onset and convenient dosing regimen, it is very appealing for the pediatric population.60 Since the weight-adjusted pediatric population in this study had similar results to those in the adult PHEONIX study, they concluded that the metabolism in patients aged 12–17 years is similar to that of adult patients.60 The more commonly reported AEs at week 60 included nasopharyngitis (34.5%), upper respiratory tract infection (12.7%), and pharyngitis (8.2%); only four patients discontinued the treatments due to AEs.60 Additionally, there were no malignancies, active tuberculosis cases, opportunistic infections, anaphylactic reactions, or serum sickness-like reactions through week 60. A recent review comparing ustekinumab and etanercept reported that ustekinumab was more efficacious at 12 weeks when compared to etanercept.61
Conclusion
Pediatric psoriasis has numerous challenges: it presents with age-specific clinical characteristics, and the presentation may evolve with age. Many treatment options approved for adults have not been studied in children; published guidelines and treatment algorithms do not include children, and adherence is difficult, especially in the toddler and adolescent age group. Larger studies are definitely needed to further investigate the safety and efficacy of all current treatment modalities. Finally, the psychosocial impact of a chronic disease in childhood has more severe implications for general function in all spheres of adult life.
Acknowledgment
All the figures were obtained with parental written consent for publication.
Disclosure
Dr Fiorillo declares a conflict of interest as she is involved with a clinical study on pediatric psoriasis sponsored by Celgene corporation. The authors report no other conflicts of interest in this work.
References
Augustin M, Glaeske G, Radtke MA, Christophers E, Reich K, Schafer I. Epidemiology and comorbidity of psoriasis in children. Br J Dermatol. 2010;162(3):633–636. | |
Christophers E. Psoriasis – epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26(4):314–320. | |
Kimball AB, Wu EQ, Guerin A, et al. Risks of developing psychiatric disorders in pediatric patients with psoriasis. J Am Acad Dermatol. 2012;67(4):651–657.e1–e2. | |
Zampieron A, Buja A, Fusco M, et al. Quality of life in patients with scalp psoriasis. G Ital Dermatol Venereol. 2015;150(3):309–316. | |
Becker L, Tom WL, Eshagh K, Benjamin LT, Paller AS. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014; 150(5):573–574. | |
Paller AS, Mercy K, Kwasny MJ, et al. Association of pediatric psoriasis severity with excess and central adiposity: an international cross-sectional study. JAMA Dermatol. 2013;149(2):166–176. | |
Bronckers IM, Paller AS, van Geel MJ, van de Kerkhof PC, Seyger MM. Psoriasis in children and adolescents: diagnosis, management and comorbidities. Paediatr Drugs. 2015;17(5):373–384. | |
Parisi R, Symmons DP, Griffiths CE, Ashcroft DM; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385. | |
Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141(12):1537–1541. | |
De Jager ME, Van de Kerkhof PC, De Jong EM, Seyger MM. Epidemiology and prescribed treatments in childhood psoriasis: a survey among medical professionals. J Dermatolog Treat. 2009;20(5):254–258. | |
Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583):263–271. | |
Tollefson MM, Crowson CS, McEvoy MT, Maradit Kremers H. Incidence of psoriasis in children: a population-based study. J Am Acad Dermatol. 2010;62(6):979–987. | |
Mercy K, Kwasny M, Cordoro KM, et al. Clinical manifestations of pediatric psoriasis: results of a multicenter study in the United States. Pediatr Dermatol. 2013;30(4):424–428. | |
Silverberg NB. Update on pediatric psoriasis. Cutis. 2015;95(3):147–152. | |
