Back to Journals » International Journal of Nanomedicine » Volume 12
Proteomic analysis of tears following acupuncture treatment for menopausal dry eye disease by two-dimensional nano-liquid chromatography coupled with tandem mass spectrometry
Authors Liu Q , Liu J, Ren C, Cai W , Wei Q, Song Y, Yu J
Received 9 November 2016
Accepted for publication 11 January 2017
Published 28 February 2017 Volume 2017:12 Pages 1663—1671
DOI https://doi.org/10.2147/IJN.S126968
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Qingyu Liu,1,* Junling Liu,1,* Chengda Ren,1 Wenting Cai,1 Qingquan Wei,2 Yi Song,3 Jing Yu1
1Department of Ophthalmology, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai, 2Department of Ophthalmology, Nanchang University, Nanchang, 3Department of Ophthalmology, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Background: The purpose of this study was to investigate whether acupuncture is effective at treating dry eye disease among postmenopausal women and to identify the possible mechanisms.
Methods: Twenty-eight postmenopausal women with dry eye disease were randomly divided into two groups: an acupuncture plus artificial tears (AC + AT) group and an artificial tears (AT) only group. After baseline examination of clinical parameters and tear sample collection, each patient received the designated modality of topical therapy for 2 months. Posttreatment documentation of clinical parameters was recorded, and tear samples were collected. Tear samples from the AC + AT group were subjected to two-dimensional nano-liquid chromatography coupled with tandem mass spectrometry (2D nano-LC-MS/MS). Western blot analysis was also performed on tear samples from both groups.
Results: After treatment, the Ocular Surface Disease Index scores, symptom assessment scores, scores of sign assessment, and tear break-up time were significantly improved in both groups (P=0.000). Symptom assessment scores were significantly improved in the AC + AT group (P=0.000) compared with the AT group. 2D nano-LC-MS/MS identified 2,411 proteins, among which 142 were downregulated and 169 were upregulated. After combined AC + AT treatment, the abundance of secreted proteins was increased, whereas that of cytoplasmic proteins decreased (Pearson’s χ2 test, P=0.000, P=0.000, respectively). Proteins involved in immunity and regulation were also more abundant (Pearson’s χ2 test, P=0.040, P=0.016, respectively), while components and proliferation-related proteins were downregulated (Pearson’s χ2 test, P=0.003, P=0.011, respectively).
Conclusion: AC + AT treatment increased protein synthesis and secretion, and improved clinical symptoms. These results indicate that acupuncture may be a complimentary therapy for treating postmenopausal dry eye disease.
Keywords: acupuncture, menopausal dry eye, proteomics
Introduction
The prevalence of dry eye disease is higher among women than men. Additionally, aging has been identified as a risk factor for this condition, which suggests that hormones may play key roles in the incidence and course of dry eye, especially in postmenopausal women.1 Common treatments for dry eye aim to increase the amount of tears at the ocular surface through the use of tear replacement with artificial tears or to reduce drainage by occlusion of the drainage system.2 However, such treatments are often palliative and inadequate with regard to providing satisfactory relief from debilitating symptoms.3 Hormone replacement therapy may improve aqueous tear production but not the quality of tears in dry eye disease, and the effect on tear production is dependent upon age.4 Nonetheless, recent studies indicate that hormone replacement therapy can increase the risk of coronary heart disease, stroke, and breast and endometrial cancers.5,6
As a complementary alternative therapy without serious side effects, acupuncture is used to treat postmenopausal symptoms including hot flashes,7 burning mouth syndrome, and sleep disturbances. According to recent studies, acupuncture may benefit midterm outcomes related to dry eye syndrome in comparison with artificial tears.8 For example, acupuncture was found to improve the signs and symptoms of dry eye in patients after 4 weeks of treatment.9 However, the effectiveness and mechanisms of acupuncture on postmenopausal dry eye have not yet been reported. In this trial, we aimed to investigate whether acupuncture is effective at treating dry eye among postmenopausal women and to identify the possible mechanisms.
