Back to Journals » Cancer Management and Research » Volume 12
PSA Kinetics as Prognostic Markers of Overall Survival in Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Abiraterone Acetate
Authors España S , Ochoa de Olza M , Sala N, Piulats JM, Ferrandiz U , Etxaniz O, Heras L, Buisan O, Pardo JC, Suarez JF, Barretina P, Comet J, Garcia del Muro X, Sumoy L , Font A
Received 17 July 2020
Accepted for publication 9 September 2020
Published 16 October 2020 Volume 2020:12 Pages 10251—10260
DOI https://doi.org/10.2147/CMAR.S270392
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Rudolph Navari
Sofia España,1 Maria Ochoa de Olza,2 Nuria Sala,3 Josep Maria Piulats,2 Ulises Ferrandiz,1 Olatz Etxaniz,1 Lucia Heras,2,4 Oscar Buisan,5 Juan Carlos Pardo,1 Jose F Suarez,6 Pilar Barretina,3 Josep Comet,7 Xavier Garcia del Muro,2 Lauro Sumoy,8 Albert Font1
1Catalan Institute of Oncology, Medical Oncology Department, University Hospital Germans Trias I Pujol, Badalona, Spain; 2Catalan Institute of Oncology, Medical Oncology Department, Bellvitge University Hospital, Hospitalet De Llobregat, Spain; 3Catalan Institute of Oncology, Medical Oncology Department, University Hospital Josep Trueta, Girona, Spain; 4Medical Oncology Department, Hospital Sant Joan Despí Moisès Broggi, Barcelona, Spain; 5Urology Department, University Hospital Germans Trias I Pujol, Badalona, Spain; 6Urology Department, Bellvitge University Hospital, Hospitalet de LLobregat, Spain; 7Urology Department, University Hospital Josep Trueta, Girona, Spain; 8High Content Genomics & Bioinformatics Unit, Germans Trias I Pujol Research Institute (IGTP), Program of Predictive and Personalized Medicine of Cancer (PMPPC), Campus Can Ruti, Badalona, Spain
Correspondence: Albert Font
Catalan Institute of Oncology, Medical Oncology Department, University Hospital Germans Trias I Pujol, Ctra Canyet, s/n, Badalona 08916, Spain
Email [email protected]
Background: Abiraterone acetate (AA) is widely used in the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC). However, a significant percentage of patients will still progress, highlighting the need to identify patients more likely to benefit from AA. Parameters linked to prostate-specific antigen (PSA) kinetics are promising prognostic markers. We have examined clinical and PSA-related factors potentially associated with overall survival (OS) in patients treated with AA.
Methods: Between 2011 and 2014, 104 patients with mCRPC treated with AA after progression to docetaxel at centers of the Catalan Institute of Oncology were included in this retrospective study. Patients were assessed monthly. Baseline characteristics and variables related to PSA kinetics were included in univariate and multivariate analyses of OS.
Results: Median OS was 16.4 months (range 12.4– 20.6) for all patients. The univariate analysis identified the following baseline characteristics as significantly associated with OS: ECOG PS, location of metastases, time between starting androgen deprivation therapy and starting AA, time between stopping docetaxel treatment and starting AA, neutrophil-lymphocyte ratio (NLR), alkaline phosphatase levels, and PSA levels. Factors related to PSA kinetics associated with longer OS were PSA response > 50%, early PSA response (> 30% decline at four weeks), PSA decline > 50% at week 12, PSA nadir < 2.4ng/mL, time to PSA nadir > 140 days, the combination of PSA nadir and time to PSA nadir, and low end-of-treatment PSA levels. The multivariate analysis identified ECOG PS (HR 37.46; p< 0.001), NLR (HR 3.7; p< 0.001), early PSA response (HR 1.22; p=0.002), and time to PSA nadir (HR 0.39; p=0.002) as independent prognostic markers.
Conclusion: Our results indicate an association between PSA kinetics, especially early PSA response, and outcome to AA after progression to docetaxel. Taken together with other factors, lack of an early PSA response could identify patients who are unlikely to benefit from AA and who could be closely monitored with a view to offering alternative therapies.
