Back to Journals » OncoTargets and Therapy » Volume 12
Pseudogene DUXAP10 acts as a diagnostic and prognostic marker and promotes cell proliferation by activating PI3K/AKT pathway in hepatocellular carcinoma
Authors Yue C , Liang C, Ge H, Yan L, Xu Y, Li G, Li P, Wei Z, Wu J
Received 30 March 2019
Accepted for publication 16 May 2019
Published 11 June 2019 Volume 2019:12 Pages 4555—4566
DOI https://doi.org/10.2147/OTT.S210623
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Chaosen Yue,1,* Chaojie Liang,2,* Hua Ge,1 Lijun Yan,1 Yingchen Xu,1 Guangming Li,1 Pengyang Li,3 Zhigang Wei,2 Jixiang Wu1
1Department of General Surgery, Beijing Tongren Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of General Surgery, First Hospital/First Clinical Medical College of Shanxi Medical University, Taiyuan, Shanxi, People’s Republic of China; 3Department of Medicine, Saint Vincent Hospital, Worcester, MA, USA
*These authors contributed equally to this work
Background: Recently, the pseudogene DUXAP10 was shown to be overexpressed in various human cancers and emerged as a key cancer regulator. However, the roles of DUXAP10 in hepatocellular carcinoma (HCC) tumorigenesis and progression remain uncharacterized.
Methods: Comprehensive analyses were performed to investigate DUXAP10 expression patterns, potential biologic functions, and clinical significance in HCC based on the data downloaded from the Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) databases. DUXAP10 expression levels in HCC tissue sections and cells were verified using quantitative real-time PCR analysis. DUXAP10-siRNA was used to silence DUXAP10 in the Hep3B cell line to determine the roles of DUXAP10 in HCC cell proliferation.
Results: DUXAP10 was significantly overexpressed in HCC, and DUXAP10 upregulation was closely associated with poor prognoses in HCC patients. DUXAP10 knockdown decreased cell proliferation and arrested HCC cells in the G1 phase of the cell cycle. Western blot analysis showed that DUXAP10 knockdown decreased p-AKT expression in HCC cells.
Conclusion: Our study demonstrates that pseudogene DUXAP10 promotes HCC cell proliferation by activating PI3K/AKT pathway and could act as a potential diagnostic and prognostic biomarker for HCC patients.
Keywords: DUXAP10, HCC, biomarker
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide, and there were about 466,100 new liver cancer cases and 422,100 liver cancer-related deaths in China in 2015.1,2 Usually, HCC is not diagnosed until the later stages of disease due to the lack of noticeable symptoms and remains a leading cause of global mortality.3,4 Identification of novel biomarkers with good clinical significance has become urgent and could provide new insights into the mechanism of tumorigenesis and metastasis to develop better therapeutic strategies.
Pseudogenes were considered to be “junk genes” for many years because of its abrogated transcription due to harbor mutations.5 However, over the past decade, non-coding RNAs including pseudogenes, long non-coding RNAs (lncRNAs), and microRNAs have been demonstrated to play key roles in tumorigenesis and cancer cell metastasis, and several studies have shown that pseudogenes could serve as key regulators of tumorigenesis by functioning as competing endogenous RNAs (ceRNAs).6–10
The pseudogene DUXAP10 was reported to be overexpressed in multiple cancers, and the molecular mechanisms of tumorigenesis of this pseudogene have been investigated in recent years. For instance, previous studies suggested that DUXAP10 could modulate bladder cancer cell proliferation via the PI3K/AKT/mTOR signaling pathway11 and regulate chronic myeloid leukemia cell apoptosis through the PTEN pathway.12 Moreover, DUXAP10 knockdown was reported to inhibit pancreatic cancer13 and colorectal cancer14 cell growth. In addition, high DUXAP10 expression was shown to be significantly associated with poor prognoses in patients suffering from lung,15 gastric,16 esophageal,17 and ovarian cancers.18 However, the expression patterns and potential biologic functions of DUXAP10 in HCC are still unknown.