Methods
Subjects
Twenty-eight patients were included in this study (Table 1). All participants were allocated into either an acupuncture plus artificial tears (AC + AT) group or an artificial tears (AT) only group. This research began on September 8, 2014, and lasted for 10 months. Menopausal women over 50 years old who were diagnosed with dry eye (with dry eye symptoms and Schirmer’s test results less than 10 mm/5 minutes or a tear break-up time [TBUT] less than 10 seconds) were eligible to participate in this study. Patients with serious eye disease, inflammation, surgery and trauma, ovariotomy or hysterectomy, and other severe body and mental diseases were excluded. Basic information for every participant was recorded or tested during their first visit, including the following: the symptoms and course of dry eye disease, the eye drops they used prior to enrollment, and the Ocular Surface Disease Index (OSDI), Generalized Anxiety Disorder 7-Item Scale (GAD-7), Patient Health Questionnaire 9-Item (PHQ-9), TBUT, and Schirmer’s test (without narcosis) scores. Venous blood and tears were also collected to detect sex hormones and for tear proteomics and Western blot analyses. The study was approved by the Ethics Committee of Shanghai Tenth People’s Hospital of Tongji University. All patients provided written informed consent to participate in this study.
Treatment procedure
The intervention lasted for 2 months. In the AC + AT group, each patient was offered acupuncture treatment, which lasted for 30 minutes each session, three times a week for 8 weeks. The acupuncture treatment, which consisted of the acupoints of Jingming (BL 1), Cuanzhu (BL 2), Sizhukong (SJ 23), Taiyang (EXTRAI), Sibai (ST 2), Hegu (LI 4), Fengchi (GB 20), Baihui (DU 20), and Chengqi (ST 1), was performed by one Chinese medicine physician (Figure 1). Participants in both intervention groups accepted artificial tear treatment. After treatment, their symptoms and OSDI, TBUT, and Schirmer’s test (without narcosis) were recorded again, and venous blood and tears were collected.
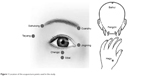  | Figure 1 Location of the acupuncture points used in the study. |
Tear collection
Tears were collected by placing a Schirmer’s test strip over the lower lid, 1/3 from the lateral canthus, without anesthesia. Patients were asked to keep their eyes closed for 5 minutes. After the wet length was recorded, the strip was placed into a 1.5 mL Eppendorf tube. Samples were immediately placed into ice transport tanks and transferred to the center lab at Shanghai Tenth People’s Hospital where they were stored at −80°C.
Protein extraction and labeling with isobaric tags for relative and absolute quantitation (iTRAQ) reagents
Proteins from patients in the AC + AT group pre- and posttreatment were extracted from the Schirmer’s test strip by incubation overnight at 4°C in 1 mL dissolution buffer (SCIEX, 4352135, Framingham, MA, USA). After centrifugation at 13,000 rpm for 10 minutes at 4°C, the supernatant was obtained and concentrated by ultrafiltration through Amicon® Ultra 0.6 mL, 3 kDa filters (Millipore, Billerica, MA, USA). Protein concentrations were measured using a bicinchoninic acid assay Protein Assay Kit (Pierce, Rockford, IL, USA). Then, 200 μg of proteins was incubated with dissolution buffer, 1 μL of 2% sodium dodecyl sulfate (SDS), and 4 μL reducing reagent (SCIEX, 4352135) for 1 hour at 60°C. After centrifugation, the precipitate was incubated with 2 μL cysteine blocking reagent (SCIEX, 4352135) for 10 minutes at room temperature. After reduction and alkylation, each sample was digested with trypsin (w(trypsin):w(protein) = 1:25) overnight at 37°C. The samples were then labeled with iTRAQ reagents (Applied Biosystems, Foster City, CA, USA) as follows: 114/115 iTRAQ reagent for pretreatment samples; 116/117 iTRAQ reagent for posttreatment samples. A total of four different isobaric tags were applied to four pooled protein samples, and each of the four labeled digested samples were mixed.
Two-dimensional liquid chromatography coupled with tandem mass spectrometry (2D nano-LC-MS/MS)
The peptide mixture was diluted 10-fold with water/formic acid (pH 3.0) and loaded onto an strong cation exchange chromatography column (SCIEX, 4326695) and fractionated into ten subgroups using isotope-coded affinity tag Cation Exchange Buffer (SCIEX, 4326747). Each strong cation exchange fraction was desalted using reverse-phase (RP) chromatography.
