Back to Journals » OncoTargets and Therapy » Volume 13
Pseudoprogression After Palbociclib with Aromatase Inhibitors Treatment in Metastatic Breast Cancer
Authors Huang W, Li C, Chen M , Lin D, Wu F, Chen X , Li N , Wang L, Liu J
Received 10 March 2020
Accepted for publication 20 July 2020
Published 5 August 2020 Volume 2020:13 Pages 7785—7792
DOI https://doi.org/10.2147/OTT.S253333
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Weiwei Huang,1 Chao Li,2 Mulan Chen,1 Duanyu Lin,3 Fan Wu,1 Xinhua Chen,1 Nani Li,1 Lili Wang,1 Jian Liu1
1Department of Medical Oncology, Fujian Cancer Hospital, Fujian Medical University Cancer Hospital, The Teaching Hospital of Fujian University of Traditional Chinese Medicine, Fuzhou, People’s Republic of China; 2Department of Pathology, Fujian Cancer Hospital, Fujian Medical University Cancer Hospital, The Teaching Hospital of Fujian University of Traditional Chinese Medicine, Fuzhou, People’s Republic of China; 3Department of Imaging, Fujian Cancer Hospital, Fujian Medical University Cancer Hospital, The Teaching Hospital of Fujian University of Traditional Chinese Medicine, Fuzhou, People’s Republic of China
Correspondence: Jian Liu Tel +86-13906912727
Email [email protected]
Abstract: A 62-year-old postmenopausal woman was diagnosed with breast cancer in her left breast and received modified radical mastectomy (molecular type: hormone-receptor-positive human epidermal growth factor receptor-2 negative). After that, she received postoperative chemotherapy and radiotherapy. After 2 years of tamoxifen adjuvant endocrine treatment, the patient inccurred recurrence with metastasis. PET-CT scanning showed metastasis in the left thoracic wall, the sixth left rib, and the right lower lobe of the lung. Multiple lymph node metastases were observed throughout the body. Palbociclib in combination with an aromatase inhibitor (AI) was used, but the metastatic lesion at the sixth left rib increased than before. Subsequently, the lesion shrunk and the clinical symptoms were relieved, which was considered as a pseudoprogression. Herein, we reported a pseudoprogression in the breast cancer patient after treatment with palbociclib plus AI.
Keywords: pseudoprogression, palbociclib, breast cancer
Introduction
Breast cancer is the most common malignant tumor in women, which accounts for nearly 25% of all the malignancies in females. It is also the primary cause of malignant tumor-induced deaths in over 100 countries. The most common subtype of breast cancer is hormone receptor-positive (HR+), also known as the luminal subtype.1 Most patients with early breast cancer are likely to survive for a long time after treatment. Nevertheless, recurrence with metastasis might occur in some patients. Once the metastasis occurs, it is almost incurable, and the 5-year survival rate is only about 24%.2
Endocrine therapy is indispensable in the treatment of advanced HR+ breast cancer patients.3 Intriguingly, primary or secondary endocrine resistance might still lead to disease progression. Most women shift to chemotherapy after the first to third lines of endocrine therapy.4 In contrast to endocrine therapy, chemotherapy usually lead to more serious adverse events. Therefore, a new therapy needs to be developed to improve the progression-free survival of advanced breast cancer patients with endocrine resistance, as well as delay the initiation of chemotherapy with not reducing the quality of life in patients.
Palbociclib is a primary optional oral reversible CDK4/6 inhibitor. Palbociclib has been approved by the US Food and Drug Administration (FDA) for the treatment of postmenopausal women with HR+HER2- breast cancer.5 According to the findings of PALOMA-1 and PALOMA-2 studies, palbociclib has been approved to be utilized with AI for initial endocrine therapy.6,7 According to the results of the PALOMA-3 study, palbociclib can be combined with fulvestrant for the treatment of HR+HER-2- advanced breast cancer patients with endocrine resistance. Consequently, the progression-free survival (PFS) benefits could be transferred to overall survival (OS), thereby indicating that the combined therapy strategy does not reduce the benefits of subsequent treatments like chemotherapy.8,9
The pseudoprogression was described as initially increased tumor lesion size and subsequently decreased tumor burden in cancer patients with immunotherapy. Yet, the pseudoprogression has not been reported in patients who received palbociclib plus endocrine therapy. Herein, we reported a postmenopausal woman with advanced breast cancer who had pseudoprogression appeared after first-line therapy in advanced breast cancer by palbociclib combined AI.