In this study, integrated analyses including data mining, receiver operating characteristic (ROC) curve analysis, summary receiver operating characteristic (SROC) curve analysis, meta-analysis, and bioinformatics analysis were performed to investigate the expression patterns and clinical significance of DUXAP10 in HCC. Moreover, in vitro cellular function assays and Western blot protein analyses were conducted to explore the roles of DUXAP10 in HCC cell proliferation.
Materials and methods
Analysis of the differentially expressed pseudogenes based on TCGA database
The RNA sequencing (RNA-Seq) count matrix and the clinical information of the HCC patients were extracted from the Cancer Genome Atlas (TCGA) portal (
DUXAP10 expression and its diagnostic value in HCC verified using the GEO database
The microarrays that contain gene expression data of HCC tissues and adjacent normal liver tissues were searched and downloaded from the Gene Expression Omnibus (GEO) database (
To verify DUXAP10 expression patterns in HCC tissues, the pooled standard mean difference (SMD) with 95% confidence interval (95% CI) was calculated using a meta-analysis. Moreover, the SROC curve analysis was performed to calculate the pooled sensitivity, pooled specificity, and the area under the curve (AUC) of the SROC curve, which was used to assess the diagnostic significance of DUXAP10 in HCC.
Tissue specimens and cell culture
Tissue specimens were collected from the Department of Hepatobiliary Surgery at Beijing Tongren Hospital, and the study protocol was approved by the institutional review board of Beijing Tongren Hospital, Capital Medical University. No patients received radiotherapy, chemotherapy, biotherapy, or other treatments before surgery. Written informed consents, in accordance with the Declaration of Helsinki, were obtained from all patients.
HCC cells (BEL-7402, BEL-7404, Hep-3B, Hep-G2, Huh-7, SMMC-7721) and normal liver cells (LO-2) were obtained from the Cell Bank of Shanghai Institute of Biochemistry & Cell Biology (Shanghai, China). Dulbecco’s modified Eagle’s medium (Gibco BRL, USA) containing 10% fetal bovine serum (FBS, Invitrogen, USA) was used to culture all of the cell lines.
Quantitative real-time polymerase chain reaction (RT-PCR)
Total RNA was extracted from tissues and HCC cells using TRIzol (Thermo Fisher Scientific, USA), and then, cDNA was synthesized using a reverse-transcription kit (Thermo Fisher Scientific, USA). An RT-PCR instrument (Thermo Fisher Scientific, USA) was used to perform RT-PCR analysis according to the manufacturer’s instructions. The relative expression of target gene was calculated by using a method of comparing Ct (2−ΔΔCT) values.
Cell transfection
Three siRNA sequences (si-DUXAP10-1#: 5ʹ- GACTATGCCTTCTGAATAT-3ʹ; si-DUXAP10-2#: 5ʹ- AGTTGTTTGTTAGAATACTGGTGCT-3ʹ; si-DUXAP10-3#: 5ʹ-GGAACTTCCCAAACCTCCATGATTT-3ʹ) were designed according to the DUXAP10 cDNA sequence extracted from GeneBank. Hep3B cells were transfected with siRNA using Lipofectamine (Invitrogen, USA) according to the manufacturer’s instructions. Stable DUXAP10 knockdown in the selected cells was confirmed using RT-PCR analysis. In addition, a negative control sequence (si-NC) was also designed and synthesized.
Cell viability and colony formation assays, and cell cycle analysis
The HCC cells of si-DUXAP10 and si-NC groups were seeded into 96-well plates and the cell proliferation assays were performed on days 1, 2, 3, and 4 using the Cell Counting Kit 8 assay (Dojindo, Japan) according to the manufacturer’s instructions. For the colony formation assay, cells were seeded into 6-well plates, and colonies which contained over 50 cells were counted after 14 days. For cell cycle analyses, harvested cells were fixed using 75% ethanol overnight at 4°C and then incubated with RNase A for 30 mins at 37°C and stained with PI. Cell cycle analysis was performed using flow cytometry.