The fractions were separated by nano-high performance liquid chromatography (Eksigent Technologies, Dublin, CA, USA) using a secondary RP analytical column (Eksigent, C18, 3 μm, 150 mm ×75 μm). Peptides were subsequently eluted using the following gradient conditions: phase B (98% acetonitrile with 0.1% formic acid) from 5% to 45% B (5–100 minutes). The total flow rate was maintained at 300 nL/minute. An electrospray voltage of 2.3 kV vs the inlet of the mass spectrometer was used. An AB SCIEX TripleTOF™ 4600 mass spectrometer (Foster city, CA, USA) was operated in information-dependent data acquisition mode to automatically switch between MS and MS/MS acquisition. MS spectra were acquired across the mass range of 350–1,250 m/z using an accumulation time of 250 ms per spectrum. MS spectra, scanned from 100 to 1,500 m/z in high-sensitivity mode with rolling collision energy, were acquired. The 30 most intense precursors were selected for fragmentation per cycle with a dynamic exclusion time of 25 seconds.
iTRAQ data analysis and statistical analysis
Protein identification and quantification of iTRAQ data were performed using ProteinPilot software version 4.5 (AB SCIEX). The paragon algorithm in ProteinPilot software was used for peptide identification and isoform-specific quantification. The signal from the iTRAQ114 group served as the internal reference for signal intensity to which all signals were normalized. The weighted average of the ratios of the respective peptides was calculated based on the protein quantitation results. False discovery rate analysis was conducted, and the detected protein threshold was set to <0.01. The proteomic database used in this study was for Homo sapiens (UniProKB) (http://www.uniprot.org/uniprot).
The differentially expressed proteins were set as follows: ≥1.2 indicating upregulated (P<0.05) and ≤0.8 indicating downregulated (P<0.05).
Western blot analysis
To confirm the 2D nano-LC-MS/MS findings, Western blot analysis of the following proteins was performed on tear samples from both groups: Annexin A1 and apolipoprotein A-I (Apo A-I). The Schirmer’s test strips for eight patients within each group were analyzed. For tear fluid extraction, the test strips were incubated in buffer (200 μL buffer containing 180 μL phosphate buffer saline and 20 μL protease inhibitor) for 24 hours at 4°C followed by centrifugation at 12,000 rpm for 30 minutes. The centrifugal force pulled the tear fluid out of the strip into the buffer. The protein concentrations were measured using an assay based on the Bradford procedure (Bio-Rad, Hercules, CA, USA). Total proteins (30 μg) were then analyzed using Western blotting, resolution via reducing 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretic transfer onto 0.1 μm pore size nitrocellulose membranes at room temperature for 1 hour at 0.8 mA/cm2. Bands were detected with a primary antibody against Annexin A1 (rabbit-anti-Annexin A1, 1:1,000, #3299, Cell Signaling Technology, Danvers, MA, USA) or ApoA1 (mouse-anti-ApoA1, 1:1,000, #3350, Cell Signaling Technology) followed by incubation with the secondary antibody (anti-rabbit IgG, 1:1,000, #5174, Cell Signaling Technology). The molecular weights of the detected protein bands were estimated using protein standards (Prestained Protein Ladder, Fermentas, Waltham, MA, USA) ranging from 10 to 170 kDa.
Statistical analysis
v2 tests, Student’s t-tests, and Pearson’s χ2 tests were performed using SPSS statistical software 24.0 (IBM Corporation, Armonk, NY, USA). P≤0.05 was considered significant.
Results
Changes in clinical outcomes
Clinical parameters
After treatment, OSDI scores, symptom assessment scores, scores of sign assessment, and TBUT scores were significantly improved in both groups (P=0.000). Conversely, Schirmer’s test results were unchanged in both groups. Compared with the AT group, symptom assessment scores were significantly improved in the AC + AT group (P=0.000), though there were no significant differences between the two groups with regard to the other clinical parameters (Table 2).
Sex hormone levels
After treatment, the levels of follicle-stimulating hormone and luteinizing hormone were increased (65.59±20.08 vs 60.302±17.831 and 28.262±9.251 vs 25.389±6.822, respectively), though the differences were not statistically significant (P=0.132 and P=0.129, respectively). Levels of testosterone were 0.23±0.18 vs 0.27±0.21 (P=0.302), and those of progesterone were 0.582±0.323 vs 0.628±0.347 (P=0.354). Estradiol levels in both groups were <18 pmol/L, which was not significantly different after treatment.
iTRAQ analysis of tear proteins
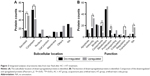
A total of 2,411 proteins were successfully identified in the iTRAQ analysis, among which 142 proteins were downregulated and 169 proteins were upregulated. To characterize the molecular features of tear proteins that exhibited alterations after treatment, subcellular location and functional categories were investigated (Figure 2A and B). Among the downregulated proteins, cytoplasmic (44.37%), secreted (10.56%), membrane, and other (both 8.45%) proteins comprised the top categories. The top three categories of upregulated proteins were secreted (28.99%), cytoplasmic (24.26%), and other proteins (15.38%). Functional category analysis showed that proteins involved in metabolism (33.80%), binding (14.79%), and cell components (13.38%) were among the top three types of downregulated proteins. The top three categories of upregulated protein functions were metabolism (31.36%), immunity (18.93%), and binding (11.83%).