Keywords: castration-resistant prostate cancer, abiraterone, PSA kinetics, survival, early PSA response
Introduction
The treatment armamentarium of metastatic castration-resistant prostate cancer (mCRPC) has increased in recent years with the FDA/EMA approval of hormonal therapies like abiraterone acetate (AA) and enzalutamide, cytotoxic therapies like cabazitaxel,1 and bone-targeted therapies like Radium-223.2,3 Given the increase in treatment options for mCRPC, the selection of optimal therapeutic regimens is crucial for obtaining the maximum survival benefit.2,4 Predictive biomarkers of response can help in the selection of an effective treatment while avoiding unnecessary toxicities and excess costs associated with a potentially ineffective drug.
AA is a prodrug of abiraterone and is a selective, irreversible inhibitor of the androgen biosynthesis enzyme CYP17, which is involved in androgen production in the testes, adrenal glands and prostate tumor tissue. AA in combination with prednisone has demonstrated an increase in overall survival (OS) in patients with mCRPC and is approved for patients who progress during or after docetaxel-based chemotherapy,5 in chemotherapy-naïve patients,6 and in metastatic castration-sensitive patients.7 However, approximately one-third of treated patients show primary resistance to AA,6 highlighting the need for early identification of these patients in order to offer them an alternative therapy before progression-related clinical deterioration renders them ineligible for subsequent therapy, including chemotherapy.
Several studies have suggested a relationship between decrease in prostate-specific antigen (PSA) levels and survival, mainly in chemotherapy treated mCRPC patients.5 However, the role of PSA decline as a surrogate marker of survival in patients treated with hormonal therapies has not been clearly established. Biosynthesis of extragonadal androgens may contribute to progression in mCRPC, which still depends on androgen receptor signals, and a relationship between PSA kinetics and response to AA has been suggested,8 indicating that PSA kinetics may be a useful predictive biomarker of response.2
In order to shed further light on the potential association of baseline patient characteristics and PSA kinetics with OS, we have performed a retrospective study of 104 mCRPC patients treated with AA after progression to docetaxel.
Patients and Methods
Study Design and Patient Population
This was a retrospective observational study that included 104 mCRPC patients treated at three centers of the Catalan Institute of Oncology (University Hospital of Germans Trias i Pujol, Badalona; Bellvitge University Hospital, l’Hospitalet de Llobregat; and University Hospital of Girona Doctor Josep Trueta, Girona) from August 2011 to October 2014. All patients had histologically confirmed prostate adenocarcinoma with evidence of metastatic disease and all had been previously treated with docetaxel. Patients received AA 1000mg orally once daily plus prednisone 5mg twice daily. Each treatment cycle was 28 days. Treatment was continued until disease progression according to the Prostate Cancer Working Group 2 (PCWG2) criteria,9 death, or unacceptable toxicity.
During treatment with AA, patients were assessed every four weeks. Clinical visits included a physical examination and routine laboratory analyses, including PSA levels, albumin, alkaline phosphatase (ALP) levels, neutrophil and lymphocyte counts, and hemoglobin levels. Thoracic-abdominopelvic computed tomography (CT) and/or a bone scan were performed before starting AA therapy and during the treatment period in accordance with the protocols of each center.
The following baseline variables were recorded and patients classified accordingly: age (<74 vs ≥74 years), Eastern Cooperative Oncology Group (ECOG) performance status (PS) (0 vs 1 vs 2), number of previous lines of chemotherapy (1 vs ≥2), metastatic sites (bone only vs lymph node only vs bone and lymph nodes vs visceral), Gleason score (≤7 vs 8–10), neutrophil-to-lymphocyte ratio (NLR) (≤4 vs >4), hemoglobin levels (<10 gr/dl vs ≥10 gr/dl), ALP levels (≤upper limit of normality [ULN] vs >ULN), PSA levels (classified by terciles), PSA doubling time (PSADT) (≤3 months vs >3 months), interval between starting androgen deprivation therapy and starting AA (≤20 months vs >20 months), interval between stopping docetaxel therapy and starting AA (≤3 months vs >3 months), and type of progression (PSA progression vs radiographic progression vs clinical deterioration). PSADT was calculated with the Memorial Sloan Kettering online calculator. NLR was the ratio of the absolute neutrophil count divided by the absolute lymphocyte count.
The following data regarding PSA kinetics were recorded during AA treatment: PSA response (>50% vs <50%); early PSA response, defined as the percentage of PSA decline at week 4 (decline ≥30% vs decline <30% or increase <20% vs increase >20%); PSA response at week 12 (>50% vs <50%); PSA nadir (≥2.4 ng/mL vs <2.4 ng/mL); time to PSA nadir; and end-of-treatment (EOT) PSA levels, defined as the levels at the last PSA assessment during AA treatment (classified by terciles).