Western blotting
Equal amounts of protein were transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, USA). Incubation with 3% non-fat milk in Tris-buffered saline by Tween-20 (TBST) at 4°C for 1 hr to block non-specific protein interactions. Next, the membranes were incubated for 2 hrs with the following primary antibodies: 1:1,000 rabbit anti-human PI3K-p110a, AKT, phospho‑AKT (Ser473) (Cell Signaling, USA). Then, the membranes were incubated with fluorescent secondary antibodies. GAPDH was used as the endogenous control.
Statistical analysis
Statistical analyses were performed using the GraphPad Prism 7 software (GraphPad Software Inc., USA), Stata 15.0 software (StataCorp LLC., USA) and SPSS 22.0 software (IBM, USA) in this study. All experiments in this study were performed in triplicate and results were represented as the mean±SD. The unpaired Student’s t-test or one-way analysis of variance test was used to perform comparisons among all groups.
Results
The clinical significance of DUXAP10 expression in HCC based on TCGA database
The expression data of 14,868 pseudogenes were extracted from TCGA database. According to the cutoff criteria (|log2FC| >2 and P<0.01), a total of 380 pseudogenes were aberrantly expressed in HCC tissues compared with adjacent liver tissues, which was found to be significant (Figure 1A). Subsequently, the clinical value of all 380 pseudogenes was evaluated by overall survival (OS) and ROC curve analyses to select potential target genes. The results revealed that DUXAP10 was significantly upregulated in HCC tissues (Figure 1B), and high DUXAP10 expression indicated poor OS in HCC patients (Figure 1C). Moreover, the ROC curve analysis showed that DUXAP10 had good diagnostic value (AUC=0.887, 95% CI 0.852–0.922) in HCC (Figure 1D).
Furthermore, we explored the relationship between DUXAP10 expression and clinicopathologic data based on the clinical data downloaded from TCGA portal. As shown in Table 1, DUXAP10 was overexpressed in HCC tissue (HCC tissues, 18.91±1.52 vs Adjacent liver tissues, 0.78±0.18; P<0.01). In addition, DUXAP10 was significantly overexpressed in patients with a high pathologic stage (Stage Ⅲ+Ⅳ, 27.99±5.14 vs StageⅠ+Ⅱ, 16.03±1.19; P<0.01) or patients with vascular invasion (Positive, 22.69±3.26 vs Negative 13.18±1.14; P<0.01). The above results indicated that DUXAP10 might play key roles in HCC tumorigenesis and development.
 | Table 1 Correlation between DUXAP10 expression and clinicopathological characteristics in TCGA |
DUXAP10 was overexpressed in HCC tissues and diagnostically useful in HCC patients based on multiple GEO datasets
Six GEO datasets (GSE6764, GSE17548, GSE19665, GSE29721, GSE55092, and GSE62232) with gene expression data from 202 HCC tissues and 181 normal liver tissues were collected in this study. Details for the 6 GEO datasets are shown in Table 2. A meta-analysis was performed to integrate the DUXAP10 expression data, which showed that the pooled SMD was 6.47 (95% CI, 3.44–9.50) using the random-effects model (Figure 2). This result certified that DUXAP10 was upregulated in HCC tissues compared with normal liver tissues.
 | Table 2 Details for GEO data |
The AUC values were calculated by ROC curve analyses to determine the ability of DUXAP10 to distinguish between HCC and normal liver tissues based on each the GEO dataset. The results are listed in Table 2. Subsequently, SROC curve analysis was performed using STATA software to integrate the AUC values. As shown in Figure 3, the pooled sensitivity, pooled specificity, and AUC of the SROC were 0.75 (95%CI, 0.61–0.85), 0.94 (95%CI, 0.89–0.97), and 0.95 (95%CI, 0.93–0.97), which indicated that DUXAP10 had good diagnostic value and might be a useful biomarker for HCC diagnoses.