The percentage and constituent ratios of the regulated proteins are shown in Table 3. After AC + AT treatment, secreted proteins were upregulated, and those residing in the cytoplasm were downregulated (Pearson’s χ2 test, P=0.000, P=0.000, respectively). In addition, proteins involved in immunity, regulation, and no annotation were upregulated (Pearson’s χ2 test, P=0.040, P=0.016, P=0.037, respectively), whereas cell components and proliferation-related proteins were downregulated (Pearson’s χ2 test, P=0.003, P=0.011, respectively).
  | Table 3 The percentage and constituent ratios of regulated proteins |
The regulated ratio was altered by more than 50%. To identify significantly regulated proteins, of which the location and functional categories are shown in Table 4, the downregulated ratio was set to <0.5, and the upregulated ratio was set to >2. These proteins included those involved in the inflammatory reaction and immune response such as polymeric immunoglobulin receptor and Apo A-I, apoptosis-related proteins such as Annexin A1 and aldehyde dehydrogenase, and cell components and metabolism-related proteins such as myosin-14 and neuroblast differentiation-associated protein AHNAK.
  | Table 4 The location and function categories of significantly regulated proteins |
Verification of proteins identified in tear fluid samples by Western blot analysis
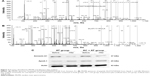
The images from MS/MS spectra of Annexin A1 and Apo A-I identified by LC-MS/MS are shown in Figure 3A and B. These two proteins were chosen to verify the observed changes by Western blotting. After treatment, Annexin A1 was downregulated and ApoA1 was upregulated in both groups, consistent with the proteomic results (Figure 3C).
Discussion
In recent studies, acupuncture therapy was determined to be effective for dry eye patients and was shown to be partially better compared with artificial tear treatment.10 Specifically, acupuncture relieved the symptoms and increased watery secretion.11 Our study validated these conclusions. In addition, we compared AC + AT treatment with artificial tear therapy alone. OSDI scores and symptoms were significantly improved in the AT + AC group compared to the AT group, indicating that acupuncture has a subjective and comprehensive beneficial effect on dry eye. One possible reason for this observation is that acupuncture can influence synthesis and secretion by the lacrimal gland. Others have postulated that acupuncture may reduce tension and alleviate pain intensity.12 Regardless, the mechanisms need to be confirmed. Acupuncture is reportedly associated with a significant increase in the secretion of serum estradiol as well as a reduction in the secretion of serum follicle-stimulating hormone and luteinizing hormone.13 However, an interesting phenomenon observed in this study was that the levels of these hormones remained the same. One possible reason for this could be that all the acupoints used in this study are located near the eye and are not gynecological acupoints.
Tear proteins have an important role in the maintenance of the ocular surface, and changes in the quality and quantity of tear components reflect changes in the health of the ocular surface. We successfully identified 2,411 proteins, among which 142 proteins were downregulated (ie, Annexin A1, myosin-14, neuroblast differentiation-associated protein AHNAK, leukocyte elastase inhibitor, and protein S100-A9) and 169 proteins were upregulated (ie, Apo A-I, complement C3, hemopexin, retinal dehydrogenase 1, clusterin, and ceruloplasmin).
Among the downregulated proteins, 44.37% are located in the cytoplasm; 28.99% of the upregulated proteins are secreted. This suggests that after treatment, more proteins are secreted into the tear fluid from the intracellular space. The pathogenesis of dry eye was traditionally ascribed to a decrease in tear production and decreased tear stability. As the mucous layer is composed of secretory, gel-forming, and transmembrane ocular surface mucus to stabilize the tear film,14 the observed upregulation of secreted proteins suggests increased production and tear stability.