Statistical Analyses
Data were recorded with Microsoft Excel 2007 and statistical analyses were carried out using R v3.6.0. We built a multivariate model starting with all untransformed variables after excluding those exceeding 15% of individuals (for which there were insufficient numbers of observations). Data were imported from Excel into R with the readxl R package. Prior to analysis, data wrangling and cleaning was performed with the tidyverse suite of packages (including dplyr, purr, lubridate, stringr, and broom). Descriptive statistics including missing data were collected and visualized with the skimr and rms R packages. Data were described in terms of ranges, medians, frequencies, and percentages. OS was defined as the time from the start of AA treatment to death from any cause. Data were censored at the time of patient death. Optimal cut-off points for possible categorical transformations were identified with the survminer R package. A univariate analysis was performed to determine the association of different factors with OS and Kaplan Meier plots were generated using the survival R package. Due to limitations in sample size, not all variables identified as significant in the univariate analysis could be included in the multivariate analysis. After imputing missing data with the Hmisc R package, a variable selection strategy was followed based on elastic net Cox proportional hazards model fitting via penalized maximum likelihood, using the glmnet R package. This was followed by AIC maximization stepwise backward variable elimination towards model reduction. All models generated on imputed data were tested against intact data. An internal cross-validation was performed by bootstrapping with 200 iterations. Imputation, Cox modeling, stepwise model selection and model validation, were done using the rms R package. Recursive tree partitioning was explored using the party R package. Significance was set at p≤0.05.
Results
Patient Characteristics and Treatment
The main patient characteristics are detailed in Table 1. Median age was 74 years (range, 49–86), 82% of patients had ECOG PS 0–1, and 51.9% had a Gleason score ≥8. Bone metastases were present in 49% of the patients, while 11.6% had only lymph node metastases and 9.7% had visceral metastases. All patients had previously been treated with docetaxel and 32% had received two or more prior chemotherapy regimens.
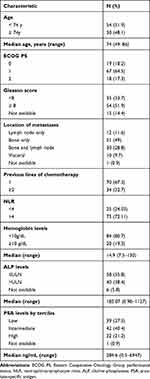 |
Table 1 Baseline Patient Characteristics |
Median duration of treatment was 7.85 months (range, 1–52 months). At the time of this analysis, 96 patients (92%) had died. Median OS was 16.4 months (95% confidence interval [CI] 12.2–20.6) for all patients (Figure 1A) and 1-year and 2-year survival rates were 66% and 33%, respectively. Median progression-free survival was 7.8 months (95% CI 5.9–9.6) (Figure 1B).
 |
Figure 1 (A) Overall survival and (B) progression-free survival from the start of abiraterone acetate treatment for all 104 patients. |
Baseline Characteristics and OS
In the univariate analysis, the following baseline characteristics were associated with longer OS: ECOG PS <2 (p<0.001) (Figure 2A); only lymph node metastases (p=0.04); interval between starting androgen deprivation therapy and starting AA ≥20 months (p=0.006); interval between stopping docetaxel and starting AA ≥3 months (p=0.04); NLR <4 (p<0.001) (Figure 2B); ALP levels ≤ULN (p=0.03); and low/intermediate PSA levels (p<0.001). No other significant association between baseline characteristics and OS was observed (Table 2).
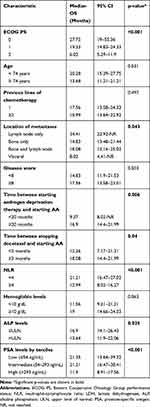 |
Table 2 Univariate Analysis of Overall Survival (OS) According to Baseline Characteristics |
 |
Figure 2 Overall survival according to (A) ECOG PS and (B) NLR. |
Prognostic Impact of PSA Kinetics
Table 3 shows the univariate analysis of OS according to factors related to PSA kinetics. PSA response >50% (p<0.001), early PSA response ≥30% (p=0.01; Figure 3), PSA response >50% at week 12 (p<0.001), PSA nadir <2.4ng/mL (p<0.001), time to PSA nadir >140 days (p<0.001), and low EOT PSA levels (p<0.001) were associated with longer OS. In addition, we observed a combinatory effect for PSA nadir plus time to PSA nadir, where patients with lower PSA nadir and longer time to PSA nadir had the longest OS (hazard ratio [HR] 0.57; 95% CI 1.43–1.75; p< 0.001) (Figure 4).