DUXAP10 related-genes in HCC for gene-enrichment analysis
Next, bioinformatics analyses were performed to investigate the potential regulatory mechanisms of DUXAP10 in HCC tumorigenesis and progression. Considering that DUXAP10 is a non-protein-coding gene, which cannot directly participate in tumorigenesis, we identified the protein-coding genes whose expression was positively or negatively correlated with that of the DUXAP10 based on gene expression data downloaded from TCGA database. The two-sided Pearson correlation coefficients and the z-tests were performed using the R software based on the gene expression data from HCC tissues downloaded from TCGA database, and |Pearson correlations| >0.40 and z-test P-values <0.001 were used as cutoff criteria.
Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed using the clusterProfiler and pathview packages (
 | Figure 4 GO (A) and KEGG pathway (B) enrichment analysis of the DUXAP10-related protein-coding genes. Abbreviations: GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes. |
We finally selected DUXAP10 for further experimental studies because this pseudogene had good diagnostic and prognostic merit and could have key regulatory roles in HCC tumorigenesis and progression according to the initial comprehensive analyses and literature review.
DUXAP10 was upregulated in HCC tissues and HCC cell lines
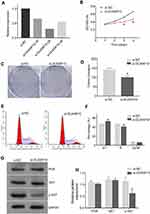
DUXAP10 expression in 6 different HCC cell types and 46 pairs of HCC and adjacent normal liver tissues were detected using RT-PCR analysis. The results indicated that DUXAP10 was significantly overexpressed in HCC tissues compared with adjacent liver tissues (Figure 5A). Moreover, the Kaplan–Meier curve analysis showed that high DUXAP10 expression indicated poor OS in HCC patients (Figure 5B). However, there were no clinicopathologic characteristics of the 46 HCC patients that were closely related to the RT-PCR-derived DUXAP10 expression levels, which might be due to the small sample size. In addition, DUXAP10 expression was higher in HCC cells compared with normal LO-2 liver cells (Figure 5C). Results of the RT-PCR analyses in our study confirmed that DUXAP10 was upregulated in HCC, and aberrantly expressed DUXAP10 was significantly associated with poor HCC patient prognoses.
The generation of DUXAP10 knockdown cell lines
Three siRNAs were transfected into Hep-3B cells using a lentiviral vector system. The transfection efficiency was evaluated using RT-PCR analyses. The siRNA (si-DUXAP10-2#: 5ʹ- AGTTGTTTGTTAGAATACTGGTGCT-3ʹ) was demonstrated to have the highest interference efficiency (Figure 6A) and was used in the subsequent experiments.
The effects of DUXAP10 on proliferation and cell cycle regulation of HCC cells
HCC cell proliferation was significantly inhibited in the DUXAP10 knockdown groups compared with the negative controls according to the CCK-8 assay growth curves (Figure 6B). Moreover, the colony formation assays revealed that HCC cells in the si-DUXAP10 groups formed fewer cell colonies compared with those of the si-NC group (Figure 6C and D). These results demonstrated that DUXAP10 knockdown inhibited HCC cell proliferation. Flow cytometric analyses were performed to identify the effect of DUXAP10 on cell cycle regulation. The proportion of cells in the G1 phase in the si-DUXAP10 group was significantly higher than those in negative control groups (Figure 6E and F), indicating that DUXAP10 knockdown could arrest HCC cells in the G1 phase of the cell cycle.
Taken together, the in vitro cellular function experiments demonstrated that DUXAP10 downregulation could arrest HCC cells in the G1 phase of the cell cycle and inhibit HCC cell proliferation.
DUXAP10 regulates the HCC cell proliferation by activating the PI3K/AKT pathway
To explore the potential mechanisms of DUXAP10 in HCC cell growth, Western blots were used to confirm PI3K/AKT signaling pathway protein expression in HCC cells of the si-DUXAP10 and negative control groups. As shown in Figure 6G and H, p-AKT protein expression was decreased in the si-DUXAP10 groups. However, DUXAP10 knockdown rarely affected PI3K and AKT protein expression in HCC cells. The results indicated that DUXAP10 could regulate HCC cell proliferation by activating the PI3K/AKT pathway.