The constituent ratio of protein function indicates that immunity-related proteins were upregulated after treatment and that those involved in apoptosis were downregulated. Dry eye has many causes, including inflammation and apoptosis, and anti-inflammation and ocular surface repair have proven to be useful in treating this condition.15 Proteins involved in transport, signal transduction, and regulation were found to be upregulated, and cell components and proliferation-related proteins were relatively downregulated. This interesting phenomenon suggests that acupuncture and artificial tear treatment may increase protein synthesis. Although the exact mechanisms of acupuncture are currently unknown, it is likely that the nervous system, neurotransmitters, and endogenous substances are involved in needle stimulation.16
Annexin A1, an endogenous anti-inflammatory molecule described as a repressor of the innate immune response,17 is a calcium-binding protein that promotes phagocyte migration and infiltration of granulocytes at wound sites and acts as an antimicrobial agent.18 Annexin A1 is upregulated under pathological conditions, such as in multiple sclerosis and hypoxic-ischemic brain damage. In addition, Annexin A1 protects against N-methyl-D-aspartate-induced brain damage, and Annexins I and V exert neurotrophic effects on cultured neurons.19 Thus, downregulated Annexin A1 after treatment indicated improvement in pathological conditions. S100-A9 is an inflammation-associated protein mainly expressed in granulocytes and epithelial cells; it is upregulated in tears of human dry eye patients and is associated with the severity of symptoms.20 A100-A9 is also upregulated in Sjögren’s syndrome, which is associated with dry eye and induces autoimmune dacryoadenitis in a rabbit model.21 As a first-line vertebrate immune defense, the polymeric immunoglobulin receptor transports polymeric IgA and IgM across the epithelium to mucosal secretions, whereby the cleaved ectodomain becomes a component of secretory antibodies or when the unbound ligand form binds and excludes bacteria.22 The fact that inflammation-associated proteins were downregulated after treatment indicates that a regional inhibitory inflammatory reaction occurred. One assumption that can be drawn from this is that the observed downregulated inflammatory proteins were induced by the stimulation of acupuncture, decreasing the release of inflammatory cytokines and the severity of dry eye.
The major apoprotein of high-density lipoprotein, Apo A-I is classically described as being synthesized only by the liver and the intestine. However, Apo A-I was recently found in the vitreous fluid and human RPE cells.23 Apo A-I has a protective effect on the corneal epithelium and may improve the epithelial conditions of the ocular surface.24 Clusterin is also present in human tears. It has been demonstrated that clusterin prevents and ameliorates ocular surface barrier disruption via a remarkable sealing mechanism that is dependent on the attainment of a critical all-or-none concentration in the tears: when the clusterin level drops below the critical all-or-none threshold, the barrier becomes vulnerable to desiccating stress.25 Clusterin indirectly enhances the colony-forming efficacy of corneal and limbal epithelial cells, suggesting its role in the epithelia–mesenchymal interaction.26 Serotransferrin is an iron-binding monomeric glycoprotein belonging to the innate immune system, and a significant decrease in transferrin in the tears of patients with early dry eye has been reported.27 These upregulated proteins observed after treatment may thus result in improvements in the ocular surface.
This study is an initial observation on the effects of acupuncture treatment on the clinical signs and tear composition of postmenopausal women with dry eye.
Limitations
The limitation of this study is its small sample size; indeed, results based on a larger sample size may be more convincing. In addition, the acupuncture course in this study was only 2 months, and we believe that a longer acupuncture course may have more obvious effects.
Conclusion
The purpose of this study was to investigate the clinical signs and changes in tear proteins after acupuncture among postmenopausal women. In the present study, AC + AT treatment increased protein synthesis and secretion and improved clinical symptoms. These results indicate that acupuncture may be a complementary therapy for postmenopausal dry eye.
Acknowledgment
This work was supported by National Natural Science Foundation of China in 2014 (project number: 81470648) and Shanghai Municipal Health Bureau Scientific Research Program in 2012 (project number: 20124100).
Disclosure
The authors report no conflicts of interest in this work.