Case Presentation
A 62-year-old postmenopausal woman was treated with modified radical mastectomy in Fujian Cancer Hospital, Fuzhou, China, for breast cancer in her left breast on April 20, 2017. The diagnosis was invasive ductal carcinoma of the left breast (pT2N3aM0 stage IIIC). Postoperative pathological examinations showed invasive ductal carcinoma in the outer upper quadrant of the left breast (mass size of 2.5×2 cm2) with cancer embolus in the vessels but not in the papilla of the specimen. Ipsilateral axillary lymph node metastases were 11/16 positive. Immunohistochemistry showed that estrogen receptor (ER) was strongly positive (95%), progesterone receptor (PR) was weakly to moderately positive (10%), Ki-67 was 35%+, CK5/6 (-), P63 (-), E-CA (+), P120 membrane (+), and Cerb-2 2+. Fluorescence in situ hybridization (FISH) was negative. After the surgery, adjuvant chemotherapy of EC×4-T×4 was conducted, followed by supplementary intensity-modulated radiation therapy (IMRT) of the lymph node drainage area in the left clavicle area and the left thoracic wall. Tamoxifen (20 mg, qd) was administered after the initiation of radiotherapy until March 12, 2019. The patient had blunt pain in the left thoracic wall, while no signs of chest distress, palpitation, cough, or dyspnea were observed. Color ultrasound examinations of the breast, thoracic wall, axillary fossa, and supraclavicular area showed solid space occupation on the lateral side of the left thoracic wall, which was close to the left axillary fossa and considered to be a metastasis. Besides, the enlarged level IV lymph nodes in the right cervix were also considered to be a metastasis. PET-CT scanning revealed the following: 1) multiple lymph node metastases in the mediastinum, bilateral portal, and left lower lung; 2) metastatic tumor in the basal segment of the right lower lobe; 3) right scapula metastasis; 4) sixth left rib metastasis accompanied by mass in the soft tissues with SUVmax of 17.2 (Figure 1A and B). The pathological examination of the biopsy of the mass in the left thoracic wall showed invasive carcinoma. In contrast, that of the level IV lymph node in the right cervix showed metastatic poor differentiated carcinoma. Immunohistochemistry showed the following findings: 1) ER 90% moderately to strongly positive, PR about 15% moderately to strongly positive, Cerb-2 2+, Ki-67 30% (+), GATA3 (+), and GCDFP-15 (-), and few mammaglobin (+) on slide 1 (Figure 2); 2) GATA3 (+), GCDFP-15 (-), and CK7 (+) on slide 2. FISH was negative. The woman had a 2-year history of type 2 diabetes, and was on the long-term oral intake of Diamicron (80 mg, bid); also, the blood glucose was well-controlled. The patient’s mother died of lung cancer, and her father, who was diagnosed with rectal cancer, died of a stroke. The patient’s physical examination results were as follows: a hard lymph node of about 1×1.5 cm2 at the right cervix was touched, and an old transverse surgical scar with 15 cm length was found in the left thoracic wall. Also, a patchy bulging mass of about 5×6 cm2 was touched at the level of the sixth rib in the left anterior thoracic wall and was found to be hard but without reddening, swelling, or disruption, although pressing pain was evident.
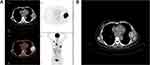 |
Figure 1 Positron emission tomography and computed tomography image (A) and computed tomography image (B) on March 18, 2019. |
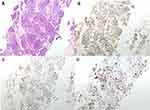 |
Figure 2 Hematoxylin-eosin staining of the first biopsy and immunohistochemistry of the thoracic mass. (A) Hematoxylin-eosin staining. (B) Estrogen Receptor. (C) Progesterone Receptor. (D) Ki-67. |
Palbociclib (125 mg po qd d1-d21, q28d) combined with exemestane (25 mg po qd) has been administered for the treatment since April 1, 2019. The pain in the left thoracic wall was significantly alleviated after ten days of the treatment. Re-examination with CT scan on June 17, 2019, after three cycles of treatment, showed that the metastasis in the sixth left rib and the mass in the soft tissue progressed, which affected the fifth and sixth intercostal spaces; while the other metastases regressed (Figure 3A). Further PET-CT scanning showed that the mass in the soft tissues of the sixth left rib increased, and the SUVmax was 12.4 (Figure 3B); the other metastases regressed, and the SUV values decreased. Physical examination did not reveal any enlargement of the superficial lymph nodes, and the mass in the left anterolateral thoracic wall was flattened without pain during pressing. Biopsy and pathological examination of the mass in the left thoracic wall showed adenocarcinoma invasion in the fibrous tissues of the left thoracic wall. Immunohistochemistry showed ER 75% strongly positive, PR 60% weakly positive, Cerb-2 2+, Ki-67 1% (+), GATA3 (+), and P120 (membrane+) (Figure 4). At first, we thought it was tumor flare, and later we found a large number of inflammatory cells infiltrated the tumor tissue and tumor cells necrosed. Meanwhile, the clinical manifestations and pathological results were improved. Therefore, we ruled out the possibility of the tumor flare, and the phenomenon were identified as pseudoprogression.