Discussion
By benefiting from a substantial number of expressed genes and clinical data regarding cancer patients in a public online database, many novel genes have been identified that are aberrantly expressed and have good clinical benefits in human cancers. For instance, a meta-analysis of data accessed from the GEO database showed that low TUBA4B levels were closely associated with short OS in various human cancers, which indicated that TUBA4B could be a novel prognostic biomarker for cancer patients.19 Also, using SROC curve analyses based on information downloaded from TCGA and GEO databases, CTD-2547G23.4 was found to have good diagnostic value for HCC patients,20 and GDF-15 was shown to have good diagnostic value for gastric cancer patients.21
In this study, we first identified 380 aberrantly expressed pseudogenes in HCC based on the gene expression data from TCGA portal. Then, Kaplan–Meier survival and ROC curve analyses were performed to explore novel pseudogenes that have good clinical significance in HCC for further investigations. DUXAP10 was selected because it provided good diagnostic and prognostic benefits and had unknown biologic functions in HCC according to our integrated analyses and literature review. Next, based on the DUXAP10 expression data and clinical information from 46 HCC patients, we showed that DUXAP10 was significantly overexpressed in HCC tissues and that DUXAP10 upregulation was closely related to a poor OS in HCC patients. Finally, we explored the regulatory mechanisms of DUXAP10 in HCC cell proliferation, in vitro.
Pseudogenes, as non-coding protein genes, have been long considered to be non-functional transcriptional relics of the genome. To date, only a few pseudogenes have been demonstrated to be HCC oncogenes, and the roles of most pseudogenes remain completely uncertain. In recent years, studies suggested that pseudogenes could epigenetically affect proteins expression in tumor cells through a ceRNA function, which contributes to tumorigenesis and cancer progression. For example, the pseudogene, OCT4-pg4, functions as a natural microRNA sponge, protecting the OCT4 transcript from being inhibited by miR-145, and promotes cell growth in HCC.9 The pseudogene, INTS6P1, and its cognate gene, INTS6, exert tumor suppressive roles by competing for oncomiR-17-5p in HCC.22 Though several studies have investigated the roles of DUXAP10 in human cancers, how DUXAP10 functions in HCC tumorigenesis and progression remain unknown. Therefore, DUXAP10-related protein-coding genes were identified in the current study to help us explore the potential regulatory roles of DUXAP10 in HCC using bioinformatics methods.
The P13K/AKT signaling pathway plays a significant role in cellular activities, such as glucose metabolism, cell proliferation, cell cycle regulation, and apoptosis. Previous studies have shown that altered PI3K/AKT signaling pathway components were closely related to human cancer events.23 AKT is a downstream PI3K molecule that can be activated by the phosphorylation of the Ser473 and Thr308 key regulatory sites.24 Several studies have demonstrated that AKT phosphorylation activation could promote cell proliferation and invasion in human cancers.25–27 Several studies have shown that non-coding genes, including lncRNA and miRNA, could epigenetically activate the PI3K/AKT pathway in HCC.28 DUXAP10 has been demonstrated to modulate cancer cell proliferation and apoptosis through the PI3K/AKT11 and PTEN pathways.12 Also, DUXAP10 was reported to promote cancer cell growth through epigenetic p2117 and PTEN14 silencing, which are involved in the cell cycle regulation of cancer cells. Interestingly, the cell cycle was the most enriched pathway in the KEGG pathway enrichment analysis for DUXAP10-related protein-coding genes in our study, which indicated that DUXAP10 might regulate HCC cell growth by epigenetically affecting genes involved in the cell cycle. Therefore, we investigated the effects of DUXAP10 on the PI3K/AKT signaling pathway, which is involved in cell cycle regulation, to identify the mechanisms involved in HCC cell proliferation.
In the present study, we found that DUXAP10 downregulation significantly decreased cell proliferation and arrested HCC cells in the G1 phase of the cell cycle, and remarkably decreased p-AKT protein expression, indicating that DUXAP10 could promote HCC cell proliferation by regulating AKT phosphorylation. However, identifying the mechanism of how DUXAP10 regulates AKT phosphorylation in HCC is still not clear. According to our bioinformatics analyses and experimental results, DUXAP10 might promote AKT phosphorylation by affecting the expression of miRNAs or proteins involved in cell cycle regulation.