 |
Table 3 Univariate Analysis of Overall Survival (OS) According to Prostate-Specific Antigen (PSA) Kinetics |
 |
Figure 3 Overall survival according to early PSA response (at week 4). |
 |
Figure 4 Overall survival according to PSA nadir plus time to PSA nadir. |
The multivariate analysis identified ECOG PS (HR 37.46; p<0.001), NLR (HR 3.7; p<0.001), early PSA response (HR 1.22; p=0.002), and time to PSA nadir (HR 0.39; p=0.002), as independent markers of OS (Table 4). Based on these findings, we generated a decision tree for survival analysis in mCRPC, which indicated that baseline ECOG PS and NLR combined could predict OS (Figure 5).
 |
Table 4 Multivariate Analysis of Overall Survival |
 |
Figure 5 Decision tree for survival of prostate cancer patients, showing ECOG PS and NLR at the beginning of abiraterone acetate treatment. |
Discussion
Several treatment options are now available for mCRPC. Novel hormonal therapies, including AA, have demonstrated activity in patients previously treated with docetaxel, but other therapies, such as cabazitaxel1 or radium-223,3 can be useful in patients refractory to hormonal therapies. Primary resistance to AA in pre-docetaxel mCRPC patients is near 20%, and the identification of patients with primary resistance may help avoid ineffective treatments.10 In the present study, we have examined the impact on OS of several baseline characteristics and parameters related to PSA kinetics in patients with mCRPC treated with AA. The multivariate analysis identified two baseline characteristics (ECOG PS and NLR) and two variables associated with PSA kinetics (early PSA response and time to PSA nadir) as independent markers of OS.
Our patients were treated off-study, yet OS was within the range of that reported in the COU-AA-302 trial,11 despite the fact that a higher percentage of our patients had PS 2 (17% vs 10%). In the COU-AA-301 trial,12 ECOG PS >1, presence of liver metastases, time between starting androgen deprivation therapy and starting AA >36 months, low serum albumin, and high serum ALP and LDH levels were associated with a poorer outcome to AA.13 Our findings regarding ECOG PS, time between starting androgen deprivation therapy and starting AA, and ALP levels were in line with these results. In addition, however, we found that low NLR, low baseline PSA levels, the absence of bone metastases, and high hemoglobin levels were also associated with longer OS.
NLR is a marker of inflammation and immune status related to carcinogenesis, and its role as a potential prognostic and predictive marker has been evaluated in many solid tumors, including mCRPC. In a study carried out at the Princess Margaret Cancer Centre,14 NLR ≥5 was significantly associated with PSA response and survival in 116 patients with mCRPC treated with AA, 53% of whom had previously been treated with docetaxel. NLR has also been identified as a prognostic marker in patients with mCRPC treated with second-line chemotherapy.15 Furthermore, a persistent NLR <3 during treatment with enzalutamide was associated with greater treatment efficacy in patients with mCRPC.16 Taken together with these previous results, our finding in the present study that a high NLR was related to shorter OS suggests that NLR is a promising prognostic marker and leads us to postulate that NLR in combination with ECOG PS can be a useful prognostic marker in patents with mCRPC. Whereas in patients with PS 0 and PS 2, survival is well defined, in the intermediate group of patients with PS 1, NLR can be useful in selecting which patients are likely to benefit from AA, especially as NLR assessment can easily be incorporated into routine clinical practice.
In contrast, age and Gleason score were not associated with OS. In fact, AA has demonstrated activity in mCRPC regardless of patient age,5 and several studies have found that Gleason score may well lose prognostic impact in heavily pretreated mCRPC patients.13,17–19 Additionally, the number of prior chemotherapy lines was not associated with OS. More than 50% of our patients who had received a second line of chemotherapy before AA were treated with docetaxel, in accordance with common practice at that time, and the interval between ending docetaxel and starting AA was more highly associated with OS than the number of previous lines.
The prognosis of mCRPC patients ranges from a few months to several years and is influenced by multiple factors. Several studies have identified predictive biomarkers of resistance to hormonal therapies. For example, Wnt pathway genes have been associated with resistance to AA20 and baseline circulating tumor cell counts have also shown promise as prognostic markers.21 Our findings on PSA kinetics can further refine these biomarker-based data and can be used in routine clinical practice to identify patients likely to be resistant to AA.