 |
Figure 3 (A) Computed tomography image at the first re-examination on June 17, 2019. (B) Positron emission tomography and computed tomography image on June 24, 2019. |
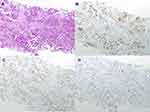 |
Figure 4 Hematoxylin-eosin staining of the second biopsy and immunohistochemistry of the thoracic mass. (A) Hematoxylin-eosin staining. (B) Estrogen Receptor. (C) Progesterone Receptor. (D) Ki-67. |
Due to reduced Ki-67 expression and the improvement of clinical manifestations and pathological results, we believed that tumor proliferation was still inhibited. Therefor the treatment of palbociclib, combined with exemestane, was continued until July 31, 2019. Then re-examination with thoracic CT scanning was conducted, which showed that the metastasis in the sixth left rib and mass in the soft tissue regressed, and bone reconstruction was observed (Figure 5). The tumor in the left thoracic wall regressed, and the patient did not experience any discomfort, such as pain in the chest and cough. The treatment was continued. The re-examination with PET-CT scanning was conducted on October 28, 2019, showing the bone destruction at the sixth left rib accompanied by the mass approximately 3.19×2.05 cm2 in size and SUVmax 12.4 that continuously regressed in the soft tissues (Figure 6). Moreover, the biopsy and pathological examination of the thoracic mass showed that the ER was about 10% weakly positive, PR about 25% weakly positive, Cerb-2 2+, KI-67 1% (+), and GATA3 (+). FISH was negative (Figure 7).
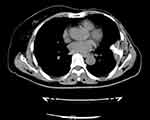 |
Figure 5 Re-examination with thoracic computed tomography scanning on July 31, 2019. |
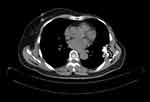 |
Figure 6 Positron emission tomography and computed tomography image on October 28, 2019. |
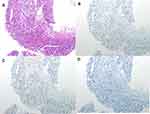 |
Figure 7 Hematoxylin-eosin staining of the third biopsy and immunohistochemistry of the thoracic mass. (A) Hematoxylin-eosin staining. (B) Estrogen Receptor. (C) Progesterone Receptor. (D) Ki-67. |
Discussion
Previous studies have shown that cyclin-dependent kinase (CDK) is the key regulator of the cell cycle process and RNA transcription. At the same time, inhibition of CDK could prevent the downstream phosphorylation of retinoblastoma protein (Rb), arrest the cells in the G1 phase, prevent the cells from entering S phase for DNA replication in vitro, and induce tumor regression in vivo.10,11 Animal studies demonstrated that a single oral dose of palbociclib induced significant regression of tumor cells of various human tumor grafts in mice.12 In the Phase II trial of palbociclib, the Rb staining of 37 recruited women with metastatic breast cancers showed positive results, ie, the intensity of staining >1+ in the primary tumor or metastatic tumor. Most patients (85%) were HR+HER- and had been previously treated with two or more cytotoxic agents (76%) or endocrine therapy (65%). In summary, the investigators reported that the clinical benefit rate of the HR+ breast cancer patients was 21%. In comparison, it increased to 29% in patients with disease progression after two lines of endocrine therapy. These findings demonstrated that palbociclib could exert a single-agent activity and mainly so in women with adaptive endocrine resistance. However, the PFS of the HR+ and HER2- women was only 3.8 months (95% CI: 1.9–5.8).13
In the present case, the patient was treated only with tamoxifen adjuvant endocrine therapy before using palbociclib. The tumor relapsed and progressed within two years, suggesting that the patient might have developed endocrine resistance. The patient has been on treatment for 8 months with palbociclib plus exemestane, and her disease had not progressed at the time of drafting this manuscript. The median PFS reported in the PALOMA-16 and PALOMA-27 were 20.2 months (Palbociclib-AI group) and 24.8 months (Palbociclib-Letrozole group) respectively. This indicated that patients might have to undergo a longer treatment period.