Several limitations of the current study should be discussed. First, this study lacked data from in vivo animal models to validate current results. Second, the upstream regulators of DUXAP10 and the regulatory roles of DUXAP10 in the downstream of PI3K/AKT signaling pathway in HCC were not explored. Without doubt, further exploration and experimental validation are needed to unravel the roles of DUXAP10 in HCC.
Conclusions
To the best of our knowledge, this is the first study focusing on the clinical significance and regulatory roles of DUXAP10 in HCC. This study showed that DUXAP10 was significantly overexpressed in HCC and that DUXAP10 upregulation was closely associated with poor prognoses in HCC patients. Moreover, we demonstrated that DUXAP10 could promote HCC cell proliferation by activating the PI3K/AKT pathway. This study identified an HCC biomarker with good diagnostic and prognostic values that could contribute to the development of pseudogene-based approaches for the treatment of HCC.
Acknowledgments
This work was supported by Beijing Tongren Hospital Clinical Medicine Development of Special Funding Support (code: TRYY-KYJJ-2017-055), Beijing Municipal Administration of Hospitals’ Youth Programme (code: QML20180203), and the priming scientific research foundation for the junior researcher in Beijing Tongren Hospital, Capital Medical University (code: 2018-YJJ-ZZL-043), Project Letter of Academic Leader of the First Hospital of Shanxi Medical University (code: YD1607) and Project Letter of Fostering Team for Precision Medical Key Innovation in the First Hospital of Shanxi Medical University (code: YT1603).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Tang A, Hallouch O, Chernyak V, Kamaya A, Sirlin CB. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom Radiol (NY). 2018;43(1):13–25. doi:10.1007/s00261-017-1209-1
4. Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156(2):477–491. doi:10.1053/j.gastro.2018.08.065
5. Proudfoot N. Pseudogenes. Nature. 1980;286:840–841.
6. An Y, Furber KL, Ji S. Pseudogenes regulate parental gene expression via ceRNA network. J Cell Mol Med. 2017;21(1):185–192. doi:10.1111/jcmm.12952
7. Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language?. Cell. 2011;146(3):353–358. doi:10.1016/j.cell.2011.07.014
8. Yue C, Ren Y, Ge H, et al. Comprehensive analysis of potential prognostic genes for the construction of a competing endogenous RNA regulatory network in hepatocellular carcinoma. Onco Targets Ther. 2019;12:561–576. doi:10.2147/OTT.S188913
9. Wang L, Guo ZY, Zhang R, et al. Pseudogene OCT4-pg4 functions as a natural micro RNA sponge to regulate OCT4 expression by competing for miR-145 in hepatocellular carcinoma. Carcinogenesis. 2013;34(8):1773–1781. doi:10.1093/carcin/bgt139
10. Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137(4):647–658. doi:10.1016/j.cell.2009.02.038
11. Lv XY, Ma L, Chen JF, et al. Knockdown of DUXAP10 inhibits proliferation and promotes apoptosis in bladder cancer cells via PI3K/Akt/mTOR signaling pathway. Int J Oncol. 2018;52(1):288–294. doi:10.3892/ijo.2017.4195
12. Yao R, Feng WT, Xu LJ, et al. DUXAP10 regulates proliferation and apoptosis of chronic myeloid leukemia via PTEN pathway. Eur Rev Med Pharmacol Sci. 2018;22(15):4934–4940. doi:10.26355/eurrev_201808_15632