References
Schaumberg DA, Buring JE, Sullivan DA, Dana MR. Hormone replacement therapy and dry eye syndrome. JAMA. 2001;286(17):2114–2119. | ||
Calonge M. The treatment of dry eye. Surv Ophthalmol. 2001;45(Suppl 2):S227–S239. | ||
Truong S, Cole N, Stapleton F, Golebiowski B. Sex hormones and the dry eye. Clin Exp Optom. 2014;97(4):324–336. | ||
Feng Y, Feng G, Peng S, Li H. The effects of hormone replacement therapy on dry eye syndromes evaluated by Schirmer test depend on patient age. Cont Lens Anterior Eye. 2016;39(2):124–127. | ||
Manson JE, Hsia J, Johnson KC, et al. Women’s Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349(6):523–534. | ||
Beral V, Bull D, Reeves G. Million Women Study Collaborators. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005;365(9470):1543–1551. | ||
Dodin S, Blanchet C, Marc I, et al. Acupuncture for menopausal hot flushes. Cochrane Database Syst Rev. 2013;(7):CD007410. | ||
Kim TH, Kang JW, Kim KH, et al. Acupuncture for the treatment of dry eye: a multicenter randomised controlled trial with active comparison intervention (artificial teardrops). PLoS One. 2012;7(5):e36638. | ||
Shin MS, Kim JI, Lee MS, et al. Acupuncture for treating dry eye: a randomized placebo-controlled trial. Acta Ophthalmol. 2010;88(8):e328–e333. | ||
Yang L, Yang Z, Yu H, Song H. Acupuncture therapy is more effective than artificial tears for dry eye syndrome: evidence based on a meta-analysis. Evid Based Complement Alternat Med. 2015;2015:143858. | ||
Jeon JH, Shin MS, Lee MS, et al. Acupuncture reduces symptoms of dry eye syndrome: a preliminary observational study. J Altern Complement Med. 2010;16(12):1291–1294. | ||
Lee MS, Shin BC, Choi TY, Ernst E. Acupuncture for treating dry eye: a systematic review. Acta Ophthalmol. 2011;89(2):101–106. | ||
Chiu HY, Hsieh YJ, Tsai PS. Acupuncture to reduce sleep disturbances in postmenopausal and postmenopausal women: a systematic review and meta-analysis. Obstet Gynecol. 2016;127(3):507–515. | ||
Mantelli F, Massaro-Giordano M, Macchi I, Lambiase A, Bonini S. The cellular mechanisms of dry eye: from pathogenesis to treatment. J Cell Physiol. 2013;228(12):2253–2256. | ||
Sosne G, Kleinman HK. Primary mechanisms of thymosin β4 repair activity in dry eye disorders and other tissue injuries. Invest Ophthalmol Vis Sci. 2015;56(9):5110–5117. | ||
Zhang A, Yan G, Sun H, et al. Deciphering the biological effects of acupuncture treatment modulating multiple metabolism pathways. Sci Rep. 2016;6:19942. | ||
Yazid S, Gardner PJ, Carvalho L, et al. Annexin-A1 restricts Th17 cells and attenuates the severity of autoimmune disease. J Autoimmun. 2015;58:1–11. | ||
Kerschbaum HH, Donato R, Hermann A. Annexin-immunoreactive proteins in the nervous system and eye of the gastropods, Aplysia and Helix. Brain Res. 1997;746:133–140. | ||
Sun J, Shao Z, Yang Y, Wu D, Zhou X, Yuan H. Annexin 1 protects against apoptosis induced by serum deprivation in transformed rat retinal ganglion cells. Mol Biol Rep. 2012;39(5):5543–5551. | ||
Zhou L, Beuerman RW, Chan CM, et al. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Proteome Res. 2009;8:4889–4905. | ||
Zhou L, Wei R, Zhao P, Koh SK, Beuerman RW, Ding C. Proteomic analysis revealed the altered tear protein profile in a rabbit model of Sjögren’s syndrome-associated dry eye. Proteomics. 2013;13(16):2469–2481. | ||
Stadtmueller BM, Huey-Tubman KE, López CJ, Yang Z, Hubbell WL, Bjorkman PJ. The structure and dynamics of secretory component and its interactions with polymeric immunoglobulins. Elife. 2016;5.pii: e10640. | ||
Simó R, Higuera M, García-Ramírez M, Canals F, García-Arumí J, Hernández C. Elevation of apolipoprotein A-I and apolipoprotein H levels in the vitreous fluid and overexpression in the retina of diabetic patients. Arch Ophthalmol. 2008;126(8):1076–1081. | ||
Nyunt AK, Ishida Y, Yu Y, Shimada S. Topical apolipoprotein A-1 may have a beneficial effect on the corneal epithelium in a mouse model of dry eye: a pilot study. Eye Contact Lens. 2008;34(5):287–292. | ||
Fini ME, Bauskar A, Jeong S, Wilson MR. Clusterin in the eye: an old dog with new tricks at the ocular surface. Exp Eye Res. 2016;147:57–71. | ||
Okada N, Kawakita T, Mishima K, et al. Clusterin promotes corneal epithelial cell growth through upregulation of hepatocyte growth factor by mesenchymal cells in vitro. Invest Ophthalmol Vis Sci. 2011;52(6):2905–2910. | ||
Versura P, Bavelloni A, Grillini M, Fresina M, Campos EC. Diagnostic performance of a tear protein panel in early dry eye. Mol Vis. 2013;19:1247–1257. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.