PSA nadir and time to PSA nadir were also associated with OS in our study. Previous studies have also reported the impact of these factors on OS, in both chemotherapy- and hormonotherapy-treated patients.22–24 Along these same lines, our univariate analysis revealed an association between PSA nadir and time to PSA nadir with OS. This effect may be due to stroma-epithelial interactions and “tumor regulating fibroblasts”, which are now being studied with promising results in vitro and in vivo.25
Several studies have found that PSA decline can be a marker of response to treatment and of longer OS,17,26–30 since PSA levels reflect disease burden. In two Phase III trials of AA – in chemotherapy-pretreated5 and in chemotherapy-naïve13 patients – PSA kinetics were significantly associated with survival.31 In our study, we also observed that PSA kinetics could predict the efficacy of treatment with AA. Importantly, we found a relationship between early PSA response and OS. This finding is in line with previous retrospective studies carried out in a limited number of patients, where early PSA changes were associated with response and survival to AA and enzalutamide. In a study of patients treated with enzalutamide after docetaxel, a PSA increase >20% at week 4 was associated with shorter progression-free survival and OS, while a PSA decline >30% was only associated with progression-free survival but not OS.32 In a study of patients treated with AA before or after docetaxel, PSA decline at week 4 was significantly associated with response at week 12 and with longer OS, both in patients who had previously received docetaxel as well as in chemonaive patients.33 Furthermore, Facchini et al reported that a very early PSA response (>50% decline at day 15 of AA treatment) was associated with longer progression-free survival and OS.8 Finally, Caffo et al found that patients treated with AA or enzalutamide who did not show a PSA decline >50% at four weeks had a 50% risk of progression within three months, compared to an 18% risk for patients with an early PSA response.6,34 The ability to predict the efficacy of AA at an early time point can be crucial in patient management, since this allows a prompt change in treatment strategy, preventing clinical deterioration which can hinder the administration of subsequent therapies. At present, the majority of physicians switch therapy based on clinical progression,35 and while the absence of early PSA response may not in itself be a sufficient rationale for changing therapy, it will help identify those patients who require closer monitoring.
Several studies have suggested prognostic models in mCRPC. Halabi et al36 classified patients in a clinical trial into high- and low-risk groups based on several baseline characteristics, while Armstrong et al37 developed a risk classification scheme based on pain, visceral metastases, anemia, and bone scan progression that was able to predict 30% PSA decline. Based on our findings in the present study, we propose a decision tree for survival analysis based on the variables of ECOG PS and NLR, both of which are easily collected from all patients. We plan to test the validity of our proposal in future studies.
As with all retrospective studies, our results must be interpreted with caution and should be validated in prospective studies including a larger number of patients. Our study has several limitations, such as the limited number of patients included and a number of patients with some missing data. Despite these limitations, however, our findings indicate that NLR can be a useful baseline marker to predict outcome in patients with mCRPC treated with AA and that early PSA response to AA may be a useful marker of treatment efficacy. While an early change in PSA is generally not a sufficient reason for modifying treatment in clinical trials,9 we propose to closely monitor patients with an early PSA increase during AA treatment, as well as taking into account other prognostic and predictive markers, when deciding on next treatment options.
Patient Consent
For this type of study, formal consent is not required.
Data Sharing Statement
Supporting data for this study is freely available upon request to the corresponding author.
Ethics Approval
This study was approved by the Ethics Committees of the Catalan Institute of Oncology at University Hospital of Germans Trias i Pujol (PI 16-183). This approval covers all three centers of the Catalan Institute of Oncology. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All patient data were anonymized and codified to ensure adequate compliance with the Spanish Data Protection Law 15/1999 and Royal Decree-Law 5/2018 of 27 July on urgent measures for the adaptation of Spanish law to European Union regulations on data protection. All data are stored in the Medical Oncology Service of the ICO-Badalona, Germans Trias i Pujol University Hospital.
Acknowledgment
Maria Ochoa de Olza is currently at Ludwig Institute for Cancer Research, University of Lausanne, and Service of Immuno-Oncology, Department of Oncology, Lausanne University Hospital, Lausanne, Switzerland. The work reported here was performed at three centers of the Catalan Institute of Oncology: University Hospital of Germans Trias i Pujol, Badalona; Bellvitge University Hospital, l’Hospitalet de Llobregat; and University Hospital of Girona Doctor Josep Trueta, Girona.