13. Lian Y, Xiao C, Yan C, et al. Knockdown of pseudogene derived from lncRNA DUXAP10 inhibits cell proliferation, migration, invasion, and promotes apoptosis in pancreatic cancer. J Cell Biochem. 2018;119(4):3671–3682. doi:10.1002/jcb.26578
14. Lian Y, Xu Y, Xiao C, et al. The pseudogene derived from long non-coding RNA DUXAP10 promotes colorectal cancer cell growth through epigenetically silencing of p21 and PTEN. Sci Rep. 2017;7(1):7312. doi:10.1038/s41598-017-07954-7
15. Wei CC, Nie FQ, Jiang LL, et al. The pseudogene DUXAP10 promotes an aggressive phenotype through binding with LSD1 and repressing LATS2 and RRAD in non small cell lung cancer. Oncotarget. 2017;8(3):5233–5246. doi:10.18632/oncotarget.14125
16. Xu Y, Yu X, Wei C, Nie F, Huang M, Sun M. Over-expression of oncigenic pesudogene DUXAP10 promotes cell proliferation and invasion by regulating LATS1 and beta-catenin in gastric cancer. J Exp Clin Cancer Res. 2018;37(1):13. doi:10.1186/s13046-018-0684-8
17. Wang Z, Ren B, Huang J, Yin R, Jiang F, Zhang Q. LncRNA DUXAP10 modulates cell proliferation in esophageal squamous cell carcinoma through epigenetically silencing p21. Cancer Biol Ther. 2018;19(11):998–1005. doi:10.1080/15384047.2018.1470723
18. Zhang Q, Wang WW, Xu TH, Xu ZF. Highly expressed long non-coding RNA DUXAP10 promotes proliferation of ovarian cancer. Eur Rev Med Pharmacol Sci. 2018;22(2):314–321. doi:10.26355/eurrev_201801_14174
19. Zhang T, Wu DM, Deng SH, et al. Integrated Analysis Reveals That Long Non-Coding RNA TUBA4B Can Be Used as a Prognostic Biomarker in Various Cancers. Cell Physiol Biochem. 2018;49(2):530–544. doi:10.1159/000492991
20. Wen DY, Lin P, Liang HW, et al. Up-regulation of CTD-2547G23.4 in hepatocellular carcinoma tissues and its prospective molecular regulatory mechanism: a novel qRT-PCR and bioinformatics analysis study. Cancer Cell Int. 2018;18:74. doi:10.1186/s12935-018-0566-3
21. Liu JY, Dong XX, Lu JN, et al. Utility of GDF-15 as a diagnostic biomarker in gastric cancer: an investigation combining GEO, TCGA and meta-analysis. FEBS Open Bio. 2019;9(1):35–42. doi:10.1002/2211-5463.12537
22. Peng H, Ishida M, Li L, et al. Pseudogene INTS6P1 regulates its cognate gene INTS6 through competitive binding of miR-17-5p in hepatocellular carcinoma. Oncotarget. 2015;6(8):5666–5677. doi:10.18632/oncotarget.3290
23. Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46(6):372–383. doi:10.3109/07853890.2014.912836
24. Nitulescu GM, Margina D, Juzenas P, et al. Akt inhibitors in cancer treatment: the long journey from drug discovery to clinical use (Review). Int J Oncol. 2016;48(3):869–885. doi:10.3892/ijo.2015.3306
25. Liu Y, Chen JB, Zhang M, et al. SGK2 promotes renal cancer progression via enhancing ERK 1/2 and AKT phosphorylation. Eur Rev Med Pharmacol Sci. 2019;23(7):2756–2767. doi:10.26355/eurrev_201904_17549
26. Liu K, Jin H, Guo Y, et al. CFTR interacts with Hsp90 and regulates the phosphorylation of AKT and ERK1/2 in colorectal cancer cells. FEBS Open Bio. 2019. doi:10.1002/2211-5463.12641
27. Pan Y, Zhan L, Chen L, Zhang H, Sun C, Xing C. POU5F1B promotes hepatocellular carcinoma proliferation by activating AKT. Biomed Pharmacother. 2018;100:374–380. doi:10.1016/j.biopha.2018.02.023
28. Zheng YF, Zhang XY, Bu YZ. LINC01133 aggravates the progression of hepatocellular carcinoma by activating the PI3K/AKT pathway. J Cell Biochem. 2019;120(3):4172–4179. doi:10.1002/jcb.27704
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.