Morris A, Rogers M, Fischer G, Williams K. Childhood psoriasis: a clinical review of 1262 cases. Pediatr Dermatol. 2001;18(3):188–198. | |
Raychaudhuri SK, Maverakis E, Raychaudhuri SP. Diagnosis and classification of psoriasis. Autoimmun Rev. 2014;13(4–5):490–495. | |
Matteo Megna and Maddalena Napolitano, Balato A, Scalvenzi M, et al. Psoriasis in children: a review. Curr Pediatr Rev. 2015;11(1):10–26. | |
Rachakonda TD, Dhillon JS, Florek AG, Armstrong AW. Effect of tonsillectomy on psoriasis: a systematic review. J Am Acad Dermatol. 2015;72(2):261–275. | |
Butbul Aviel Y, Tyrrell P, Schneider R, et al. Juvenile Psoriatic Arthritis (JPsA): juvenile arthritis with psoriasis? Pediatr Rheumatol Online J. 2013;11(1):11. | |
Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149(7):795–801. | |
Shah KN. Diagnosis and treatment of pediatric psoriasis: current and future. Am J Clin Dermatol. 2013;14(3):195–213. | |
Manzoni AP, Weber MB, Nagatomi AR, Pereira RL, Townsend RZ, Cestari TF. Assessing depression and anxiety in the caregivers of pediatric patients with chronic skin disorders. An Bras Dermatol. 2013;88(6):894–899. | |
Ganemo A, Wahlgren CF, Svensson A. Quality of life and clinical features in Swedish children with psoriasis. Pediatr Dermatol. 2011;28(4):375–379. | |
de Jager ME, de Jong EM, van de Kerkhof PC, Seyger MM. Efficacy and safety of treatments for childhood psoriasis: a systematic literature review. J Am Acad Dermatol. 2010;62(6):1013–1030. | |
Oranje AP, Marcoux D, Svensson A, et al. Topical calcipotriol in childhood psoriasis. J Am Acad Dermatol. 1997;36(2 pt 1):203–208. | |
van Geel MJ, Mul K, Oostveen AM, van de Kerkhof PC, de Jong EM, Seyger MM. Calcipotriol/betamethasone dipropionate ointment in mild-to-moderate paediatric psoriasis: long-term daily clinical practice data in a prospective cohort. Br J Dermatol. 2014;171(2):363–369. | |
Gooderham M, Debarre JM, Keddy-Grant J, Xu Z, Kurvits M, Goodfield M. Safety and efficacy of calcipotriol plus betamethasone dipropionate gel in the treatment of scalp psoriasis in adolescents 12–17 years of age. Br J Dermatol. 2014;171(6):1470–1477. | |
Oostveen AM, de Jong EM, Donders AR, van de Kerkhof PC, Seyger MM. Treatment of paediatric scalp psoriasis with calcipotriene/betamethasone dipropionate scalp formulation: effectiveness, safety and influence on children’s quality of life in daily practice. J Eur Acad Dermatol Venereol. 2015;29(6):1193–1197. | |
Brune A, Miller DW, Lin P, Cotrim-Russi D, Paller AS. Tacrolimus ointment is effective for psoriasis on the face and intertriginous areas in pediatric patients. Pediatr Dermatol. 2007;24(1):76–80. | |
Steele JA, Choi C, Kwong PC. Topical tacrolimus in the treatment of inverse psoriasis in children. J Am Acad Dermatol. 2005;53(4):713–716. | |
Amichai B. Psoriasis of the glans penis in a child successfully treated with Elidel (pimecrolimus) cream. J Eur Acad Dermatol Venereol. 2004;18(6):742–743. | |
Mansouri P, Farshi S. Pimecrolimus 1 percent cream in the treatment of psoriasis in a child. Dermatol Online J. 2006;12(2):7. | |
Zamberk P, Velazquez D, Campos M, Hernanz JM, Lazaro P. Paediatric psoriasis – narrowband UVB treatment. J Eur Acad Dermatol Venereol. 2010;24(4):415–419. | |
Lara-Corrales I, Ramnarine S, Lansang P. Treatment of childhood psoriasis with phototherapy and photochemotherapy. Clin Med Insights Pediatr. 2013;7:25–33. | |
Pavlovsky M, Baum S, Shpiro D, Pavlovsky L, Pavlotsky F. Narrow band UVB: is it effective and safe for paediatric psoriasis and atopic dermatitis? J Eur Acad Dermatol Venereol. 2011;25(6):727–729. | |
Patrizi A, Raone B, Ravaioli GM. Management of atopic dermatitis: safety and efficacy of phototherapy. Clin Cosmet Investig Dermatol. 2015;8:511–520. | |
Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327–349. | |
Tay YK, Morelli JG, Weston WL. Experience with UVB phototherapy in children. Pediatr Dermatol. 1996;13(5):406–409. | |
Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62(1):114–135. | |
van Geel MJ, Oostveen AM, Hoppenreijs EP, et al. Methotrexate in pediatric plaque-type psoriasis: long-term daily clinical practice results from the Child-CAPTURE registry. J Dermatolog Treat. 2015;26(5):406–412. | |
van Geel MJ, Mul K, de Jager ME, van de Kerkhof PC, de Jong EM, Seyger MM. Systemic treatments in paediatric psoriasis: a systematic evidence-based update. J Eur Acad Dermatol Venereol. 2015;29(3):425–437. | |
Wright NA, Piggott CD, Eichenfield LF. The role of biologics and other systemic agents in the treatment of pediatric psoriasis. Semin Cutan Med Surg. 2010;29(1):20–27. | |
Kilic SS, Hacimustafaoglu M, Celebi S, Karadeniz A, Ildirim I. Low dose cyclosporin A treatment in generalized pustular psoriasis. Pediatr Dermatol. 2001;18(3):246–248. | |
Zweegers J, de Jong EM, Nijsten TE, et al. Summary of the Dutch S3-guidelines on the treatment of psoriasis 2011. Dutch Society of Dermatology and Venereology. Dermatol Online J. 2014;20(3):21769. | |
Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43–S54. | |
Pereira TM, Vieira AP, Fernandes JC, Sousa-Basto A. Cyclosporin A treatment in severe childhood psoriasis. J Eur Acad Dermatol Venereol. 2006;20(6):651–656. | |
Marqueling AL, Cordoro KM. Systemic treatments for severe pediatric psoriasis: a practical approach. Dermatol Clin. 2013;31(2):267–288. | |
Rosinska D, Wolska H, Jablonska S, Konca I. Etretinate in severe psoriasis of children. Pediatr Dermatol. 1988;5(4):266–272. | |
Al-Shobaili H, Al-Khenaizan S. Childhood generalized pustular psoriasis: successful treatment with isotretinoin. Pediatr Dermatol. 2007;24(5):563–564. | |
Lee CS, Koo J. A review of acitretin, a systemic retinoid for the treatment of psoriasis. Expert Opin Pharmacother. 2005;6(10):1725–1734. | |
Diluvio L, Campione E, Paterno EJ, Mordenti C, El Hachem M, Chimenti S. Childhood nail psoriasis: a useful treatment with tazarotene 0.05%. Pediatr Dermatol. 2007;24(3):332–333. | |
Fabrizi G, Guerriero C, Pagliarello C. Etanercept in infants: suberythrodermic, recalcitrant psoriasis in a 22 month-old child successfully treated with etanercept. Eur J Dermatol. 2007;17(3):245. | |
Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358(3):241–251. | |
Paller AS, Siegfried EC, Eichenfield LF, et al. Long-term etanercept in pediatric patients with plaque psoriasis. J Am Acad Dermatol. 2010;63(5):762–768. | |
Alvarez AC, Rodriguez-Nevado I, De Argila D, et al. Recalcitrant pustular psoriasis successfully treated with adalimumab. Pediatr Dermatol. 2011;28(2):195–197. | |
Callen JP, Jackson JH. Adalimumab effectively controlled recalcitrant generalized pustular psoriasis in an adolescent. J Dermatolog Treat. 2005;16(5–6):350–352. | |
Bellodi Schmidt F, Shah KN. Biologic response modifiers and pediatric psoriasis. Pediatr Dermatol. 2015;32(3):303–320. | |
Hiremath G, Duffy L, Leibowitz I. Infliximab-induced psoriasis in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2011;52(2):230–232. | |
Pourciau C, Shwayder T. Occurrence of pustular psoriasis after treatment of Crohn disease with infliximab. Pediatr Dermatol. 2010;27(5):539–540. | |
Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73(4):594–603. | |
Young MS, Horn EJ, Cather JC. The ACCEPT study: ustekinumab versus etanercept in moderate-to-severe psoriasis patients. Expert Rev Clin Immunol. 2011;7(1):9–13. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.