Funding
This study received no outside funding.
Disclosure
Dr Nuria Sala reports grants from Astellas, she has participated at advisored board of Pfizer, Sanofi, Jansen and Astellas, outside the submitted work. Dr Xavier Garcia del Muro reports personal fees from Astellas, outside the submitted work. The authors report no other conflicts of interest for this work.
References
1. de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381(26):2506–2518. doi:10.1056/NEJMoa1911206
2. Sartor O, de Bono JS, Longo DL. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645–657. doi:10.1056/NEJMra1701695
3. Miller K, Heinrich D, O’Sullivan JM, et al. Radium-223 (Ra-223) therapy after abiraterone (Abi): analysis of symptomatic skeletal events (SSEs) in an international early access program (iEAP) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol. 2018;29:viii287. doi:10.1093/annonc/mdy284.033
4. Lorente D, Fizazi K, Sweeney C, de Bono JS. Optimal treatment sequence for metastatic castration-resistant prostate cancer. Eur Urol Focus. 2016;2(5):488–498. doi:10.1016/j.euf.2016.10.008
5. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi:10.1056/NEJMoa1014618
6. Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. doi:10.1056/NEJMoa1209096
7. James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338–351. doi:10.1056/NEJMoa1702900
8. Facchini G, Caffo O, Ortega C, et al. Very early PSA response to abiraterone in mCRPC patients: a novel prognostic factor predicting overall survival. Front Pharmacol. 2016;7:123. doi:10.3389/fphar.2016.00123
9. Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148–1159. doi:10.1200/JCO.2007.12.4487
10. Fuerea A, Baciarello G, Patrikidou A, et al. Early PSA response is an independent prognostic factor in patients with metastatic castration-resistant prostate cancer treated with next-generation androgen pathway inhibitors. Eur J Cancer. 2016;61:44–51. doi:10.1016/j.ejca.2016.03.070
11. Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled Phase 3 study. Lancet Oncol. 2015;16(2):152–160. doi:10.1016/S1470-2045(14)71205-7
12. Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13(10):983–992. doi:10.1016/S1470-2045(12)70379-0
13. Chi KN, Kheoh T, Ryan CJ, et al. A prognostic index model for predicting overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate after docetaxel. Ann Oncol. 2016;27(3):454–460.
14. Leibowitz-Amit R, Templeton AJ, Omlin A, et al. Clinical variables associated with PSA response to abiraterone acetate in patients with metastatic castration-resistant prostate cancer. Ann Oncol. 2014;25(3):657–662. doi:10.1093/annonc/mdt581
15. Lorente D, Mateo J, Templeton AJ, et al. Baseline neutrophil-lymphocyte ratio (NLR) is associated with survival and response to treatment with second-line chemotherapy for advanced prostate cancer independent of baseline steroid use. Ann Oncol. 2015;26(4):750–755. doi:10.1093/annonc/mdu587
16. Conteduca V, Crabb SJ, Jones RJ, et al. Persistent neutrophil to lymphocyte ratio >3 during treatment with enzalutamide and clinical outcome in patients with castration-resistant prostate cancer. PLoS One. 2016;11(7):e0158952. doi:10.1371/journal.pone.0158952
17. Rescigno P, Dolling D, Conteduca V, et al. Early post-treatment prostate-specific antigen at 4 weeks and abiraterone and enzalutamide treatment for advanced prostate cancer: an international collaborative analysis. Eur Urol Oncol. 2020;3(2):176–182. doi:10.1016/j.euo.2019.06.008
18. Chi K, Hotte SJ, Joshua AM, et al. Treatment of mCRPC in the AR-axis-targeted therapy-resistant state. Ann Oncol. 2015;26(10):2044–2056. doi:10.1093/annonc/mdv267
19. Alvim CM, Mansinho A, Paiva RS, et al. Prognostic factors for patients treated with abiraterone. Future Sci OA. 2019;6(2):Fso436.