The imaging of our patient at the first re-examination after treatment showed transient enlargement of the metastasis in the left thoracic wall accompanied by bone metastasis. However, the consequent re-examinations showed that these lesions continuously shrunk, and the pathological results and clinical manifestations rapidly improved. These phenomena seem to indicate that this was a tumor flare, but we found the infiltration of a large number of inflammatory cells. Thus, we speculated that the rapid disruption of the tumor cells in the local lesion and invasion of inflammatory cells could lead to the release of abundant cytokines, which was accompanied by active bone reconstruction in the bone metastasis, thereby increasing the size of the mass. To the best of our knowledge, such pseudoprogression has not yet been reported in patients with palbociclib plus exemestane. The following follow up showed that the woman responded adequately to the treatment, the tumor shrunk continuously, and the clinical manifestations rapidly improved. Besides, re-examination with biopsy and pathological examinations of the enlarged tumor showed decreased proliferation index of the tumor.
Pseudoprogression is a common phenomenon in patients using immunotherapy, which is defined as an increase in the size of the primary tumor followed by tumor regression. An important feature of pseudoprogression is infiltration and recruitment of various immune cells in tumor tissues.14 CDK4/6 inhibitors may trigger anti-tumor immunity by enhancing tumor antigen presentation and suppressing the proliferation of regulatory T cells. These effects might promote cytotoxic T-cell-mediated clearance of tumor cells and caused pseudoprogression.15
Conclusion
This is the first clinical case of pseudoprogression in a patient with metastatic breast cancer treated with palbociclib. The tumor size increased in a short time after the treatment but then rapidly shrunk, the Ki-67 also decreased, followed by continuous benefits. In the case of patients with imaging progression, clinical manifestations improved during the treatment with palbociclib. Yet, further observations are needed, and re-examination with pathologic biopsy should be conducted to confirm the progression. Also, the drug should not be hastily discontinued.
Abbreviations
CDK, cyclin-dependent kinase; ER, estrogen receptor; FDA, Food and Drug Administration; FISH, fluorescence in situ hybridization; HR+, hormone receptor-positive; IMRT, intensity-modulated radiation therapy; LHRH, luteinizing hormone-releasing hormone; OS, overall survival; PFS, progression-free survival; PR, progesterone receptor; Rb, retinoblastoma protein.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
In this study, the standard care was performed, thus ethical approval is not applicable. Written informed consent was obtained from the patient.
Acknowledgment
We appreciate the valuable review comments provided by Pfizer Inc. medical affairs.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5993 breast cancers. Am J Clin Pathol. 2005;123(1):21–27. doi:10.1309/4WV79N2GHJ3X1841
2. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi:10.3322/caac.21349
3. Ellis M, Hays D, Ellis L. Treatment of metastatic breast cancer. In: Harris J, Lippman M, Morrow M, Osborne C, editors. Disease of the Breast. Philadelphia: Lippincott Williams; 2004:1101–1159.
4. Clarysse A. Hormone-induced tumor flare. Eur J Cancer Clin Oncol. 1985;21(5):545–547. doi:10.1016/0277-5379(85)90077-X
5. Beaver JA, Amiri-Kordestani L, Charlab R, et al. FDA approval: palbociclib for the treatment of postmenopausal patients with estrogen receptor-positive, HER2-negative metastatic breast cancer. Clin Cancer Res. 2015;21(21):4760–4766. doi:10.1158/1078-0432.CCR-15-1185
6. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised Phase 2 study. Lancet Oncol. 2015;16(1):25–35. doi:10.1016/S1470-2045(14)71159-3
7. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi:10.1056/NEJMoa1607303
8. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, Phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi:10.1016/S1470-2045(15)00613-0
9. Walker AJ, Wedam S, Amiri-Kordestani L, et al. FDA approval of palbociclib in combination with fulvestrant for the treatment of hormone receptor-positive, HER2-negative metastatic breast cancer. Clin Cancer Res. 2016;22(20):4968–4972. doi:10.1158/1078-0432.CCR-16-0493
10. Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77. doi:10.1186/bcr2419
11. Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24(11):1770–1783. doi:10.1200/JCO.2005.03.7689
12. Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3(11):1427–1438.
13. DeMichele A, Clark AS, Tan KS, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res. 2015;21(5):995–1001. doi:10.1158/1078-0432.CCR-14-2258
14. Ma Y, Wang Q, Dong Q, Zhan L, Zhang J. How to differentiate pseudoprogression from true progression in cancer patients treated with immunotherapy. Am J Cancer Res. 2019;9(8):1546–1553.
15. Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548(7668):471–475. doi:10.1038/nature23465
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