20. Wang L, Dehm SM, Hillman DW, et al. A prospective genome-wide study of prostate cancer metastases reveals association of wnt pathway activation and increased cell cycle proliferation with primary resistance to abiraterone acetate-prednisone. Ann Oncol. 2018;29(2):352–360. doi:10.1093/annonc/mdx689
21. Lorente D, Olmos D, Mateo J, et al. Circulating tumour cell increase as a biomarker of disease progression in metastatic castration-resistant prostate cancer patients with low baseline CTC counts. Ann Oncol. 2018;29(7):1554–1560. doi:10.1093/annonc/mdy172
22. Pei X, Wu K, Sun Y, et al. PSA time to nadir as a prognostic factor of first-line docetaxel treatment in castration-resistant prostate cancer: multicenter validation in patients from the Chinese Prostate Cancer Consortium. Urol Oncol. 2020;38(1):
23. Sasaki T, Onishi T, Hoshina AN. PSA level and time to PSA nadir following primary androgen deprivation therapy are the early survival predictors for prostate cancer patients with bone metastasis. Prostate Cancer Prostatic Dis. 2011;14(3):248–252. doi:10.1038/pcan.2011.14
24. Petrylak DP, Ankerst DP, Jiang CS, et al. Evaluation of prostate-specific antigen declines for surrogacy in patients treated on SWOG 99-16. J Natl Cancer Inst. 2006;98(8):516–521. doi:10.1093/jnci/djj129
25. Sasaki T, Sugimura Y. The importance of time to Prostate-Specific Antigen (PSA) nadir after primary androgen deprivation therapy in hormone-naive prostate cancer patients. J Clin Med. 2018;7(12):565. doi:10.3390/jcm7120565
26. Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for Phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17(11):3461–3467. doi:10.1200/JCO.1999.17.11.3461
27. Caffo O, Pappagallo G, Brugnara S, et al. Multiple rechallenges for castration-resistant prostate cancer patients responding to first-line docetaxel: assessment of clinical outcomes and predictive factors. Urology. 2012;79(3):644–649. doi:10.1016/j.urology.2011.11.043
28. Kijima T, Fujii Y, Yokoyama M, et al. Prostate-specific antigen response to deferred combined androgen blockade therapy using bicalutamide predicts survival after subsequent oestrogen and docetaxel therapies in patients with castration-resistant prostate cancer. BJU Int. 2012;110(8):1149–1155. doi:10.1111/j.1464-410X.2012.10959.x
29. Petrylak DP. New paradigms for advanced prostate cancer. Rev Urol. 2007;9(Suppl 2):S3–S12.
30. Schiff JP, Cotogno P, Feibus A, et al. Early prostate-specific antigen response post-abiraterone as predictor of overall survival in metastatic castrate-resistant prostate cancer. BMC Cancer. 2019;19(1):524. doi:10.1186/s12885-019-5729-7
31. Xu XS, Ryan CJ, Stuyckens K, et al. Correlation between prostate-specific antigen kinetics and overall survival in abiraterone acetate-treated castration-resistant prostate cancer patients. Clin Cancer Res. 2015;21(14):3170–3177. doi:10.1158/1078-0432.CCR-14-1549
32. Conteduca V, Crabb SJ, Scarpi E, et al. Association between early PSA increase and clinical outcome in patients treated with enzalutamide for metastatic castration resistant prostate cancer. Mol Diagn Ther. 2016;20(3):255–263. doi:10.1007/s40291-016-0196-1
33. Rescigno P, Lorente D, Bianchini D, et al. Prostate-specific antigen decline after 4 weeks of treatment with abiraterone acetate and overall survival in patients with metastatic castration-resistant prostate cancer. Eur Urol. 2016;70(5):724–731. doi:10.1016/j.eururo.2016.02.055
34. Caffo O, Veccia A, Maines F, Bonetta A, Spizzo G, Galligioni E. Potential value of rapid prostate-specific antigen decline in identifying primary resistance to abiraterone acetate and enzalutamide. Future Oncol. 2014;10(6):985–993. doi:10.2217/fon.14.24
35. Lorente D, Ravi P, Mehra N, et al. Interrogating metastatic prostate cancer treatment switch decisions: a multi-institutional survey. Eur Urol Focus. 2018;4(2):235–244. doi:10.1016/j.euf.2016.09.005
36. Halabi S, Lin CY, Kelly WK, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32(7):671–677. doi:10.1200/JCO.2013.52.3696
37. Armstrong AJ, Tannock IF, de Wit R, George DJ, Eisenberger M, Halabi S. The development of risk groups in men with metastatic castration-resistant prostate cancer based on risk factors for PSA decline and survival. Eur J Cancer. 2010;46(3):517–525. doi:10.1016/j.ejca.2009.11.007
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
